Abstract
Context GC-MS analysis is the best way to characterize volatile sulphur-containing compounds. Ferula (Apiaceae) is a genus of perennial herbs. Due to the occurrence of essential oils or oleoresins in the Ferula species, these plants usually possess strong aromatic scent. Terpenoid compounds were the most abundant constituents of Ferula oils, however, in some of Ferula species, the essential oils were dominated by volatile sulphur-containing compounds.
Objectives Ferula alliacea Boiss. is considered one of the sources of the oleo-gum-resin asafoetida. In this study, we analyzed the hydrodistilled essential oil from its dried roots and provide new data about retention indices and mass fragmentation patterns of some volatile sulphur-containing compounds that are useful for future studies on this class of compounds.
Materials and methods The roots of F. alliacea were collected during the flowering stage of plant, from Bezgh, Kashmar to Neishabour road, Khorasan-Razavi province, Iran, in June 2012. The oil was obtained by hydrodistillation using a Clevenger apparatus and analyzed by GC-MS.
Results This is the first report on phytochemical analysis of F. alliacea roots. Seventy-six components, representing 99.5% of the oil, were characterized. The major components were 10-epi-γ-eudesmol (22.3%), valerianol (12.5%), hinesol (8.3%), guaiol (7.3%) and Z-propenyl-sec-butyl trisulphide (6.5%). Predominant mass fragment ions of the identified sulphur-containing compounds are explained in this paper.
Conclusion The volatile oil of F. alliacea mostly contains oxygenated sesquiterpenes, however, its odour was dominated by sulphur-containing compounds. The most abundant sulphur-containing compound includes Z-propenyl-sec-butyl trisulphide (6.5%).
Introduction
Ferula (Apiaceae), a genus of perennial herbs, comprises about 170 species distributed from central Asia (particularly Iran and Afghanistan) westward throughout the Mediterranean region to northern Africa (Pimenov & Leonov Citation1993). Thirty species of Ferula have been represented in Iranian flora, of which 15 species are endemic to Iran (Mozaffarian Citation1983, Citation1996). To date, more than 70 species of this genus have been chemically investigated (Iranshahi et al. Citation2008c, Citation2010a,Citationb; Sahebkar & Iranshahi Citation2011; Kasaian et al. Citation2014). Based on the findings, Ferula spp. have been turned out to be a source of bioactive phytochemicals such as sesquiterpene/sesquiterpene coumarin derivatives (Iranshahi et al. Citation2004, Citation2007, Citation2008d), and sulphur-containing compounds (Iranshahi et al. Citation2003, Citation2006, Citation2008b; Sahebkar et al. Citation2010; Iranshahi Citation2012).
The most important medicinal Ferula species include F. assa-foetida Boiss. & Buhse, F. gummosa Boiss. F. foetida Regel, F. latisecta Rech. f. & Aellen and F. persica Willd. Previous research also unveiled a number of medicinal properties form Ferula spp. including antibacterial, antifungal, antioxidant, anti-inflammatory, antinociceptive, anticonvulsant, antispasmodic and hypotensive activities (Bakkali et al. Citation2008; Asili et al. Citation2009; Maggi et al. Citation2009; Bagheri et al. Citation2010; Franz Citation2010; Chibani et al. Citation2012; Labed-Zouad et al. Citation2015).
Volatile sulphur compounds have been reported from different terrestrial plants (Iranshahi, Citation2012). One of the most well-known volatile sulphur compounds is allicin from Allium species. A large number of biological activities have been attributed to allicin and its analogues including antibacterial, antifungal, antiviral and antiprotozoal properties (Borlinghaus et al. Citation2014). However, the scaffold of volatile sulphur-containing compounds of Ferula species is unique and has only been reported from the genus Ferula (Iranshahi Citation2012). There are a few reports on the biological activities of volatile sulphur compounds from Ferula species such as their antifungal property against human dermatophytes (Iranshahi et al. Citation2008a).
Ferula alliacea Bioss. is used in Iranian folklore as one of asafoetida sources with similar medicinal applications to those of F. assa-foetida. This plant possessed a strong sulphurous odour due to the presence of volatile sulphur-containing compounds. No phytochemical investigation has been conducted on the volatile sulphur compounds of this species. In this study, we aimed to identify chemical composition of the essential oil of F. alliacea roots for the first time. The spectral mass fragmentation patterns of sulphur-containing compounds are also discussed in this paper. These mass fragmentation patterns can provide useful information for researchers who are interested in identifying the volatile sulphur-containing compounds of this genus.
