Abstract
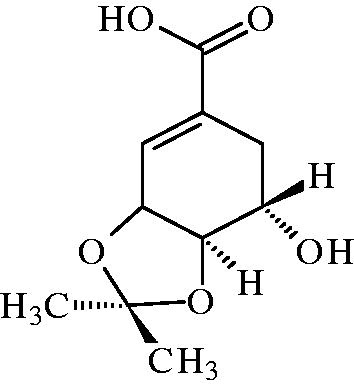
Context 3,4-Oxo-isopropylidene-shikimic acid (ISA) is an analog of shikimic acid (SA). SA is extracted from the dry fruit of Illicium verum Hook. f. (Magnoliaceae), which has been used for treating stomachaches, skin inflammation and rheumatic pain.
Objective To investigate the anti-inflammatory, analgesic and antioxidant activities of ISA.
Materials and methods Analgesic and anti-inflammatory activities of ISA were evaluated using writhing, hot plate, xylene-induced ear oedema, carrageenan-induced paw oedema and cotton pellets-induced granuloma test, meanwhile the prostaglandin E2 (PGE2) and malondialdehyde (MDA) levels were assessed in the oedema paw tissue. ISA (60, 120 and 240 mg/kg in mice model and 50, 120 and 200 mg/kg in rat model) was administered orally, 30 min before induction of inflammation/pain. Additionally, ISA was administered for 12 d in rats from the day of cotton pellet implantation. The active oxygen species scavenging potencies of ISA (10−3–10−5 M) were evaluated by the electron spin resonance spin-trapping technique.
Results ISA caused a reduction of inflammation induced by xylene (18.1–31.4%), carrageenan (7.8–51.0%) and cotton pellets (11.4–24.0%). Furthermore, ISA decreased the production of PGE2 and MDA in the rat paw tissue by 1.0–15.6% and 6.3–27.6%, respectively. ISA also reduced pain induced by acetic acid (15.6–48.9%) and hot plate (10.5–28.5%). Finally, ISA exhibited moderate antioxidant activity by scavenging the superoxide radical and hydroxyl radical with IC50 values of 0.214 and 0.450 μg/mL, respectively.
Discussion and conclusion Our findings confirmed the anti-inflammatory, analgesic and antioxidant activities of ISA.
Introduction
Inflammation is the most common biological reaction to a variety of stimuli and local injury. Inflammatory reactions can be triggered by physical or chemical trauma, invading organisms and antigen–antibody reactions, and is often exacerbated by the resultant swelling or oedema of tissue, pain or even cell damage. Although many anti-inflammatory drugs are in application in clinic including the steroidal and non-steroidal structures, they all cause undesired and sometimes serious side effects (Babu et al. Citation2009). Therefore, continuous screening and development of more effective anti-inflammatory drugs without adverse reaction are still needed.
Chinese star anise, the dry fruit of Illicium verum Hook. f. (Magnoliaceae), has long been used in traditional Chinese medicine and food industry for a long history; it is listed in the Chinese Pharmacopoeia (Citation2010) and has been applied for the treatment of vomiting, stomachaches, insomnia, skin inflammation and rheumatic pain (Itoigawa et al. Citation2004). Modern pharmacology studies demonstrated that its crude extracts and active compounds possess wide pharmacological actions, especially in antimicrobial, antioxidant, insecticidal, analgesic, sedative and anticonvulsive activities (Wang et al. Citation2011). Shikimic acid (SA), one of the constituent of Chinese star anise, previously showed significantly analgesic activity (Ma et al. Citation2000). However, the poor absorption of SA in the gastrointestinal tract due to its hydrophilicity decreases its bioavailability. 3,4-Oxo-isopropylidene-shikimic acid (ISA), a semisynthetic derivative of SA, possessed the advantage over SA of being readily absorbed from the gastrointestinal tract (Yao et al. Citation2009). Therefore, in this present study, we evaluate the analgesic and anti-inflammatory activities of ISA in vivo.
