Abstract
Context Although olive mill wastewater (OMWW) is a good source of bioactive phenolic compounds, disposing OMWW is a serious environmental challenge. Production of wine via fermenting OMWW may be a promising alternative to deal with OMWW. However, whether or not olive wine from OMWW still reserves its original bioactivities remains unclear.
Objective This study examines antioxidant activity of olive wine fermented from OMWW.
Materials and methods Hydroxytyrosol in olive oil was determined by HPLC. Total flavonoid, total polyphenol and in vitro antioxidant activities were measured by spectrophotometry. Aged mice were intragastricly administered 7, 14 and 28 mL/kg olive wine consecutively for 30 d. Afterward, levels of malonaldehyde (MDA), protein carbonyl, reduced glutathione (GSH) and activity of superoxide dismutase (SOD) were assayed in mouse plasma and liver.
Results Contents of hydroxytyrosol, total flavonoid and total polyphenol in olive wine were 0.14 ± 0.01, 0.29 ± 0.06 and 0.43 ± 0.03 mg/mL, respectively. The IC50 value of olive wine to scavenge DPPH and hydroxyl free radicals was 2.5% and 3.2% (v/v), respectively. Compared with the solvent control group, olive wine with a dose of 28 mL/kg remarkably lowered mouse MDA concentration in liver, and reduced protein carbonyl level in plasma (p < 0.05). Meanwhile, olive wine at doses of 7 and 28 mL/kg notably enhanced SOD activity in both mouse plasma and liver (p < 0.05). The beneficial effect on liver was superior to that of γ-tocopherol.
Conclusion The study demonstrated that olive wine from OMWW has potential for treating oxidative stress-associated diseases.
Introduction
Olive oil, a popular component of the Mediterranean diet, possesses a variety of biological activities. For example, apart from its antioxidant, anti-inflammatory and anticancer functions (Cardeno et al. Citation2013), olive oil is able to improve cognition (Martinez-Lapiscina et al. Citation2013), protect neurodegradation (Grossi et al. Citation2013), reduce the risk of developing chronic diseases, such as depression, metabolic syndrome, cardiovascular diseases (Chedraui & Perez-Lopez Citation2013), and so on (Lakshminarayana & Baskaran Citation2013; Parzonko et al. Citation2013). The manufacture of olive oil includes the crushing of olives, followed by the aqueous extraction of the oil and the subsequent separating oil from the aqueous phase by centrifugation (Dermechea et al. Citation2013; Frankel et al. Citation2013). As a result of three-phase decanter systems, huge volumes of olive mill wastewater (OMWW) are generated as a byproduct. The disposal of OMWW is becoming a major challenge for producers. Improper discharge of OMWW on soil will influence soil characteristics, plant growth and phytotoxicity (Barbera et al. Citation2013). For example, due to sodium and potassium replacement of soil cations, the salt level of an alkaline soil increased after it was polluted by OMWW, along with the elevated soil C/N ratio (Paredes et al. Citation1987). OMWW alters the original bacteria balance in soil as well. On one hand, the enrichment of C prompts the growth of some microflora. On the other hand, polyphenols abundant in OMWW may be toxic to certain microorganisms (Barbera et al. Citation2013).
Since olive oil has multiple beneficial functions, OMWW may also possess some unique properties that can be exploited. Studies verified that OMWW was capable of acting as a surfactant to reduce hydrophobicity of sandy soil (Diamantis et al. Citation2013). Due to its high content of organic ingredients, OMWW is a potential raw material to manufacture bio-ethanol through fermentation (Zanichelli et al. Citation2007). Apart from its possible application in agriculture, OMWW displayed some important pharmacological activities. OMWW enriched in hydroxytyrosyl (HT) successfully alleviated skeletal muscle function decline originated from age-related oxidative stress (Pierno et al. Citation2014). HT-rich OMWW attenuated the cytotoxicity to murine brain cells triggered by Fe2+ and NO, indicating its potential brain protection activity (Schaffer et al. Citation2007). Owing to diverse polyphenols abundant in OMWW, it exhibited potent antioxidant (Leger et al. Citation2000; Ranalli et al. Citation2003; Ghanbari et al. Citation2012; Taheri et al. Citation2014), hypoglycaemic (Hamden et al. Citation2009) and hypocholesterolaemic capacity (Fki et al. Citation2007).