Materials and methods
Plant material
The roots of F. alliacea were collected during the flowering stage of plant, from Bezgh, Kashmar to Neishabour road, Khorasan-Razavi province, Iran, in June 2012. The plant was identified by Mr. Joharchi and a voucher specimen (No. 38927) was deposited at the Herbarium of the Department of Pharmacognosy, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Essential oil preparation
Essential oil (EO) was isolated using hydro-distillation of air-dried material for 3 h, with a Clevenger-type apparatus. The oil was obtained as a greenish yellow liquid with a yield of 0.13% (v/w). The oil was dried over anhydrous sodium sulphate and stored at 4 °C in dark until use. The EO was dissolved in n-hexane for gas chromatography and mass spectrometry analyses.
GC-MS analysis
The GC-MS analyses were performed using an Agilent 5975 apparatus (Agilent Technologies, Santa Clara, CA) with a HP-5 MS column (30 m × 0.25 mm i.d., 0.25 m film thicknesses) interfaced with a quadruple mass detector and a computer equipped with Wiley 7n. Library. Other analytical settings were: oven temperature: 50 °C (5 min), 50–250 °C (3 °C/min), 250 °C (10 min); injector temperature 250 °C; injection volume: 0.1 L; split ratio: 1:50; carrier gas: helium at 1.1 mL/min; ionization potential: 70 eV; ionization current: 150 A; and mass range: 35–465 mui. Identification of individual compounds was made by comparison of their mass spectra and retention indices (RI) with those of authentic samples and those given in the literature (Khajeh et al. Citation2005; Adams Citation2007). Quantification of the relative amount of each individual component was performed according to the area percentage method without consideration of calibration factor.
Results and discussion
The oil of the roots of Ferula alliacea was greenish yellow, in a total yield of 0.13% v/w. Seventy-six components were identified from the oil of this species (), representing 99.5% of the total oil. GC-MS analysis of the essential oil of F. alliacea roots led to the identification of mono- and sesquiterpenes as well as volatile sulphur-containing compounds. Although oxygenated sesquiterpenes were determined to be the most abundant constituents in the essential oil, the odour of the oil was dominated by volatile sulphur-containing compounds. The essential oil contained oxygenated sesquiterpenes (74.7%), sulphur-containing compounds (16.6%), sesquiterpene hydrocarbons (3.7%) and oxygenated monoterpenes (2.6%). The major components of the oil were determined to be 10-epi-γ-eudesmol (22.3%), valerianol (12.5%), hinesol (8.3%), guaiol (7.3%) and Z-propenyl-sec-butyl trisulphide (6.5%). The sulphur-containing compounds (16.6% of the oil, ) were identified to be 3,4-dimethyl thiophene (trace), 2,3,4-trimethyl thiophene (trace), propyl sec-butyl disulphide (0.1%), Z-1-propenyl sec-butyl disulphide (2.2%), E-1-propenyl sec-butyl disulphide (1.9%), di-sec-butyl disulphide (0.1%), methyl 1-(methylthio) propyl disulphide (0.1%), Z-1-propenyl propyl trisulphide (0.3%), E-1-propenyl propyl trisulphide (0.4%), Z-1-propenyl sec-butyl trisulphide (6.5%), E-1-propenyl sec-butyl trisulphide (2.7%), sec-butenyl sec-butyl trisulphide (0.1%) and mintsulphide (0.3%). The volatile sulphur-containing compounds were characterized on the basis of their retention indices (Iranshahi Citation2012) and mass spectral fragmentation patterns ( and ).
Figure 1. Major mass fragmentation ions of the volatile sulphur-containing compounds from Ferula alliacea.
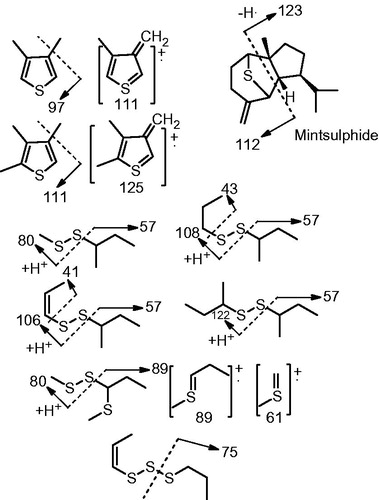
Table 1. Chemical composition of the essential oil from the roots of Ferula alliacea Boiss.