The dynamic equilibrium between generation and scavenging of reactive oxygen species (ROS), such as superoxide radical and hydroxyl radical, is essential in human normal metabolism (Kryston et al. Citation2011). It will result in cell and tissue damage when the amounts of ROS exceed the antioxidant capacity of the organism, which is believed to be linked with inflammation and many other human diseases (Valko et al. Citation2007). Many antioxidant agents from natural products can possibly be used in the prevention of oxidative stress and inflammation-related disorders (Luo et al. Citation2011; Mao et al. Citation2011). In the present study, we also determine the antioxidant activity of ISA in vitro.
Materials and methods
Animals
ICR mice (18–24 g) of both sexes and male Sprague–Dawley rats (110–160 g) were obtained from Beijing Vital River Laboratory Animals, China. The animals were housed under a 12 h light–dark cycle at a constant ambient temperature (22–25 °C), with normal rat chow and water ad libitum. They were allowed to acclimatize for 1 week before the experiments started. All animal care and experimental protocols complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China and the guidelines of animal ethics committee at Xi’an Jiaotong University (Protocol no. 20130225).
Drugs and chemicals
ISA (purity > 98%) were provided by Beijing University of Traditional Chinese Medicine, China. Indomethacin was obtained from Tianjin Pharmaceuticals Group Co., China. Morphine sulphate was obtained from Shenyang Pharmaceuticals Group Co., China. 5,5-Dimethyl-l-pyrroline-N-oxide, diethylenetriaminepentaacetic acid, xanthine oxidase, hypoxanthine and carrageenan were obtained from Sigma, St. Louis, MO. Ferrous sulphate, hydrogen peroxide (H2O2) and ascorbic acid were obtained from Beijing Chemical Reagent Co., Beijing, China. All the other reagents were also of analytic grade and used as received. The chemical structure of ISA is shown in .
Anti-inflammatory activity
Xylene-induced ear oedema
The xylene-induced ear oedema test was carried out as described in the previous study (Hu et al. Citation2008; Wei et al. Citation2010). In the experiment, mice were randomly divided into five groups of ten animals each group. Thirty minutes after oral treatment of mice with distilled water (20 mL/kg), indomethacin (10 mg/kg) and ISA (60, 120 and 240 mg/kg), oedema was induced in each mouse by applying 0.03 mL of xylene to the anterior and the posterior surface of the right ear. The left ear was considered as a control. One hour later, mice were killed under ether anaesthesia and both ears were removed. Circular sections were taken using a cork borer (7 mm in diameter) and weighed. Oedematous response was measured as the weight difference between the two earplugs.
Carrageenan-induced paw oedema
The carrageenan-induced paw oedema test was carried out as described by Xu et al. (Citation2012). Rats were divided into five groups of ten animals each group. Thirty minutes after oral treatment of rats with distilled water (10 mL/kg), indomethacin (10 mg/kg) and ISA (50, 100 and 200 mg/kg), oedema was induced by injection of carrageenan (0.1 mL, 1%, w/v in saline) into the right hind paw subplantar of each rat. The oedema volume was recorded at 1, 2, 3, 4 and 5 h after carrageenan injection using plethysmometer (MK-101P, NatureGene Corp., Beijing, China). The average volume of the right hind paw of each rat was calculated from three readings.
In the secondary experiment, the animals were killed under ether anaesthesia and the carrageenan-induced oedema paw was dissected, and the paw tissue was weighed and rinsed in ice-cold normal saline, and immediately placed in cold normal saline four times their volume and homogenized at 4 °C. Then the homogenate was centrifuged at 10,000 g for 5 min. The supernatant was obtained and stored at −20 °C refrigerator for the prostaglandin E2 (PGE2) and malondialdehyde (MDA) assays. PGE2 levels were determined by the method of Qin et al. (Citation2000), and PGE2 levels are expressed as Absorbance278nm/g tissue. MDA was determined by the thiobarbituric acid reacting substance method (Xing et al. Citation2012), and MDA levels are expressed as nmol/g tissue.
