Abstract
Context Asiatic acid, a triterpenoid compound extracted from the tropical medicinal plant Centella asiatica (Family: Apiaceae), has exhibited various biological activities.
Objective This study was performed to investigate the cytotoxic effects of asiatic acid on human ovarian cancer cells.
Materials and methods SKOV3 and OVCAR-3 ovarian cancer cells were exposed to different concentrations of asiatic acid (10–100 μg/mL) for 72 or 48 h. Cell viability, colony formation, cell cycle distribution, apoptotic response were examined. Involvement of the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway was tested.
Results At the concentration of 40 μg/mL, asiatic acid caused about 50% reduction in the viability of ovarian cancer cells, but had little effect on the viability of normal human ovarian epithelial cells. Asiatic acid at 10 μg/mL reduced colony formation of ovarian cancer cells by 25–30%. Asiatic acid-treated cells showed a cell cycle arrest at the G0/G1 phase and 7- to 10-fold increase in apoptosis. The phosphorylation levels of PI3K, Akt and mTOR were remarkably lower in asiatic acid-treated cells. Overexpression of constitutively active Akt partially reversed the cytotoxic effects of asiatic acid, as evidenced by increased cell viability and colony formation. Furthermore, knockdown of Akt mimicked the growth-suppressive activity of asiatic acid.
Discussion and conclusion These results provide first the evidence for the anticancer potential of asiatic acid in ovarian cancer cells, partially via inactivation of the PI3K/Akt/mTOR pathway. Asiatic acid may represent a potential therapeutic agent for ovarian cancer.
Introduction
Ovarian cancer is one of the most frequent malignancies affecting the females, with a high mortality rate (Clarke-Pearson Citation2009; Siegel et al. Citation2013). Although the relative survival for all stages of ovarian cancer has been improved in the past decades (Wright et al. Citation2015), the prognosis for patients with advanced ovarian cancer is still poor. A combination of surgical resection and platinum-based chemotherapy has been currently used as a standard treatment for advanced ovarian cancer. However, most of the cases receiving adjuvant platinum-based chemotherapy will experience drug resistance after an initial response (Kim et al. Citation2012; Sayal et al. Citation2015). Therefore, there is a urgent need for identifying novel agents for the treatment of ovarian cancer.
Asiatic acid, a triterpenoid compound extracted from the tropical medicinal plant Centella asiatica (Family: Apiaceae), has exhibited various biological activities (Jew et al. Citation2000; Soo Lee et al. Citation2003; Bunbupha et al. Citation2015). Asiatic acid has been found to confer protection against ultraviolet-A-induced photoaging (Soo Lee et al. Citation2003) and β-amyloid-induced neurotoxicity (Jew et al. Citation2000) and attenuate cardiovascular remodelling in Nω-nitro-l-arginine methyl ester hydrochloride (L-NAME)-induced hypertensive rats (Bunbupha et al. Citation2015). In recent years, asiatic acid has attracted increasing attention due to its broad anticancer spectrum. For instance, asiatic acid shows the ability to inhibit cell growth and induce apoptosis in colon cancer (Tang et al. Citation2009). Chen et al. (Citation2014) reported that asiatic acid exerts growth suppressive effects on human hepatoma cells. However, the anticancer activity of asiatic acid in ovarian cancer has not been determined.
Therefore, this study was undertaken to investigate the effect of asiatic acid on proliferation, apoptosis and cell cycle progression of human ovarian cancer cells. Several molecular pathways such as mitogen-activated protein kinase and phosphoinositide 3-kinase (PI3K)/Akt signalling (Hsu et al. Citation2005; Ramachandran & Saravanan Citation2015; Wu et al. Citation2015) have been found to mediate the biological activity of asiatic acid. The PI3K/Akt/mammalian target of rapamycin (mTOR) pathway is commonly activated in ovarian cancer and has been suggested as a promising therapeutic target for this disease (Mabuchi et al. Citation2015). The effect of asiatic acid on the PI3K/Akt/mTOR pathway was also examined in ovarian cancer cells.