As it is well known, due to the high content of resveratrol and polyphenols, juice and wine made from grape reduce the risk of cardiovascular diseases. Since HT-enriched OMWW shares significant similarity with grape juice in compositions and functions, it was hypothesized that wine fermented from OMWW may also reserve its original biological activities and present marketing opportunities in the health care sector, as grape wine does. Moreover, the low alcohol content in wine helps to preserve the active ingredients and suppress the growth of spoilage bacteria and mildew. For this consideration, another utility of OMWW, to make olive wine, began to arise in some olive oil manufacturers of China. However, whether or not olive wine indeed reserves the bioactivity of OMWW has not yet been well documented. In this study, both in vitro and in vivo antioxidant activities of olive wine fermented from OMWW was assessed to determine its possible application as a functional wine.
Materials and methods
Materials
Olive wine, fermented from OMWW by yeast, with batch number of 130312, was kindly donated by Sichuan Tianyuan Olive Corporation. The manufacture process includes the following steps: after the treatment with pectinase, OMWW was filtered and collected in a container. Then glucose and honey was added to adjust flavour, followed by fermentation using yeast twice at different time intervals. The fermented solution stood under ambient temperature for a sufficient period to produce enough alcohol, and was made clear via clarificants and filtering.
The stable free radical diphenyl-β-picrylhydrazyl (DPPH) was purchased from Aldrich (Tokyo, Japan). Rutin, a control for total flavonoids measurement, was obtained from National Institutes for Drug and Food Control, Beijing, China. HT used in HPLC analysis was from Xian Tianyi Biological Co., Ltd, Xian, China. Xanthine was from Shanghai Suolaibao Bio-Technology Co., Ltd, Shanghai, China. Xanthine oxidase was from Shanghai Enzyme Couple Bio-Technology Co., Ltd, Shanghai, China. Reagents for HPLC assay were of chromatographic purity. Others were purchased from Chengdu Kelun Reagent Company, Chengdu, China, and were of analytical grade.
Analysis of HT, total flavonoids, total polyphenol and alcohol in olive wine
HT content of olive wine was determined by HPLC. Samples were separated on a Dima C18 column (250 mm × 4.6 mm, 5 μm). The column temperature was maintained at 30 °C. The mobile phase consisted of methanol:0.2% phosphate solution (12:88) with a flow rate of 1 mL/min. Detection was carried out at wavelength of 230 nm. Samples of 20 μL were injected into the system and HT concentration was calculated via the external standard curve (Li et al. Citation2014).
To determine total polyphenol content in olive wine, Folin–Ciocalteu’s phenol reagent was prepared following the procedures described by Eidi et al. (Citation2013). Total polyphenol was denoted as mg/mL gallic acid equivalents. Gallic acid standard solutions at concentration of 0.01, 0.02, 0.03, 0.04 and 0.05 mg/mL were prepared with methanol, and a 0.5 mL aliquot was mixed with 2.5 mL of 10-fold diluted Folin–Ciocalteu’s reagent and 2 mL of 7.5% sodium carbonate (w/v), respectively. After standing at room temperature for 30 min, the solutions were subject to absorbance measurement at 760 nm. Likewise, olive wine was diluted 10-fold with methanol and manipulated in the same manner. Polyphenol content of olive wine was derived from standard curve of gallic acid.
Content of total flavonoids was assayed according to the spectrometric approach of Samatha et al. (Citation2012) with some modification. In general, test substance of 3 mL was drawn to a volumetric flask of 25 mL, mixed with 1.0 mL of 5% sodium nitrite (w/v) and stood for 6 min. Then 1 mL of 10% aluminium nitrate (w/v) was added and stood for another 6 min. After the addition of 5 mL of 4% sodium hydroxide (w/v), the solution was diluted to graduation with distilled water. The flask was shaken intermittently to blend the solution and placed at ambient temperature for 15 min, after which the absorbance at 506 nm was read. A blank sample without aluminium nitrate was prepared and assayed as described to eliminate the interference from sample colour. Total flavonoids in samples were calculated with respect to the control solution of rutin.