Cotton pellets-induced granuloma
The cotton pellets-induced granuloma test was carried out as described by Gupta et al. (Citation2005). The rats were divided into five groups of 10 rats each group. After shaving the fur, the rats were anaesthetized with diethyl ether and 50 mg of sterile cotton pellets were inserted, one in each axilla. Distilled water (10 mL/kg), indomethacin (10 mg/kg) and ISA (50, 100 and 200 mg/kg) were orally administered for 12 consecutive days from the day of cotton pellet implantation. The animals were anaesthetized on the 13th day and cotton pellets were removed surgically and made free from extraneous tissues. The moist pellets were dried at 60 °C for 24 h, after that dried pellets were weighed. Increment in the dry weight of the pellets was taken as measure of granuloma formation.
Analgesic activity
Hot-plate test
The hot-plate test was carried out as described by Asongalem et al. (Citation2004). Mice used in this experiment were initially screened by placing the animals in turn on a hot plate pain threshold detector (YLS-6B, Chengdu TME, China) with the temperature adjusted to 55 ± 1 °C and animals which failed to lick the hind paw or jump (nociceptive responses) within 30 s were discarded. Eligible animals were divided into five groups of eight mice each group and pretreatment reaction time for each mouse was determined. Thirty minutes after treatment of mice with distilled water (20 mL/kg, p.o.), ISA (60, 120 and 240 mg/kg, p.o.) and morphine sulphate (10 mg/kg, s.c.), the reaction time of animals was again recorded at 30, 60 and 90 min. The time between placement and licking of the paws or jumping was recorded as latency second.
Acetic acid-induced writhing test
This test was carried out by using the method described by Sawadogo et al. (Citation2006). Fifty mice were randomly divided into five groups, 10 mice in each group. Writhing was induced by intraperitoneal injection of 0.6% acetic acid (v/v). Thirty minutes after oral treatment of mice with distilled water (20 mL/kg), indomethacin (10 mg/kg) and ISA (60, 120 and 240 mg/kg), the number of writhes was counted for 20 min following acetic acid injection.
Antioxidant activity
The scavenging effects of ISA on the superoxide and hydroxyl radicals were carried out by using the method described by Guo et al. (Citation1999). Superoxide radical and hydroxyl radical were supplied enzymatically from hypoxanthine–xanthine oxidase reaction and hydrogen peroxide-ferrous sulphate (Fenton reaction), respectively. ISA (10−3–10−5 M) and ascorbic acid (10−6 M) were evaluated by the electron spin resonance (ESR) spin-trapping technique. The scavenging effects of ISA and ascorbic acid on the superoxide and hydroxyl radicals were calculated by
Where, h0 and hx were the ESR signal intensities of samples with and without ISA or ascorbic acid, respectively.
Statistical analysis
All statistical analyses were performed with SPSS version 11.5 for Windows (SPSS Inc., Chicago, IL). The parameters were compared by one-way ANOVA followed by the Tukey test. All results were expressed as mean ± SEM. Statistical significance was set at p < 0.05.
Results
Anti-inflammatory activity
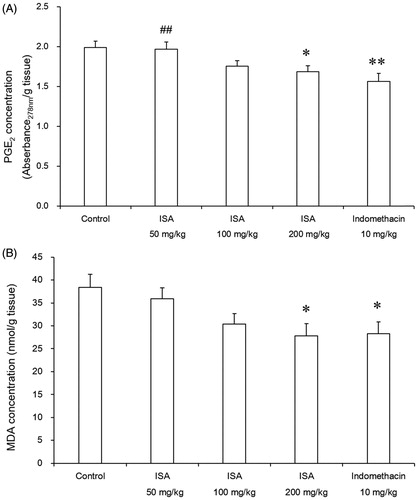
Effects of ISA on xylene-induced ear oedema in mice
Effects of ISA on mouse ear oedema induced by xylene are shown in . ISA inhibited ear oedema by 18.1, 28.1 and 31.4% (p < 0.05) at the dose of 60, 120 and 240 mg/kg, respectively. The effect of ISA at 240 mg/kg was comparable and not significantly different (p > 0.05) from that produced by 10 mg/kg indomethacin (40.5%).