Materials and methods
Materials
Asiatic acid (>95% in purity), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT), propidium iodide (PI) and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). Primary antibodies anti-cyclin-dependent kinase 2 (CDK2), anti-CDK4, anti-CDK6, anti-cyclin D1, anti-cyclin E, anti-phospho-PI3K, anti-PI3K, anti-Akt, anti-phospho-Akt (Ser473), anti-phospho-mTOR and anti-mTOR were purchased from Cell Signaling Technology (Danvers, MA). Anti-p21, anti-p27, anti-Bax, anti-Bcl-2, anti-caspase-3, anti-caspase-9, anti-poly (ADP-ribose) polymerase (PARP), anti-β-actin antibodies, as well as horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Dulbecco’s modified Eagle medium (DMEM), foetal bovine serum (FBS) and BLOCK-iT Alexa Fluor Red Fluorescent Oligo were purchased from Invitrogen (Carlsbad, CA). Annexin V-FITC Apoptosis Kit was purchased from Calbiochem (San Diego, CA), BCA Protein Quantitative Analysis Kit from BioRad Laboratories (Hercules, CA), and enhanced chemiluminescence (ECL) kit from Pierce (Rockford, IL). The pCDNA3-myr-HA-Akt1 plasmid, which expresses a constitutively activated Akt mutant, was obtained from Addgene (Cambridge, MA). Akt siRNA and negative control siRNA (C-siRNA) were purchased from Cell Signaling Technology.
Cell culture
Two human ovarian cancer cell lines (SKOV3 and OVCAR-3) and normal human ovarian surface epithelial (OSE) cells were obtained from the Institute of Cell and Biochemistry Research of Chinese Academy of Science (Shanghai, China). Cells were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2.
Asiatic acid was dissolved in DMSO and further diluted in culture medium. SKOV3 and OVCAR-3 cells were treated with different concentrations of asiatic acid (0, 10, 20, 40, 60, 80 and 100 μg/mL) for 72 or 48 h. Then cells were collected and subjected to further analysis.
Cell transfection
Cells seeded onto six-well plates (4 × 105 cells/2 mL/well) were transiently transfected with pCDNA3-myr-HA-Akt1 plasmid (1 μg) or empty vector (1 μg) using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. After incubation for 24 h, cells were treated with asiatic acid for additional 48 h before analysis. Transfection efficiency, which was determined in parallel wells by transfection with a green fluorescence protein (GFP)-expressing plasmid (pmaxGFP, Amaxa, Lonza, Walkersville, MD), was 75–80%.
For knockdown experiments, cells were seeded onto 12-well plates (1 × 105 cells/1 mL/well) and transfected with control siRNA or Akt siRNA (50 nM for each) using Lipofectamine 2000. After incubation for 24 h, cells were subjected to gene expression and cell viability analysis. siRNA transfection efficiency, which was estimated by co-transfection with the BLOCK-iT Alexa Fluor red fluorescent oligonucleotide, was ∼85% in this study.
Cell viability assay
Cells were seeded onto 96-well plates, with 0.1 mL/well at a density of 5 × 104 cells/mL, and treated with indicated concentrations of asiatic acid for 72 h. Then, MTT (0.5 mg/mL) was added to each well and incubated for 4 h at 37 °C. DMSO (100 μL) was added to each well to dissolve the MTT formazan. The absorbance was measured at 540 nm.
Colony formation assay
Cells were seeded onto six-well plates, with 2 mL/well at a density of 1 × 103 cells/mL, and treated with indicated concentrations of asiatic acid (0, 10, 20 and 40 μg/mL). After incubation for 10 days, cells were washed three times with phosphate buffered saline (PBS) and stained with 0.1% crystal violet. Colonies were photographed using a light microscope and those containing >50 cells were counted using ImagePro software (Version 4.0, Media Cybernetics, Silver Spring, MD). Each cell group was assayed in triplicates.