Alcohol concentration in olive wine was analyzed by GC. HP-5 (30 m × 0.53 mm i.d., 2.65 μm) was employed as the analytical column with FID detector. Column temperature was maintained at 33 °C for 5 min, then raised to 80 °C at the rate of 5 °C/min, followed by further elevation to 260 °C at 35 °C/min. Column temperature was kept at 260 °C for 20 min. The temperature of injector and the detector was set at 220 °C and 260 °C, respectively. Injection volume was 2 μL (Yao et al. 2001). Ethanol content in olive wine was obtained through external standard method.
Antioxidant activities in vitro
In vitro antioxidant activities of olive wine, including the capacity for scavenging DPPH free radicals, hydroxyl free radicals and ferric reducing antioxidant power (FRAP) were evaluated. Ascorbic acid at various concentrations was employed as control solutions in the following experiments. Meanwhile, olive wine was diluted to different concentrations with water. Clearance rate of DPPH by olive wine was determined in accordance with the approach proposed by Taheri et al. (Citation2014). Briefly, 2.0 mL of the sample was mixed with 2.0 mL of 80 μg/L DPPH-ethanol solution, and was kept under room temperature for 30 min to achieve reaction equilibrium. The absorbance of the solution at 517 nm was then measured.
The ability of various concentrations of olive wine to scavenge hydroxy radicals was examined using the method of Gu et al. (Citation2008). In general, 1.0 mL of the test sample was mixed with 2.0 mL of 0.028% FeSO4 (w/v) and 1.5 mL of 0.025% salicylic acid–ethanol solution (w/v), followed by the addition of 0.1 mL of 0.3% (v/v) H2O2 to trigger the reaction. After the reaction system was maintained at 37 °C for 30 min, the absorbance of 510 nm was assayed.
The FRAP of olive wine was determined by the method reported by Yen and Chen (Citation1995). In brief, the test sample was blended with 2.5 mL of pH 6.6 phosphate buffer and 2.5 mL of 1% K3Fe(CN)6 solution (w/v), and was kept at 50 °C for 20 min. Afterward, the solution was allowed to cool to ambient temperature and mixed with 2.5 mL of 10% trichloroacetic acid (w/v), from which a 2.5 mL aliquot was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1% FeCl3 (w/v). After standing at room temperature for 30 min, the absorbance at 700 nm of the solution was read.
Animals
Female Kunming mice, weighing 20–22 g, were purchased from Sichuan Experimental Animal Center and raised under 25 °C with a humidity of 60–65% and the cycle of 12 h day light and 12 h darkness. Both raising conditions and experimental protocol conformed to the experimental animal guidance of Chinese Good Laboratory Animal Care and Use Practice as well as the principles in Declaration of Helsinki.
When mouse weights exceeded 25 g, they were randomly divided into seven groups, eight mice per group. The administration protocol was carried out according to . Subcutaneous injection was performed every day from the beginning till the end of the experiment when all mice were sacrificed. Intragastric administration (ig) started on day 15 of subcutaneous injection and lasted consecutively, once a day, for 30 d. On day 30 of ig feeding, all mice were sacrificed by being exposed to carbon dioxide. Blood was collected in tubes pre-coated with heparin sodium. Livers were isolated, washed with saline and dried on filter paper for the experiment.
Table 1. Administration protocol.
Measurement of malonaldehyde (MDA) level in blood and liver
Whole blood of one mouse was withdrawn to a tube and diluted with distilled water to constitute 2% haemolysate solution. Liver was cut into small pieces, from which a portion was placed into tissue homogenizer and ground with phosphate buffer solution (PBS) to prepare 2% homogenized liver solution. The measurement of MDA in blood and liver was carried out following the method reported by Nie et al. (Citation2013). In general, MDA reacts with thiobarbituric acid to produce a pink complex with a maximum absorbance of 532 nm. The intensity of the absorbance is proportional to MDA content.