Figure 2. Effects of ISA at different doses on xylene-induced ear oedema in mice (n = 10/group). Error bars indicate standard error of mean. *p < 0.05, **p < 0.01, compared with the control group. The control group received distilled water (20 mL/kg), and the reference drug was indomethacin (10 mg/kg).
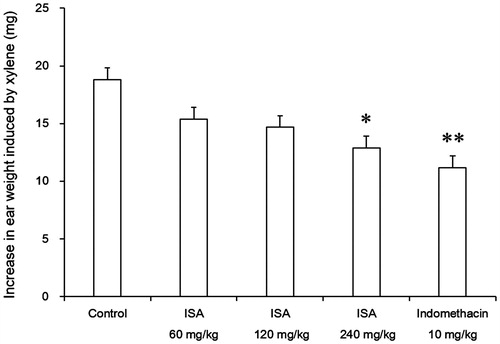
Effects of ISA on carrageenan-induced paw oedema in rats
As shown in , ISA reduced carrageenan-induced paw oedema in both early and late phases of the experiment. ISA at the dose of 200 mg/kg inhibited the oedema formation by 32.6% in early phase (2 h). This effect also extended and significantly increased at the 4th h (p < 0.01). ISA at the dose of 200 mg/kg showed its inhibition by 35.6% at the 3rd h, increased to 51.0% at the 4th h and decreased to 41.3% at the 5th h in the late phase. ISA at the dose of 200 mg/kg produced anti-inflammatory activity and the results were slightly comparable with that of 10 mg/kg indomethacin.
Figure 3. Effects of ISA at different doses on carrageenan-induced foot oedema in rats (n = 10/group). Error bars indicate standard error of mean. *p < 0.05, **p < 0.01, compared with the control group. #p < 0.05, compared with the indomethacin group. †p < 0.05, compared with the ISA 50 mg/kg group. The control group received distilled water (10 mL/kg), and the reference drug was indomethacin (10 mg/kg).
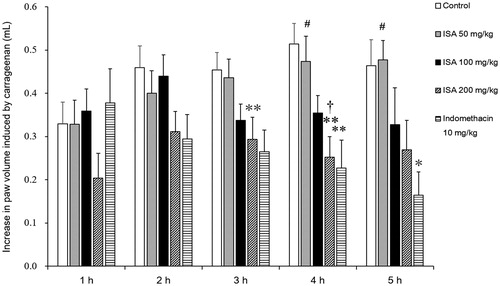
Effects of ISA on the levels of PGE2 and MDA in paw tissue in rats
As shown in , ISA decreased PGE2 levels in the oedema paw tissue by 12.1 and 15.6% (p < 0.05) at the dose of 100 and 200 mg/kg, respectively. ISA decreased MDA levels in the oedema paw tissue by 20.8 and 27.6% (p < 0.05) at the dose of 100 and 200 mg/kg, respectively. The effect of ISA at 200 mg/kg was comparable and not significantly different (p > 0.05) from that produced by 10 mg/kg indomethacin (21.6% for PGE2 and 26.3% for MDA).
Figure 4. Effect of ISA at different doses on levels of PGE2 (A) and MDA (B) of carrageenan-induced foot oedema in rats (n = 10/group). Error bars indicate standard error of mean. *p < 0.05, **p < 0.01, compared with the control group. ##p < 0.01, compared with the indomethacin group. The control group received distilled water (10 mL/kg), and the reference drug was indomethacin (10 mg/kg).
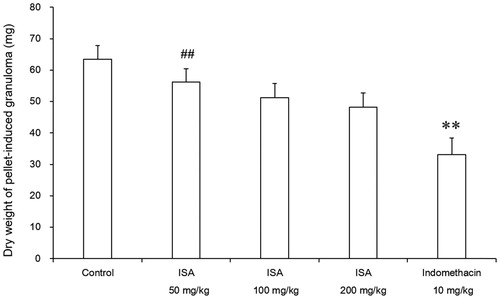
Effects of ISA on cotton pellets-induced granuloma in rats
The effects of ISA on the proliferative phase of inflammation are shown in . ISA inhibited the granuloma formation by 11.4, 19.1 and 24.0% at 50, 100 and 200 mg/kg, respectively. The inhibition produced by indomethacin 10 mg/kg (47.8%) was greater than that produced by ISA 200 mg/kg (24.0%).