Cell cycle and apoptosis analysis
Cells were seeded onto 12-well plates, with 1 mL/well at a density of 1 × 105 cells/mL. After treatment with different concentrations of asiatic acid for 48 h, cells were collected and fixed in 75% ice-cold ethanol at 4 °C overnight. Then, cells were washed with PBS and resuspended in 20 mg/mL PI solution containing 200 mg/mL RNAse for 30 min at 4 °C in the dark. Cellular DNA content was examined by a flow cytometer.
Cell apoptosis was measured with the Annexin V-FITC Apoptosis Kit according to the manufacturer’s instructions. Briefly, after asiatic acid treatment, cells were harvested, washed with PBS twice and stained with annexin V and PI. Then, cells were analysed by flow cytometry.
Western blot analysis
Whole cell extracts were prepared by suspending cells in ice-cold lysis buffer (50 mM Tris–HCl, pH 7.4; 150 mM NaCl; 1% NP-40 and 0.5% sodium deoxycholate) with protease inhibitors (Roche Diagnostics, Indianapolis, IN). Protein samples (30 μg) were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% fat-free milk. After blocking, the membranes were probed with primary antibody overnight at 4 °C, followed by incubation with appropriate HRP-conjugated secondary antibodies for 1 h. Blots were developed using the ECL kit according to the manufacturer’s instructions. Densitometric analysis of the blots was performed using the QuantityOne software (Bio-Rad).
Statistical analysis
Data are expressed as the mean ± standard deviation and were analysed by one-way analysis of variance followed by the Tukey’s test using SPSS 16.0 software (SPSS Inc., Chicago, IL). p Values of less than 0.05 were considered as statistically significant.
Results
Asiatic acid induces growth suppression in ovarian cancer cells
To check whether asiatic acid exerts cytotoxic effects against ovarian cancer cells, two human ovarian cancer cell lines (SKOV3 and OVCAR-3) were treated with various concentrations of asiatic acid. MTT assay revealed that compared with the vehicle control, asiatic acid significantly (p < 0.05) suppressed SKOV3 and OVCAR-3 cell viability (). Moreover, such suppression was in a concentration-dependent manner. At the concentration of 40 μg/mL, asiatic acid caused about 50% reduction in the viability of both SKOV3 and OVCAR-3 cells. In contrast, asiatic acid up to 40 μg/mL had no significant effect on the viability of normal OSE cells (). These results suggest that low concentrations of asiatic acid can be selectively cytotoxic to ovarian cancer cells.
Figure 1. Asiatic acid inhibits cell growth in ovarian cancer cells. (A) Chemical structure of asiatic acid. (B) OSE, SKOV3 and OVCAR-3 cells were incubated with different concentrations of asiatic acid for 72 h and tested for cell viability by the MTT assay. (C) Cells were treated with different concentrations of asiatic acid and incubated for 10 days. Representative images of colony formation assay were shown in the upper panel. Bar graphs in the lower panel show the quantification of colony formation efficiency. Results are expressed as the percentage of colony formation relative to controls without asiatic acid treatment. *p < 0.05 versus vehicle-treated controls.
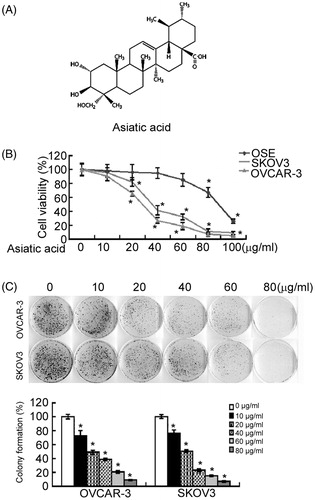
To determine the inhibitory effect of asiatic acid on cell growth, the colony formation assay was performed in SKOV3 and OVCAR-3 cells after exposure to different concentrations of asiatic acid. As shown in , cells treated with asiatic acid (10 μg/mL) formed significantly (P < 0.05) fewer colonies, compared with vehicle-treated controls. However, higher concentrations of asiatic acid appeared to exert cytotoxic effect rather than inhibition of colony formation.