Measurement of protein carbonyl level in blood and liver
Masses (0.1 g) of liver were ground in 0.24% cold HEPES buffer (pH 7.4) to yield a 10% homogenate. The homogenate was then centrifuged at 880 g for 10 min, and 450 μL of supernatant was withdrawn and mixed with 50 μL of 100 g/L streptomycin sulphate. After being kept under room temperature for 10 min, the solution was centrifuged at 11 780 g for a further 10 min. The supernatant of liver sample as well as plasma was applied for the assay. The measurement procedures were conducted as Razo-Estrada et al. (Citation2013) proposed. In short, oxidization of proteins leads to an increased content of carbonyl groups. Carbonyl groups react with 2,4-dinitrophenylhydrazine to generate 2,4-dinitrophenylhydrazone (DNTP), a red brown sediment which is capable of dissolving in guanidine hydrochloride and yielding the absorbance at 370 nm.
Determination of superoxide dismutase (SOD) activity in blood and liver
To prepare blood samples for the determination of SOD activity, 10 μL of whole blood was diluted with 0.5 mL of 0.9% saline (w/v), followed by centrifugation at 880 g for 3 min. After the supernatant was discarded, 0.2 mL of cold double distilled water was mixed well with the precipitant. Then 0.1 mL of 95% ethanol was added and vibrated for 30 s. The solution was extracted with 0.1 mL of chloroform using a vortex mixer. Afterward, centrifugation was carried out at 1560 g for 3 min and the upper layer was the extract solution containing SOD.
To measure SOD activity in liver, a portion of liver was weighed, cut into pieces and ground with cold saline in tissue homogenizer. The homogenization was repeated three times, 10 s per time with 30 s intervals between operations. In this way, 1% liver homogenate was prepared along with disrupted mitochondria. The solution was then centrifuged at 1560 g for 5 min and 20 μL of supernatant was used for further analysis.
The assay of SOD activity was conducted following the method reported by Das et al. (Citation2000) and Dey et al. (Citation2007). During the process, xanthine produces superoxide anions under the catalysis of xanthine oxidase. Superoxide anions attacked hydroxylamine hydrochloride to yield nitrite, which further reacts with methyl naphthylamine to form diazo compounds, which combine with p-aminobenzenesulphonic acid to generate red azoic compounds with a maximum absorbance of 530 nm. If there is SOD existing in samples, superoxide anions will be scavenged, resulting in the decreased absorbance at 530 nm.
Measurement of reduced glutathione (GSH) in blood and liver
To assay GSH concentration in blood, 0.1 mL of whole blood was thoroughly mixed with 0.9 mL of double distilled water until the solution became transparent. A 0.5 mL aliquot was then mixed with 0.1 mL of 4% sulphosalicylic acid (w/v), and centrifuged at 1200 g for 10 min. The supernatant was subjected to analysis.
To assess GSH concentration in liver, 10% liver homogenate was prepared by saline, from which a 0.5 mL aliquot was mixed with 0.5 mL of 4% sulphosalicylic acid, and centrifuged at 1200 g for 10 min. The supernatant was retained for analysis.
The measurement of GSH conformed to what Akerboom and Sies (Citation1981) and Forman et al. (Citation2009) described. In brief, under the catalysis of glutathione peroxidase, GSH reacts with 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) to form yellow 5-thio-nitro formic acid anion that has the maximum absorbance of 420 nm. The intensity is dependent on GSH concentration.
Statistical analysis
The data were expressed as mean ± standard deviation. Result comparisons were carried out using one-way ANOVA analysis. Variation was considered statistical significance when p < 0.05.