Figure 5. Effects of ISA at different doses on cotton pellet-induced granuloma in rats (n = 10/group). Error bars indicate standard error of mean. **p < 0.01, compared with the control group. ##p < 0.01, compared with the indomethacin group. The control group received distilled water (10 mL/kg), and the reference drug was indomethacin (10 mg/kg).
Analgesic activity
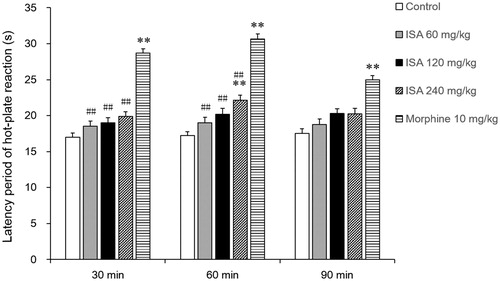
Effects of ISA on hot-plate reaction time in mice
As shown in , in the hot-plate test, ISA increased the mean of latency time to discomfort reaction, of which the duration of action was more than 90 min. The peak value of analgesic activity for 240 mg/kg ISA was observed at the interval of 30, 60 and 90 min, the latency time was 19.9 ± 0.7 s, 22.1 ± 0.8 s (p < 0.01) and 20.2 ± 0.9 s, separately, compared with the control group at 17.0 ± 0.6 s, 17.2 ± 0.6 s and 17.5 ± 0.7 s.
Figure 6. Effects of ISA at different doses on hot-plate reaction time in mice (n = 8/group). Error bars indicate standard error of mean. **p < 0.01, compared with the control group. ##p < 0.01 compared with the morphine sulphate group. The control group received distilled water (20 mL/kg), and the reference drug was morphine sulphate (10 mg/kg).
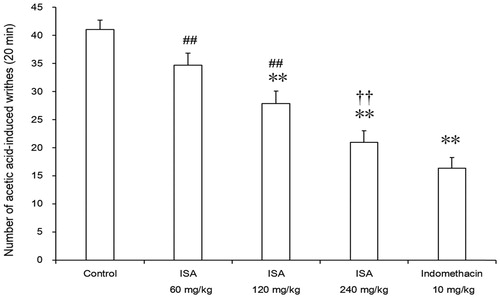
Effects of ISA on acetic acid-induced writhing in mice
As shown in , ISA at doses of 120 and 240 mg/kg produced significant inhibition of writhing response induced by acetic acid with values of 32.1 (p < 0.01) and 48.9% (p < 0.01), respectively. However, 10 mg/kg indomethacin was found to exhibit the highest analgesic activity (60.0%) when compared with the 240 mg/kg ISA (48.9%).
Figure 7. Effects of ISA at different doses on acetic acid-induced writhing in mice (n = 10/group). Error bars indicate standard error of mean. **p < 0.01, compared with the control group. ##p < 0.01 compared with the indomethacin group. ††p < 0.01, compared with the ISA 60 mg/kg group. The control group received distilled water (20 mL/kg), and the reference drug was indomethacin (10 mg/kg).
Antioxidant activity
Superoxide anion radical scavenging activities
Superoxide anion-scavenging activity of three different concentrations of ISA was investigated by using the hypoxanthine–xanthine oxidase reaction system. In the range of 10−3–10−5 M, ISA displayed an inhibition on the superoxide anion. The inhibition ratio of ISA was 34.9% at the concentration of 10−3 M and the inhibition ratio of ascorbic acid was 86.7% at the concentration of 10−6 M ().
Table 1. The scavenging effect of ISA on superoxide anion radicals in xanthine oxidase system (mean ± SEM, n = 6).