Asiatic acid arrests the cell cycle at G0/G1 phase in ovarian cancer cells
Next, the effect of asiatic acid on cell cycle distribution of ovarian cancer cells was examined. Compared with vehicle-treated cells, asiatic acid treatment led to a concentration-dependent increase in the percentage of cells at G0/G1 phase (; p < 0.05). This increase was accompanied by a significant decrease in the percentage of cells at S and G2/M phases. These results suggested that the growth-suppressive effect of asiatic acid was partly due to a G0/G1-phase arrest.
Figure 2. Asiatic acid induces G0/G1 cell cycle arrest in ovarian cancer cells. SKOV3 and OVCAR-3 cells were incubated with asiatic acid (0, 10, 20 and 40 μg/mL) for 48 h. (A) Cells were collected for DNA content by flow cytometry. Representative flow histograms of PI-stained cells. (B) Percentages of cells in different cell cycle phases were determined. *p < 0.05. (C) Western blot analysis was performed to analysed the expression of cell cycle-related proteins. Representative blots from three independent experiments are shown.
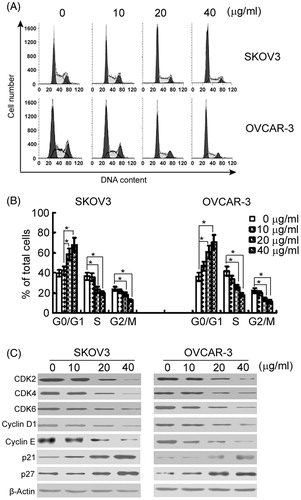
Western blot analysis revealed that asiatic acid treatment strongly decreased the protein levels of CDK2, CDK4, CDK6, cyclin D1 and cyclin E in a concentration-dependent manner (). Moreover, the CDK inhibitors p21 and p27 were markedly increased after asiatic acid treatment.
Asiatic acid promotes apoptosis in ovarian cancer cells
Next, it was determined whether asiatic acid can induce apoptotic death in ovarian cancer cells. Flow cytometry analysis showed that treatment with different concentrations of asiatic acid induced a concentration-dependent apoptosis in SKOV3 and OVCAR-3 cells (p < 0.05; ). The percentage of apoptotic cells was 7- to 10-fold higher in the cells treated with 40 μg/mL asiatic acid than that in vehicle-treated cells.
Figure 3. Asiatic acid induces apoptosis in ovarian cancer cells. (A) After treatment with asiatic acid for 48 h, SKOV3 and OVCAR-3 cells were stained with annexin-V and PI and analysed by flow cytometry. Representative flow cytometric dot-plots are shown in the upper panel. Bar graphs in the lower panel show quantification of annexin-V-positive apoptotic cells. *p < 0.05. (B) Western blot analysis of apoptosis-related proteins. Representative blots from three independent experiments are shown. CF: cleaved form.
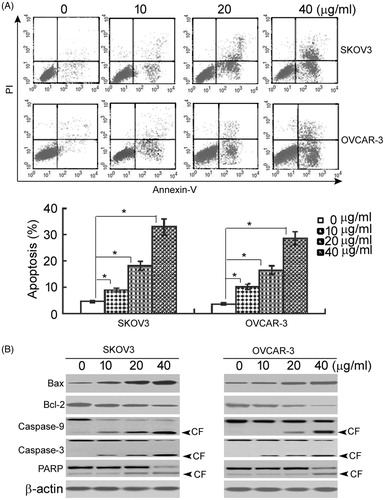
To determine the involvement of caspases in the pro-apoptotic activity of asiatic acid, the levels of active (cleaved) caspase-9 and caspase-3 were measured. As shown in , there was a marked increase in the amounts of cleaved caspase-9 and caspase-3 in both SKOV3 and OVCAR-3 cells treated with asiatic acid. Moreover, a major downstream target of caspase-3, namely PARP, was cleaved in response to asiatic acid treatment. These data suggested that asiatic acid-induced apoptosis of ovarian cancer cells involves the activation of caspase-9 and caspase-3 and cleavage of PARP. Additionally, asiatic acid treatment induced the expression of Bax and reduced the expression of Bcl-2 in a concentration-dependent manner ().
