Abstract
Context: In folk medicine, the stem bark of Streblus asper Lour. (Moraceae) has been reported to possess anticonvulsant activity. However, no systematic/scientific validation is available.
Objective This study explores the constituents in the stem bark, their biosassy-guided isolation and their efficacy in neuro-pharmacological disorders, for validating the traditional claims.
Materials and methods: Microwave-assisted extraction (MAE) technique was employed to obtain the crude extract. The n-hexane, dichloromethane and aqueous fractions were prepared and phytoconstituents were ascertained by phytochemical tests. The isolated compound, betulin, was characterized by different physicochemical and spectral methods, including HPTLC. Finally, neuro-pharmacological evaluations were conducted at 100, 200, 400 mg/kg b.w., p.o. (25, 50, 100 mg/kg b.w. for betulin) doses in BALB/c mice.
Results: The n-hexane fraction (400 mg/kg), and isolated compound betulin (100 mg/kg), showed maximum anticonvulsant activity in maximal electroshock (87.84% and 85.14% seizure inhibition), and isoniazid induced convulsion models (88.85% and 83.18% seizure inhibition), respectively. A dose-dependent attenuation of epileptic seizures was observed, probably through GABArgic mechanism of anticonvulsant action. Moreover, the antidepressant study was also conducted using behavioural models and the results expound that n-hexane and dichloromethane fractions (400 mg/kg) significantly reduced the duration of immobility, as compared to the control.
Discussion and conclusion: This study reports some novel aspects like applying an environmentally benign/green approach of MAE, neuro-pharmacological screening and use of docking studies, for the first time, on the plant S. asper. The findings present a rational explanation for its use in traditional medicine, for the management of neuro-pharmacological disorders.
Introduction
The current anticonvulsant therapies are generally directed at the symptomatic treatment, by suppressing excitability within the brain. Consequently, they elicit unfavourable effects such as cognitive impairment, dependence and abuse. The need for more effective, and less toxic, anticonvulsants has generated renewed interest in natural products for the treatment of convulsions (Kim & Oh Citation2012). Streblus asper Lour. (Moraceae) is a well-known ethno-medicinal plant, distributed throughout the dry areas of India. Most parts of the plant have been used traditionally in the treatment of different ailments such as filariasis, leprosy, piles, diarrhea, dysentery and cancer (Warrier Citation1996; Wealth of India… Citation1998; Nadkarni Citation2002). Scientific investigations have revealed various activities of S. asper such as anti-hepatitis B, analgesic, anticancer, anti-inflammatory, antioxidant and antimicrobial (Wongkham et al. Citation2001; Phutdhawong et al. Citation2004; Sripanidkulchai et al. Citation2009; Basuri Citation2011; Chen et al. Citation2012; Kumar et al. Citation2012; Ibrahim et al. Citation2013; Rao et al. Citation2014). The literature also mentions that the stem bark of S. asper is a rich source of phyto-constituents, from which a number of compounds such as α-amyrin acetate, lupeol acetate, β-sitosterol, α-amyrin, lupeol and diol, strebloside and mansonin have been isolated (Prakesh et al. Citation1992; Warrier Citation1996; Khare Citation2004; Rastogi et al. Citation2006). A pregnane glycoside, sioraside (Mukherjee & Roy Citation1983), has also been isolated. n-Triacontane, tetraiacontan-3-one, β-sitosterol, stigmasterol, betulin and oleanolic acid have been identified in the aerial parts (Gaitonde et al. Citation1964; Saxena & Chaturvedi Citation1985). Chemically, pentacyclic triterpenes, viz., betulin, betulinic acid, lupeol, etc. share similar structural moieties, comprising of four 6-membered rings and one 5-membered ring, and are widely distributed throughout the plant kingdom. A number of non-sterol triterpenes have been reported from the plant S. asper (), with betulin being most abundant followed by betulinic acid and lupeol, present mainly in the outer bark of the plant.
Muceniece et al. (Citation2008) hypothesized that lipophilic molecules (betulin and lupeol) may also penetrate the blood–brain barrier to show central nervous system (CNS) activity, as has already been demonstrated for betulinic acid. They studied the influence of triterpenes on GABAA-receptor antagonist bicuculline-induced seizures in mice and expressed distinct GABAA-receptor related properties of lupane type triterpenes in vivo and in vitro. The findings of the study showed that betulin competed with GABA for binding to the corresponding sites on the GABAA receptor, through its central and peripheral administration, whereas betulinic acid and lupeol did not show any significant binding (Muceniece et al. Citation2008).
In folk medicine, the stem bark of S. asper has been reported to possess anticonvulsant activity. However, there are no systematic and scientific records of this activity. Taking a lead from the previous studies on non-sterol triterpenes, the present investigation was aimed towards the scientific exploration of the constituents present in the stem bark, their bioassay guided isolation and their efficacy in neuro-pharmacological disorders, to establish the authenticity of the traditional claims. Further, to justify the binding interactions of betulin at the GABAA receptor site, molecular docking studies were also proposed.