Hydroxyl radical scavenging activities
Hydrogen peroxide-scavenging activity of three different concentrations of ISA was investigated on the ISA using the Fenton reaction mechanism. In the range of 10−3–10−5 M, the ISA displayed an inhibition on the hydrogen peroxide. The inhibition ratio of ISA was 18.7% at the concentration of 10−3 M and the inhibition ratio of ascorbic acid was 83.2% at the concentration of 10−6 M ().
Table 2. The scavenging effect of ISA on hydroxyl radicals produced Fenton reaction (mean ± SEM, n = 6).
Discussion
The present study indicates that oral administration of ISA possesses significant systemic and topical anti-inflammatory, analgesic and antioxidant effects.
The ear oedema induced by xylene is useful for screening and investigating the anti-inflammatory activity of drugs on the acute phase of inflammation (Hu et al. Citation2008). Xylene causes instant irritation of the mouse ear, which provokes the release of pro-inflammatory mediators such as histamine and serotonin that promote vasodilatation, leukocytes infiltration and plasma leakage (Ferreira et al. Citation2010). Our results showed that ISA could inhibit the inflammation response induced by xylene (). It suggested that ISA might exhibit anti-inflammatory activity by inhibiting the vasodilatation, leukocytes infiltration.
Carrageenan-induced rat paw oedema is widely used to discover and evaluate anti-inflammatory drugs, since the relative potency estimates obtained from most drugs tend to reflect clinical experience (Xu et al. Citation2012). The inflammatory process induced by injection of carrageenan is believed to be a biphasic event. In the early phase, histamine and serotonin are released, then peak at 3 h to release of kinin-like substances, while in the late phase, prostaglandins, proteases and lysozymes are released (Arfan et al. Citation2010). The results obtained in this study indicated that ISA significantly inhibited the formation of rat paw oedema, both in the early and late phases (). This anti-oedematous effect was significantly maintained during the late phase of oedema development. In comparison with our previous study, a similar dose of SA presented a significantly low inhibition (18.5% at 5 h). This may be due to the inhibition of cyclooxygenase enzymes that are involved in the formation of prostaglandins. In this study, the treatment with ISA significantly decreased in PGE2 level ().
In addition, the carrageenan-induced inflammatory response has been linked to neutrophil infiltration and the production of neutrophil-derived free radicals, such as hydrogen peroxide, superoxide and hydroxyl radicals, as well as the release of other neutrophil-derived mediators (Mazzon et al. Citation2008). Some research has demonstrated that the inflammatory reaction induced by carrageenan is associated with the production of free radicals. Free radicals and prostaglandin will be released when administrating with carrageenan for 1–6 h (Dudhgaonkar et al. Citation2006). The oedema effect was raised to the maximum at 3 h. Janero (Citation1990) demonstrated that MDA production is due to free radical attack plasma membrane. Thus, inflammatory effect would result in the accumulation of MDA. In this study, there was a significant decrease in MDA level with ISA treatment (). We assumed that the suppression of MDA production was probably due to the activities of ISA in scavenging superoxide anion and hydroxyl radicals.
The cotton pellet granuloma method is widely used to evaluate the transudative and proliferative components of the chronic inflammation. The dry weight of the pellet correlates with the amount of the granulomatous tissue (Olajide et al. Citation1999, Citation2000). In this study, the administration of ISA inhibited the dry weight of granuloma by 11.4–24.0% (), but without significant difference when compared with the control group (p > 0.05). This result indicated that ISA had weak effect on inhibiting the granuloma formation. This effect may be due to the cellular migration to injured sites and the accumulation of collagen and mucopolysaccharide.
The hot plate test has been found to be suitable for the evaluation of centrally acting analgesics. The validity of this test has been shown even in the presence of substantial impairment of motor performance (Plummer et al. Citation1991). The present study findings indicate that the analgesic activity of ISA may be central action (). Also, the analgesic activity was much better than that of SA, which only produced a 9.4% of inhibition in the 1st h at the same dose.