The PI3K/Akt/mTOR pathway is involved in the growth-suppressive activity of asiatic acid
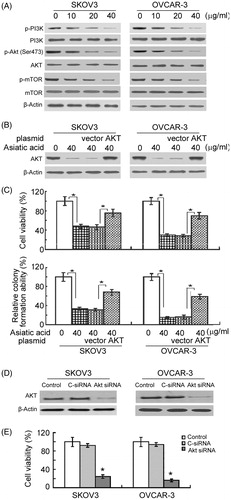
Next, it was tested whether asiatic acid exerts its cytotoxic effects against ovarian cancer cells via inhibition of the PI3K/Akt/mTOR pathway. Western blot analysis showed that the phosphorylation levels of PI3K, Akt and mTOR were remarkably lower in asiatic acid-treated cells than in vehicle-treated cells (). For rescue experiments, a constitutively active form of Akt was delivered into SKOV3 and OVCAR-3 cells before exposure to 40 μg/mL asiatic acid (). Notably, expression of constitutively active Akt partially rescued cell viability and clonogenic growth in the presence of asiatic acid (). To confirm the role of Akt signalling in the growth of ovarian cancer cells, siRNA-mediated knockdown of Akt was performed (). As expected, downregulation of Akt was found to cause a significant decline in the viability of ovarian cancer cells (), which mimics the growth-suppressive effects elicited by asiatic acid.
Figure 4. The PI3K/Akt/mTOR pathway is involved in the growth-suppressive activity of asiatic acid in ovarian cancer cells. (A) Western blot analysis of indicated proteins in cells with or without asiatic acid treatment for 48 h. (B and C) Cells were transiently transfected with active Akt-expressing plasmid or empty vector 24 h before treatment with asiatic acid (40 μg/mL) for 48 h. (B) Western blot analysis of Akt expression. (C) Cell viability (upper panels) and colony formation ability (bottom panels) were measured as described in the section “Materials and methods”. Bar graphs represent data from three independent experiments. *p < 0.05. (D and E) Knockdown of Akt suppresses the viability of ovarian cancer cells. (D) Western blot analysis of Akt expression in cells transfected with control siRNA (C-siRNA) or Akt siRNA. (E) The viability of cells transfected with C-siRNA or Akt siRNA was determined after culturing for 72 h. Bar graphs represent data from three independent experiments. *p < 0.05.
Discussion
Asiatic acid has shown suppressive activity in several types of cancer cells such as colon cancer (Tang et al. Citation2009), hepatoma (Chen et al. Citation2014), glioblastoma (Cho et al. Citation2006). This study provides first evidence for cytotoxic effects of asiatic acid against ovarian cancer cells. It was found that asiatic acid treatment resulted in a concentration-dependent inhibition of the viability of two ovarian cancer cell lines. Moreover, clonogenic growth was hindered in asiatic acid-treated ovarian cancer cells. Examination of cell cycle distribution after asiatic acid treatment provided a mechanistic insight into the growth suppressive property of this compound. Interestingly, asiatic acid significantly raised the percentage of G0/G1-phase cells at the expense of a significant reduction in S- and G2/M-phase cells. Taken together, asiatic acid exerts growth suppression on ovarian cancer cells, at least partially, through induction of a G0/G1 phase arrest. Hsu et al. (Citation2005) reported that asiatic acid can induce S-G2/M arrest in human breast cancer cells. In multiple myeloma RPMI 8226 cells, asiatic acid treatment also led to a G2/M phase arrest (Zhang et al. Citation2013). Therefore, asiatic acid seems not to induce a specific cell cycle phase arrest. It is well accepted that cell cycle progression is regulated by multiple cyclins, CDKs and CDK inhibitors (Nam & Kim Citation2008; Masamha & Benbrook Citation2009). Our data revealed that asiatic acid treatment markedly inhibited the expression of CDK2, CDK4, CDK6, cyclin D1 and cyclin E and increased the expression of the CDK inhibitors p21 and p27 in ovarian cancer cells. These results confirmed the activity of asiatic acid in the regulation of cell cycle progression of ovarian cancer cells.