Table 2. Mass fragmentation ions observed for volatile sulphur-containing compounds from the essential oil of Ferula alliacea roots. Predominant ions have shown in bold.
In general, sec-butyl part of sulphur-containing compounds is considered as the chemotaxonomic marker of the family Apiaceae. sec-Butyl derivatives of sulphur-containing compounds have previously been reported from Ferula species including F. assa-foetida (Iranshahy & Iranshahi Citation2011), F. latisecta, (Iranshahi et al. Citation2008b; Sahebkar et al. Citation2010), F. persica (Iranshahi et al. Citation2003, Citation2006) and F. foetida (Chitsazian-Yazdi et al. Citation2015). As shown in and , the m/z = 89 ion peak is produced by the [sec-butyl-S] ion that could be formed by splitting the molecular ion at the SS or SSS bonds. This ion peak is usually observed as base peak. The m/z = 75 ion peak is also produced by the [propyl-S] ions and observed as a predominant peak in propyl-containing sulphur compounds. Considering thiophene derivatives, [M-H]+ (111 and 125) and [M-CH3]+ (97 and 111) are predominant mass fragmentation ions in their mass spectra (). Other common fragmentation ion peaks in the mass spectra of sulphur-containing compounds include 57, 41 and 43 attributable to [isobutyl], [allyl] and [propyl] ions, respectively.
In total, GC-MS analysis is the best approach to characterize sulphur-containing compounds. The characterization of these compounds is not commonly carried out using NMR techniques due but their small quantities and volatility. In this study, we tried to provide new information about the retention indices and mass fragmentation patterns of some volatile sulphur-containing compounds that are useful for future studies on this class of compounds.
The volatile oil of F. alliacea mostly contains oxygenated sesquiterpenes, however, its odour was dominated by sulphur-containing compounds. The most abundant sulphur-containing compound includes Z-propenyl-sec-butyl trisulphide (6.5%).
This plant is used by local people as an anthelminthic and antispasmodic herb for gastrointestinal disorders. There is a gap in our knowledge about the biological activities of sulphur components of F. alliacea and other Ferula species that needs further investigation; however, sulphur compounds may play a role, at least in part, in biological activities of this plant.
Funding information
This research was supported by Mashhad University of Medical Sciences (MUMS) Research Council.
Acknowledgements
The authors would like to thank Mr. H. Joharchi for his assistance in plant identification.
Disclosure statement
The authors report no declarations of interest.
References
- Adams RP. 2007. Identification of essential oil components by gas chromatography quadropole mass spectroscopy. IL: Allured Publ Corp Carol Stream.
- Asili J, Sahebkar A, Fazly Bazzaz BS, Sharifi S, Iranshahi M. 2009. Identification of essential oil components of Ferula badrakema fruits by GC-MS and 13C-NMR methods and evaluation of its antimicrobial activity. J Essent Oil-Bearing Plants. 12:7–15.
- Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. 2010. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 48:242–246.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. 2008. Biological effects of essential oils – a review. Food Chem Toxicol. 46:446–475.
- Borlinghaus J, Albrecht F, Gruhlke MCH, Nwachukwu ID, Slusarenko AJ. 2014. Allicin: chemistry and biological properties. Molecules. 19:12591–12618.
- Chibani S, Bensouici C, Kabouche A, Aburjai T, Touzani R, Kabouche Z. 2012. Analysis of the essential oil of aerial parts of Ferula lutea Poiret from Algeria. J Essent Oil-Bearing Pl. 15:682–685.
- Chitsazian-Yazdi M, Agnolet S, Lorenz S, Schneider B, Es'haghi Z, Kasaian J, Khameneh B, Iranshahi M. 2015. Foetithiophenes C-F, thiophene derivatives from the roots of Ferula foetida. Pharm Biol. 53:710–714.
- Franz CM. 2010. Essential oil research: past, present and future. Flav Fragr J. 25:112–113.
- Iranshahi M. 2012. A review of volatile sulfur-containing compounds from terrestrial plants: biosynthesis, distribution and analytical methods. J Essent Oil Res. 24:393–434.
- Iranshahi M, Amin G, Amini M, Shafiee A. 2003. Sulfur containing derivatives from Ferula persica var. latisecta. Phytochemistry. 63:965–966.