Acetic acid-induced abdominal writhing, which is a visceral pain model, is used to evaluate peripheral analgesic activity of drugs. The acetic acid provokes the release of endogenous mediator such as serotonin, bradykinin, histamine, prostaglandins and some cytokines (Guginski et al. Citation2009; Ruangsang et al. Citation2010). In this study, the oral administration of ISA produced significant inhibition of the acetic acid induced abdominal writhing in a dose-dependent manner in mice (). Previously, the same dose of SA showed a lower inhabitation (35.2%). These results suggested that ISA may produce peripheral analgesic effect by inhibiting the release of chemical mediators and/or cytokines.
During inflammation, activation of mast cells, macrophages, eosinophils and neutrophils are known to produce the ROS such as superoxide radicals and hydroxyl radicals (Fernandes et al. Citation2004). These radicals can also act as secondary messengers, thereby provoking the production of other mediators involved in the inflammatory response. In addition, the activation of arachidonic acid metabolism by ROS produces prostaglandins and cytokines (Ricciotti & FitzGerald Citation2011). In this study, the scavenging effect of ISA on superoxide anion radicals and hydroxyl radicals at 10−3 mol/L was 34.9% and 18.7% ( and ), respectively, which showed that ISA had a certain scavenging effect. The ISA scavenged free radicals through the inhibition of nicotinamide adenine dinucleotide phosphate oxidase during the inflammation. Thus, the antioxidant property of ISA would produce an additional therapeutic benefit enhancing its anti-inflammatory effects.
The results of this study demonstrated that ISA possessed anti-inflammatory and analgesic effects. In addition, ISA was able to scavenge the free radicals during the inflammatory response process, which enhanced its anti-inflammatory activity. This study thus suggested that ISA may be developed as a novel analgesic and anti-inflammatory drug.
Funding information
This work was supported by the National Natural Science Foundation of China (No 30973578).
Disclosure statement
The authors report no declarations of interest.
References
- Arfan M, Amin H, Khan N, Khan I, Saeed M, Khan MA, Fazal-ur-Rehman. 2010. Analgesic and anti-inflammatory activities of 11-O-galloylbergenin. J Ethnopharmacol. 131:502–504.
- Asongalem EA, Foyet HS, Ngogang J, Folefoc GN, Dimo T, Kamtchouing P. 2004. Analgesic and antiinflammatory activities of Erigeron floribundus. J Ethnopharmacol. 91:301–308.
- Babu NP, Pandikumar P, Ignacimuthu S. 2009. Anti-inflammatory activity of Albizia lebbeck Benth., an ethnomedicinal plant, in acute and chronic animal models of inflammation. J Ethnopharmacol. 125:356–360.
- Dudhgaonkar SP, Tandan SK, Bhat AS, Jadhav SH, Kumar D. 2006. Synergistic anti-inflammatory interaction between meloxicam and aminoguanidine hydrochloride in carrageenan-induced acute inflammation in rats. Life Sci. 78:1044–1048.
- Editorial Committee of Chinese Pharmacopoeia. 2010. Chinese pharmacopoeia. Beijing, China: China Med. Sci. Technol. Publisher and Distributor.
- Fernandes E, Costa D, Toste SA, Lima JL, Reis S. 2004. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic Biol Med. 37:1895–1905.
- Ferreira FS, Brito SV, Saraiva RA, Araruna MK, Menezes IR, Costa JG, Coutinho HD, Almeida WO, Alves RR. 2010. Topical anti-inflammatory activity of body fat from the lizard Tupinambis merianae. J Ethnopharmacol. 130:514–520.
- Guginski G, Luiz AP, Silva MD, Massaro M, Martins DF, Chaves J, Mattos RW, Silveira D, Ferreira VM, Calixto JB, et al. 2009. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol Biochem Behav. 93:10–16.
- Guo Q, Zhao BL, Shen SG, Hou J, Hu J, Xin W. 1999. ESR study on the structure–antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta. 1427:13–23.
- Gupta M, Mazumder UK, Kumar RS, Gomathi P, Rajeshwar Y, Kakoti BB, Selven VT. 2005. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J Ethnopharmacol. 98:267–273.