Induction of apoptosis is an important anticancer mechanism for asiatic acid (Tang et al. Citation2009; Wu et al. Citation2015). In this study, the pro-apoptotic activity of asiatic acid in ovarian cancer was tested. Exposure to asiatic acid resulted in a concentration-dependent apoptosis in SKOV3 and OVCAR-3 cells, compared to vehicle-treated cells. Moreover, asiatic acid treatment markedly increased the levels of cleaved caspases-9, caspases-3 and PARP and altered the Bax-to-Bcl-2 ratio in ovarian cancer cells. The Bcl-2 family of proteins contains both pro- and anti-apoptotic molecules (Kvansakul & Hinds Citation2015). Dysregulation of the Bcl-2 family proteins has been suggested to initiate the intrinsic apoptotic pathway, where mitochondrial cytochome c is released to the cytoplasm, leading to activation of caspases-9 and -3, thus initiating apoptosis (Li & Dewson Citation2015). In agreement with our results, asiatic acid treatment triggered the intrinsic apoptotic pathway in breast cancer cells, as evidenced by alteration of Bax/Bcl-2 ratios, cytochrome c release and caspase-9 activation (Hsu et al. Citation2005). Similarly, Park et al. (Citation2005) reported that asiatic acid-induced apoptosis of SK-MEL-2 human melanoma cells is mediated through modulation of Bax/Bcl-2 ratios and activation of caspase-3. Taken together, asiatic acid can induce apoptotic death in ovarian cancer cells, largely via the intrinsic apoptotic pathway.
The PI3K/Akt/mTOR pathway is critical for cell proliferation and survival in ovarian cancer cells (Gao et al. Citation2014; Mabuchi et al. Citation2015). Inhibition of this pathway has been suggested to account for anticancer effects elicited by various drugs (He et al. Citation2014; Pang et al. Citation2014). In the present work, activation of the PI3K/Akt/mTOR pathway in response to asiatic acid treatment was checked. Of note, the phosphorylation levels of PI3K, Akt and mTOR were reduced in asiatic acid-treated cells, indicating the inactivation of the PI3K/Akt/mTOR pathway by asiatic acid. Most importantly, overexpression of constitutively active Akt partially rescued cell viability and clonogenic growth in asiatic acid-treated cells. Furthermore, the growth-suppressive activity of asiatic acid was recapitulated by knockdown of Akt. Taken together, asiatic acid targets the PI3K/Akt/mTOR pathway to prevent the growth and induce apoptosis of ovarian cancer cells.
Some limitations of this study should be noted. First, some other signalling pathways may also be involved in the anticancer potential of asiatic acid in ovarian cancer cells, as overexpression of constitutively active Akt did not completely reverse the suppressive effects of asiatic acid. Second, there is no in vivo evidence for the anticancer activity of asiatic cancer in ovarian cancer.
In conclusion, these data underscore the cytotoxic effects of asiatic acid on ovarian cancer cells, via induction of G0/G1 cell cycle arrest and mitochondrial apoptosis. This anticancer activity is partially mediated through inactivation of the PI3K/Akt/mTOR pathway. This work warrants further investigation of the anticancer efficacy of asiatic acid in animal models of ovarian cancer.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bunbupha S, Prachaney P, Kukongviriyapan U, Kukongviriyapan V, Welbat JU, Pakdeechote P. 2015. Asiatic acid alleviates cardiovascular remodelling in rats with L-NAME-induced hypertension. Clin Exp Pharmacol Physiol. 42:1189–1197.
- Chen JY, Chen JY, Xu QW, Xu H, Huang ZH. 2014. Asiatic acid promotes p21(WAF1/CIP1) protein stability through attenuation of NDR1/2 dependent phosphorylation of p21(WAF1/ CIP1) in HepG2 human hepatoma cells. Asian Pac J Cancer Prev. 15:963–967.
- Cho CW, Choi DS, Cardone MH, Kim CW, Sinskey AJ, Rha C. 2006. Glioblastoma cell death induced by asiatic acid. Cell Biol Toxicol. 22:393–408.