Results and discussion
Analysis of HT, total flavonoids and alcohol in olive wine
HT, total polyphenol and total flavonoids contents of olive wine used for the assessment of antioxidant activity in vitro as well as in vivo, was 0.14 ± 0.01, 0.43 ± 0.03, and 0.29 ± 0.06 mg/mL, respectively, implying that olive wine fermented from OMWW is abundant in antioxidant ingredients. Oleuropein was also assayed by HPLC, however, oleuropein was not detected in olive wine. It seemed that most oleuropein was hydrolyzed to HT during fermentation. Alcohol concentration determined by GC was 17% (v/v). Thus, the solvent control group in the animal experiment was given 17% alcohol through i.g., along with subcutaneous injection of galactose, to examine the potential antioxidant activity of olive wine.
Antioxidant activity in vitro
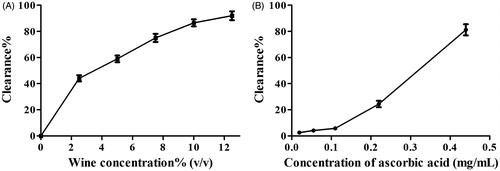
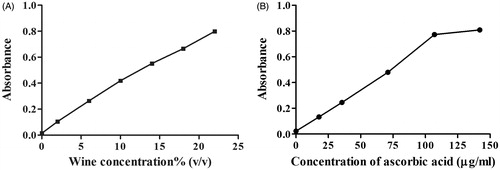
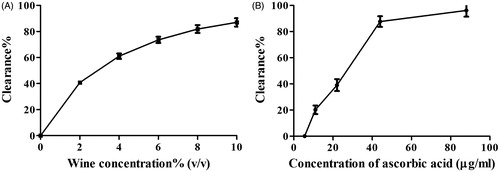
Antioxidant activities of olive wine in vitro, including scavenging DPPH free radicals and hydroxyl free radicals, as well as FRAP, are displayed in . It implied that the antioxidant activity of olive wine increased in a concentration-dependent manner. The IC50 value of olive wine to scavenge DPPH and hydroxyl free radicals was 2.5% and 3.2% (v/v), respectively, while the corresponding values for ascorbic acid were 30 μg/mL and 0.32 mg/mL ( and ), respectively, manifesting the potent power of olive wine in clearing hydroxyl free radicals. Olive wine also exhibited excellent activity in FRAP assay. When the concentration of olive wine and ascorbic acid was increased to 13% (v/v) and 72 μg/mL, respectively, the FRAP absorbance of both solutions amounted to 0.5. In all experiments, the activity of solvent control, 17% alcohol, was measured in parallel. Contribution of the solvent to the activities was deducted from all sample results.
Figure 1. Activity comparison of olive wine (A) and ascorbic acid (B) in scavenging DPPH free radicals.
Measurement of malonaldehyde level in blood and liver
Based on the proposed daily dose of 100 mL olive wine for a person with 70 kg weight, administration protocol in animal experiment was determined. MDA is the final peroxidation product of lipid in cell membrane. Its content reveals the extent of lipid peroxidation (Nielsena et al. Citation1997; Tug et al. Citation2005; Bhutia et al. Citation2011). At the end of animal experiment, plasma levels of MDA in the negative control group (subcutaneously injected with saline) and in the model group (injected with galactose) were 26.31 ± 12.13 and 39.47 ± 17.32 nmol/mL, respectively (p < 0.05), showing that after subcutaneous injection of galactose consecutively for 45 d, MDA concentration in plasma of the model group had been elevated substantially, establishing the validity of the aging model. MDA levels of other groups are shown in . Compared with the solvent control, the positive control group fed with γ-tocopherol lowered MDA concentration a little in plasma, while remarkably decreased MDA level in liver (p < 0.05). There was no obvious difference regarding MDA level in plasma between the mice fed with olive wine and those with solvent. However, hepatic MDA concentrations in mice given olive wine decreased in a dose-dependent mode. A dose of 28 mL/kg of olive wine significantly reduced hepatic MDA level (p < 0.05).
Table 2. MDA levels in plasma and liver of mice with different administration protocols.