- Hu XJ, Jin HZ, Xu WZ, Chen M, Liu XH, Zhang W, Su J, Zhang C, Zhang WD. 2008. Anti-inflammatory and analgesic activities of Edgeworthia chrysantha and its effective chemical constituents. Biol Pharm Bull. 31:1761–1765.
- Itoigawa M, Ito C, Tokuda H, Enjo F, Nishino H, Furukawa H. 2004. Cancer chemopreventive activity of phenylpropanoids and phytoquinoids from Illicium plants. Cancer Lett. 214:165–169.
- Janero DR. 1990. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 9:515–540.
- Kryston B, Georgiev AB, Pissis P, Georgakilas AG. 2011. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res-Fund Mol M. 711:193–201.
- Luo AX, Fan YJ, Luo AS. 2011. In vitro free radicals scavenging activities of polysaccharide from Polygonum multiflorum Thunb. J Med Plants Res. 5:966–972.
- Ma Y, Sun JN, Xu QP, Guo YJ. 2000. Inhibitory effects of shikimic acid on platelet aggregation and blood coagulation. Acta Pharm Sin. 35:1–3.
- Mao XY, Cheng X, Wang X, Wu SJ. 2011. Free-radical-scavenging and anti-inflammatory effect of yak milk casein before and after enzymatic hydrolysis. Food Chem. 126:484–490.
- Mazzon E, Esposito E, Di Paola R, Muià C, Crisafulli C, Genovese T, Caminiti R, Meli R, Bramanti P, Cuzzocrea S. 2008. Effect of tumour necrosis factor-alpha receptor 1 genetic deletion on carrageenan-induced acute inflammation: a comparison with etanercept. Clin Exp Immunol. 153:136–149.
- Olajide OA, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, Okpako DT. 2000. Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J Ethnopharmacol. 71:179–186.
- Olajide OA, Makinde JM, Awe SO. 1999. Effects of the aqueous extract of Bridelia ferruginea stem bark on carrageenan-induced oedema and granuloma tissue formation in rats and mice. J Ethnopharmacol. 66:113–117.
- Plummer JL, Cmielewski PL, Gourlay GK, Owen H, Cousins MJ. 1991. Assessment of antinociceptive drug effects in the presence of impaired motor performance. J Pharmacol Methods. 26:79–87.
- Qin L, Peng X, Zhang SH, Wang L, Liu F. 2000. Influence of monkshood root-peony root combination on inflamation-induced agents and free radicals. China J Chin Mater Med. 25:370–373.
- Ricciotti E, Fitz Gerald GA. 2011. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 31:986–1000.
- Ruangsang P, Tewtrakul S, Reanmongkol W. 2010. Evaluation of the analgesic and anti-inflammatory activities of Curcuma mangga Valand Zijp rhizomes. Zijp. J Nat Med. 64:36–41.
- Sawadogo WR, Boly R, Lompo M, Somé N, Lamien CE, Guissou IP, Nacoulma OG. 2006. Anti-inflammatory, analgesic and antipyretic activities of Dicliptera verticillata. Int J Pharmacol. 2:435–438.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39:44–84.
- Wang GW, Hu WT, Huang BK, Qin LP. 2011. Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. J Ethnopharmacol. 136:10–20.
- Wei W, Wu XM, Li YJ. 2010. Experimental methodology of pharmacology. Beijing, China: People's Medical Publishing House. Publisher and Distributor.
- Xing JF, Sun JY, You HS, Lv J, Sun JN, Dong YL. 2012. Anti-inflammatory effect of 3,4-oxo-isopropylidene-shikimic acid on acetic acid-induced colitis in rats. Inflammation. 35:1872–1879.
- Xu Z, Zhou JR, Cai JM, Zhu Z, Sun XJ, Jiang CL. 2012. Anti-inflammation effects of hydrogen saline in LPS activated macrophages and carrageenan induced paw oedema. J Inflamm. 9:2–9.
- Yao JC, Ni J, Sun JN. 2009. Tissue distribution of 3,4-oxo-isopropylidene-shikimic acid in Kunming mice. Chin J Pharm Anal. 29:1273–1276.