- Clarke-Pearson DL. 2009. Clinical practice. Screening for ovarian cancer. N Engl J Med. 361:170–177.
- Gao X, Liu Y, Deeb D, Arbab AS, Gautam SC. 2014. Anticancer activity of pristimerin in ovarian carcinoma cells is mediated through the inhibition of prosurvival Akt/NF-κB/mTOR signaling. J Exp Ther Oncol. 10:275–283.
- He F, Wu HN, Cai MY, Li CP, Zhang X, Wan Q, Tang SB, Cheng JD. 2014. Inhibition of ovarian cancer cell proliferation by Pien Tze Huang via the AKT-mTOR pathway. Oncol Lett. 7:2047–2052.
- Hsu YL, Kuo PL, Lin LT, Lin CC. 2005. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J Pharmacol Exp Ther. 313:333–344.
- Jew SS, Yoo CH, Lim DY, Kim H, Mook-Jung I, Jung MW, Choi H, Jung YH, Kim H, Park HG. 2000. Structure–activity relationship study of asiatic acid derivatives against beta amyloid (A beta)-induced neurotoxicity. Bioorg Med Chem Lett. 10:119–121.
- Kim A, Ueda Y, Naka T, Enomoto T. 2012. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 31:14.
- Kvansakul M, Hinds MG. 2015. The Bcl-2 family: structures, interactions and targets for drug discovery. Apoptosis. 20:136–150.
- Li MX, Dewson G. 2015. Mitochondria and apoptosis: emerging concepts. F1000Prime Rep. 7:42.
- Mabuchi S, Kuroda H, Takahashi R, Sasano T. 2015. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 137:173–179.
- Masamha CP, Benbrook DM. 2009. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 69:6565–6572.
- Nam EJ, Kim YT. 2008. Alteration of cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol Cancer. 18:1169–1182.
- Pang Y, Si M, Sun B, Niu L, Xu X, Lu T, Yuan H, Lou H. 2014. DHA2, a synthesized derivative of bisbibenzyl, exerts antitumor activity against ovarian cancer through inhibition of XIAP and Akt/mTOR pathway. Food Chem Toxicol. 69:163–174.
- Park BC, Bosire KO, Lee ES, Lee YS, Kim JA. 2005. Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett. 218:81–90.
- Ramachandran V, Saravanan R. 2015. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum Exp Toxicol. 34:884–893.
- Sayal K, Gounaris I, Basu B, Freeman S, Moyle P, Hosking K, Iddawela M, Jimenez-Linan M, Abraham J, Brenton J. 2015. Epirubicin, cisplatin, and capecitabine for primary platinum-resistant or platinum-refractory epithelial ovarian cancer: results of a retrospective, single-institution study. Int J Gynecol Cancer. 25:977–984.
- Siegel R, Naishadham D, Jemal A. 2013. Cancer statistics, 2013. CA Cancer J Clin. 63:11–30.
- Soo Lee Y, Jin DQ, Beak SM, Lee ES, Kim JA. 2003. Inhibition of ultraviolet-A-modulated signaling pathways by asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur J Pharmacol. 476:173–178.
- Tang XL, Yang XY, Jung HJ, Kim SY, Jung SY, Choi DY, Park WC, Park H. 2009. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol Pharm Bull. 32:1399–1405.
- Wright JD, Chen L, Tergas AI, Patankar S, Burke WM, Hou JY, Neugut AI, Ananth CV, Hershman DL 2015. Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet Gynecol. 125:1345–1352.
- Wu Q, Lv T, Chen Y, Wen L, Zhang J, Jiang X, Liu F. 2015. Apoptosis of HL-60 human leukemia cells induced by asiatic acid through modulation of B-cell lymphoma 2 family proteins and the mitogen-activated protein kinase signaling pathway. Mol Med Rep. 12:1429–1434.
- Zhang J, Ai L, Lv T, Jiang X, Liu F. 2013. Asiatic acid, a triterpene, inhibits cell proliferation through regulating the expression of focal adhesion kinase in multiple myeloma cells. Oncol Lett. 6:1762–1766.