Measurement of protein carbonyl level in blood and liver
Side chain of amino acids on protein will generate carbonyls under the attack of hydrogen peroxide and superoxide anion. When hydroxyl radicals attack peptide chain, the chain will break, alter protein primary structure and yield carbonyls at its cracking position (Rossner et al. Citation2007). Proteins with carbonyls tend to assemble into macromolecules through crosslinking with each other, resulting in attenuation or even loss of the original protein functions (Stadtman & Berlett Citation1997). For this reason, the content of protein carbonyl directly represents the degree of damage to the protein (Dalle-Donne et al. Citation2003). Protein carbonyl levels in plasma and liver of both control and test groups are displayed in . It indicated that compared with the solvent control group, protein carbonyl level in plasma of mice treated with olive wine declined in a dose-dependent mode. A dose of 28 mL/kg significantly reduced the plasma concentration of protein carbonyl (p < 0.05). However, this capacity of olive wine was less potent than γ-tocopherol (). Both γ-tocopherol and olive wine failed to diminish the formation of hepatic protein carbonyls.
Table 3. Protein carbonyl levels in plasma and liver of mice with different administration protocols.
Determination of SOD activity in blood and liver
SOD is capable of catalyzing superoxide anions into hydrogen peroxide, which is further catalyzed into water by glutathione peroxidase (GSH-Px) and catalase. In this way, SOD detoxifies superoxide anions (Gregory & Fridovich Citation1973; Fukai & Ushio-Fukai Citation2011). The capacity of the body to scavenge radicals is proportional to SOD activity, and this ability declines with aging (Valko et al. Citation2006). Mice treated with different agents exhibited various SOD activities in plasma and liver (). With respect to the solvent control, while the effect on plasma SOD was not evident following administration of 14 mL/kg of olive wine, other groups fed with olive wine demonstrated the dramatically enhanced SOD activity in both plasma and liver. This beneficial function toward liver exceeded that of γ-tocopherol, manifesting the distinctive capacity of olive wine in improving SOD activity.
Table 4. SOD activity in plasma and liver of mice administrated according to the proposed protocol.
Measurement of GSH in blood and liver
GSH is a primary non-protein sulphhydryl compound in body which is capable of scavenging superoxide anions and hydrogen peroxide under catalysis of glutathione peroxidase (GSH-Px) (Cavas & Tarhan Citation2003). The amount of GSH is frequently used to evaluate body antioxidant capacity. GSH concentrations in the plasma and liver of mice administrated in accordance with the proposed protocol are shown in . The same trend was noted in all groups that the GSH level in plasma was far beyond that in liver. In contrast to the solvent group, oral administration of γ-tocopherol notably reduced GSH concentration in plasma (p < 0.05). The lowering of plasma GSH by olive wines was less than that achieved by γ-tocopherol (p > 0.05). Neither γ-tocopherol nor olive oil had discernible impact on GSH concentration of liver. An unexpected finding was that administration of antioxidant components may decrease GSH level in vivo. The possible explanation is that to some extent GSH content reflects the activity of GSH-Px. When GSH-Px is in an active state, considerable GSH will be consumed to scavenge free radicals, resulting in less prototype GSH remaining in blood and tissue. Similarly, due to the relatively higher hepatic concentrations of enzymes, GSH concentrations sharply declined in liver.
Table 5. GSH levels in plasma and liver of mice administrated according to the proposed protocol.
In conclusion, olive wine fermented from OMWW was abundant in antioxidant components. Concentrations of HT, total polyphenol and total flavonoids amounted to 0.14 ± 0.01, 0.43 ± 0.03 and 0.29 ± 0.06 mg/mL, respectively. In vitro antioxidant experiments confirmed the capacity of olive wine to scavenge DPPH radicals and hydrogen radicals, as well as strong FRAP. In vivo antioxidant activity study indicated that compared with the solvent control group, olive wine dosed at 28 mL/kg substantially reduced mouse MDA level in liver and protein carbonyl concentration in plasma (p < 0.05). In addition, olive wine at different doses exhibited potent ability to enhance hepatic SOD activity (p < 0.05), which is even more vigorous than γ-tocopherol. The study demonstrates that olive wine shows prospective opportunity in acting as a functional wine to treat oxidant stress-related diseases.