- Iranshahi M, Amin G, Salehi-Sourmaghi MH, Shafiee A, Hadjiakhoondi A. 2006. Sulphur-containing compounds in the essential oil of the root of Ferula persica Willd.var. persica. Flav Fragr J. 21:260–261.
- Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, Piacente S, Pizza C. 2007. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 68:554–561.
- Iranshahi M, Fata A, Emami B, Shahri BMJ, Bazzaz BSF. 2008a. In vitro antifungal activity of polysulfides-rich essential oil of Ferula latisecta fruits against human pathogenic dermatophytes. Nat Prod Commun. 3:1543–1546.
- Iranshahi M, Hassanzadeh-Khayat M, Fazly Bazzaz BS, Sabeti Z, Enayati F. 2008b. High content of polysulphides in the volatile oil of Ferula latisecta Rech. F. et Aell. fruits and antimicrobial activity of the oil. J Essent Oil Res. 20:183–185.
- Iranshahi M, Hassanzadeh-Khayyat M, Sahebkar A, Famili A. 2008c. Chemical composition of the fruit oil of Ferula flabelliloba. J Essent Oil-Bearing Plants. 11:143–147.
- Iranshahi M, Kalategi F, Rezaee R, Shahverdi AR, Ito C, Furukawa H, Tokuda H, Itoigawa M. 2008d. Cancer chemopreventive activity of terpenoid coumarins from Ferula species. Planta Med. 74:147–150.
- Iranshahi M, Kalategi F, Sahebkar A, Sardashti A, Schneider B. 2010b. New sesquiterpene coumarins from the roots of Ferula flabelliloba. Pharm Biol. 48:217–220.
- Iranshahi M, Masullo M, Asili A, Hamedzadeh A, Jahanbin B, Festa M, Capasso A, Piacente S. 2010a. Sesquiterpene coumarins from Ferula gumosa. J Nat Prod. 73:1958–1962.
- Iranshahi M, Shahverdi AR, Mirjani R, Amin G, Shafiee A. 2004. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratiamarcescens. Z Naturforsch C. 59:506–508.
- Iranshahy M, Iranshahi M. 2011. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assafoetida oleo-gum-resin) – a review. J Ethnopharmacol. 134:1–10.
- Kasaian J, Iranshahy M, Masullo M, Piacente S, Ebrahimi F, Iranshahi M. 2014. Sesquiterpene lactones from Ferula oopoda and their cytotoxic properties. J Asian Nat Prod Res. 16:248–253.
- Khajeh M, Yamini Y, Bahramifar N, Sefidkon F, Pirmoradei MR. 2005. Comparison of essential oils compositions of Ferula assa-foetida obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem. 91:639–644.
- Labed-Zouad I, Labed A, Laggoune S, Zahia S, Kabouche A, Kabouche Z. 2015. Chemical composition and antibacterial activity of four essential oils from Ferula vesceritensis Coss. & Dur. against clinical isolated and food-borne pathogens (Apiaceae). Rec Nat Prod. 9:518–525.
- Maggi F, Cecchini C, Cresci A, Coman MM, Tirillini B, Sagratini G, Papa F. 2009. Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L. (F. communis L. subsp. glauca) growing in Marche (central Italy). Fitoterapia. 80:68–72.
- Mozaffarian V. 1983. The family of Umbelliferae in Iran-Keys and distribution, vol. 54. Tehran, Iran: Research Institute of Forests and Rangelands Press. p. 378–415.
- Mozaffarian V. 1996. A dictionary of Iranian plant names. Tehran: Farhang-eMoaser. p. 228–230.
- Pimenov MG, Leonov MV. 1993. The genera of the Umbelliferae. Kew: Royal Botanic Gardens.
- Sahebkar A, Hassanzadeh-Khayat M, Iranshahi M. 2010. Qualitative analysis of the hydro-distilled essential oil of Ferula latisecta Rech. f. and Aell. roots from Iran. J Essent Oil Bearing Plants. 13:340–346.
- Sahebkar A, Iranshahi M. 2010. Biological activities of essential oils from the genus Ferula (Apiaceae). Asian Biomed. 4:835–847.
- Sahebkar A, Iranshahi M. 2011. Volatile constituents of the genus Ferula (Apiaceae): a review. J Essent Oil-Bearing Plants. 14:504–531.