Funding information
This study was financially supported by key program of Education Department of Sichuan Province (Grant no. 16ZA0298) and fund of National Education Department for overseas returnees (Grant no. 20131792).
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Akerboom TPM, Sies H. 1981. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Meth Enzymol. 77:373–382.
- Barbera AC, Maucieri C, Cavallaro V, Ioppolo A, Spagna G. 2013. Effects of spreading olive mill wastewater on soil properties and crops, a review. Agric Water Manage. 119:43–53.
- Bhutia Y, Ghosh A, Sherpa ML, Pal R, Mohanta PK. 2011. Serum malondialdehyde level: surrogate stress marker in the Sikkimese diabetics. J Nat Sci Biol Med. 2:107–112.
- Cardeno A, Sanchez-Hidalgo M, Alarcon-de-la-Lastra C. 2013. An up-date of olive oil phenols in inflammation and cancer: molecular mechanisms and clinical implications. Curr Med Chem. 20:4758–4776.
- Cavas L, Tarhan L. 2003. Glutathione redox system, GSH-Px activity and lipid peroxidation (LPO) levels in tadpoles of R. r. ridibunda and B. viridis. Cell Biochem Funct. 21:75–79.
- Chedraui P, Perez-Lopez FR. 2013. Nutrition and health during mid-life: searching for solutions and meeting challenges for the aging population. Climacteric. 16:85–95.
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. 2003. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 329:23–38.
- Das K, Samanta L, Chainy GBN. 2000. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind J Biochem Biophys. 37:201–204.
- Dermechea S, Nadoura M, Larrocheb C, Moulti-Mati F, Michaud P. 2013. Olive mill wastes: biochemical characterizations and valorization strategies. Process Biochem. 48:1532–1552.
- Dey SK, Dey J, Patra S, Pothal D. 2007. Changes in the antioxidative enzyme activities and lipid peroxidation in wheat seedlings exposed to cadmium and lead stress. Braz J Plant Physiol. 19:53–67.
- Diamantis V, Pagorogon L, Gazani E, Doerr SH, Pliakas F, Ritsema CJ. 2013. Use of olive mill wastewater (OMW) to decrease hydrophobicity in sandy soil. Ecol Eng. 58:393–398.
- Eidi A, Moghadam JZ, Mortazavi P, Mortazavi P, Rezazadeh S, Olamafar S. 2013. Hepatoprotective effects of Juglans regia extract against CCl4-induced oxidative damage in rats. Pharm Biol. 51:558–565.
- Fki I, Sahnoun Z, Sayadi S. 2007. Hypocholesterolemic effects of phenolic extracts and purified hydroxytyrosol recovered from olive mill wastewater in rats fed a cholesterol-rich diet. J Agric Food Chem. 55:624–631.
- Forman HJ, Zhang H, Rinna A. 2009. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 30:1–12.
- Frankel E, Bakhouche A, Lozano-Sánchez J, Segura-Carretero A, Fernández-Gutiérrez A. 2013. Literature review on production process to obtain extra virgin olive oil enriched in bioactive compounds. Potential use of byproducts as alternative sources of polyphenols. J Agric Food Chem. 61:5179–5188.
- Fukai T, Ushio-Fukai M. 2011. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 15:1583–1606.
- Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N. 2012. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.) –a review. Int J Mol Sci. 13:3291–3340.
- Gregory EM, Fridovich I. 1973. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 114:1193–1197.
- Grossi C, Rigacci S, Ambrosini S, Dami TE, Luccarini I, Traini C, Failli P, Berti A, Casamenti F, Stefani M. 2013. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. Plos One. 8: Article ID e71702.3
- Gu HF, Li CM, Xu YJ, Hua WF, Chena MH, Wan QH. 2008. Structural features and antioxidant activity of tannin from persimmon pulp. Food Res Int. 41:208–217.
- Hamden K, Allouche N, Damak M, Elfek A. 2009. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem Biol Interact. 180:421–432.
- Lakshminarayana R, Baskaran V. 2013. Influence of olive oil on the bioavailability of carotenoids. Eur J Lipid Sci Tech. 115:1085–1093.
- Leger CL, Kadiri-Hassani N, Descomps B. 2000. Decreased superoxide anion production in cultured human promonocyte cells (THP-1) due to polyphenol mixtures from olive oil processing wastewaters. J Agric Food Chem. 48:5061–5067.
- Li CY, Yan J, Chen FZ, Guo XQ, Gou XJ. 2014. Extraction and purifiction of hydroxytyrosol from olive leaves. Food Ferment Ind. 40:227–232. (In Chinese)
- Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, Julián BS, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MÁ. 2013. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatr. 84:1318–1325.
- Nie XP, Liu BY, Yu HJ, Liu WQ, Yang YF. 2013. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata. Environ Pollut. 172:23–32.
- Nielsena F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. 1997. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 43:1209–1214.
- Paredes MJ, Moreno E, Ramos-Cormenzana A, Martinez J. 1987. Characteristics of soil after pollution with wastewaters from olive oil extraction plants. Chemosphere. 16:1557–1564.
- Parzonko A, Czerwinska ME, Kiss AK, Naruszewicz M. 2013. Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine. 20:1088–1094.
- Pierno S, Tricarico D, Liantonio A, Mele A, Digennaro C, Rolland JF, Bianco G, Villanova L, Merendino A, Camerino GM, et al. 2014. An olive oil-derived antioxidant mixture ameliorates the age-related decline of skeletal muscle function. Age. 36:73–88.
- Ranalli A, Lucera L, Contento S. 2003. Antioxidizing potency of phenol compounds in olive oil mill wastewater. J Agric Food Chem. 51:7636–7641.
- Razo-Estrada AC, García-Medina S, Madrigal-Bujaidar E, Gómez-Oliván LM, Galar-Martínez M. 2013. Aluminum-induced oxidative stress and apoptosis in liver of the common carp, Cyprinus carpio. Water Air Soil Pollut. 224:1510–1518.
- Rossner P Jr, Terry MB, Gammon MD, Agrawal M, Zhang FF, Ferris JS, Teitelbaum SL, Eng SM, Gaudet MM, Neugut AI, et al. 2007. Plasma protein carbonyl levels and breast cancer risk. J Cell Mol Med. 11:1138–1148.
- Samatha T, Shyamsundarachary R, Srinivas P, Swamy NR. 2012. Quantification of total phenolic and total flavonoid contents in extracts of Oroxylum indicum L. Kurz. Asian J Pharm Clin Res. 5:177–179.
- Schaffer S, Podstawa M, Visioli F, Bogani P, Müller WE, Eckert GP. 2007. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J Agric Food Chem. 55:5043–5049.
- Stadtman ER, Berlett BS. 1997. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 10:485–494.
- Taheri A, Sabeena Farvin KH, Jacobsen C, Baron CP. 2014. Antioxidant activities and functional properties of protein and peptide fractions isolated from salted herring brine. Food Chem. 142:318–326.
- Tug T, Karatas F, Terzi SM, Ozdemir N. 2005. Comparison of serum malondialdehyde levels determined by two different methods in patients with COPD: HPLC or TBARS methods. Labmedicine. 36:41–44.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 160:1–40.
- Yen GC, Chen HY. 1995. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 43:27–32.
- Yao Q, Li ZW, Zhang Q, Ye LM. 2001. Study on gas chromatographic method for the evaluation of residual solvents by using wide bore open tubular columns. Chin J Chromatogr. 19:141–143. (In Chinese).
- Zanichelli D, Carloni F, Hasanaj E, D’Andrea N, Filippini A, Setti L. 2007. Production of ethanol by an integrated valorization of olive oil byproducts – the role of phenolic inhibition. Environ Sci Pollut Res. 14:5–6.