Materials and methods
Plant material
The plant material (stem bark) of S. asper was collected from the local areas of Faizabad District (U.P.), India in October 2012. The plant material was identified and authenticated at the Herbarium Division of National Botanical Research Institute (NBRI), Lucknow, India. The plant specimen has been preserved in the herbarium with accession no. LWG009. The stem bark was washed with distilled water and dried in shade. It was then powdered using a homogenizer and mixer. All the solvents used were of AR grade from S.D. Fine Chem. Ltd., Mumbai, Maharashtra, India. The reference standard (betulin) was obtained from Sigma Aldrich (St. Louis, MO).
Extraction, fractionation and phytochemical tests
Application of microwaves, which increases the kinetics of extraction, for heating the solvents and plant tissues in extraction process is called microwave-assisted extraction (MAE). MAE involves the rapid heating of solvent, in contact with a sample, in order to partition analytes from the solvent matrix. MAE has a number of advantages over traditional methods of extraction of compounds from various matrices, especially natural products, for example shorter extraction time, less volume of solvent, higher extraction rate and lower cost (Delazar et al. Citation2012). The powdered plant material was extracted in a microwave synthesis system (Raga’s Scientific Microwave Systems, Ragatech, Pune, Maharashtra, India), at a power of 350 W for 20 min, using ethanol as the solvent. The solvent:sample loading ratio was 10:1 and the pre-leaching time was 10 min. The crude extract obtained was collected and concentrated in a rotary vacuum evaporator (Bai et al. Citation2007; Fan et al. Citation2010; Das et al. Citation2012) and stored in a desiccator.
The solid mass obtained (crude extract) was dissolved in distilled water and fractionated, in a separating funnel, with n-hexane. The upper layer was separated and concentrated in a rotary vacuum evaporator to obtain the n-hexane fraction. The remaining aqueous layer was then fractionated with dichloromethane and the lower layer was separated and concentrated in a vacuum rotary evaporator to obtain dichloromethane fraction. The upper layer, after separating the dichloromethane fraction, was concentrated in a rotary vacuum evaporator to obtain the aqueous fraction.
The extracts of the plant material were screened for various classes of natural products by phyto-chemical tests for alkaloids, steroids, glycosides, proteins, phenolic compounds, amino acids and carbohydrates (Vishnoi Citation2007).
Isolation and characterization
The isolation of the compounds was done using column chromatography. The column was packed by the wet packing method. The most active, n-hexane fraction, of the stem bark of S. asper was charged in the glass column, above the stationary phase of silica gel. The column was eluted with petroleum ether and petroleum ether–ethyl acetate, in increasing order of polarity. The various fractions were collected and analysed using thin-layer chromatography (TLC), using n-hexane:ethyl acetate (8:2) as the solvent system. The fractions obtained after eluting the column with petroleum ether:ethyl acetate (85:15) showed an Rf value similar to that of standard betulin, and these similar fractions were combined. The isolated compound was further characterized using spectral techniques and HPTLC (Pandey et al. Citation2007; Khan et al. Citation2012).
IR spectra were recorded on a Shimadzu 8400S FTIR spectrophotometer (Shimadzu Corporation, Kyoto, Japan), using KBr discs, and the values are expressed in cm−1. 1H NMR spectra were recorded on a Bruker DRX-300, (Bruker Instruments Inc.) spectrometer at 300 MHz and the chemical shifts are reported in parts per million (δ value), taking TMS (δ 0 ppm for 1H NMR) as the internal standard. 13C NMR spectra were recorded at 75 MHz, on a Bruker DRX spectrometer (Bruker Instruments Inc.). Mass spectra were recorded on an Agilent 6520-QTOF LCMS mass spectrometer, using the ESI technique. Elemental analysis was performed on a Vario EL III Elemental analyser (Elementar, Hanau, Germany).
HPTLC profiling
Specifications
HPTLC plates: Silica gel GF254 (Merck) 10 cm × 10 cm; mobile phase: n-hexane:ethyl acetate (8:2) (Rout et al. Citation2011; Hridya et al. Citation2012); wavelength: 295 nm; HPTLC system: CAMAG TLC Scanner 3, with winCATS software (Muttenz, Switezerland).
Preparation of standard and test samples
A stock solution of betulin (1000 μg/mL) was prepared by dissolving 10.0 mg of accurately weighed betulin in methanol, and diluting it to 10.0 mL with methanol. Further dilutions were made with methanol to obtain working standards of 20, 40, 60, 80, 100 μg/mL.
The test samples of crude extract, n-hexane fraction and isolated compound were prepared, each with a concentration of 100 μg/mL.
Methodology
The TLC plate was activated by placing it in an oven, at a temperature of 110 °C for 20 min. The plate was spotted with standard and test preparations, maintaining a distance of 15 mm from the edge of the TLC plate. It was developed up to 75 mm in a twin trough chamber, using mobile phase, dried in an oven and subjected to TLC scanning at 295 nm (Verma et al. Citation2015).
Pharmacological evaluation
Study animals
Male albino mice of BALB/c strain, weighing 25–35 g, were used for the different studies of the crude extract and the various fractions of S. asper. These animals were obtained from the Animal House, Faculty of Pharmacy, BBDNIIT, Lucknow, U.P., India. The animals were housed in polypropylene cages with steel net, in a temperature controlled room under standard living condition of 25 ± 5 °C and relative humidity of 55 ± 5%, with regular 12 h dark/light cycles, respectively, and were given free access to food and water. All the animals were treated humanely in accordance with the guidelines laid down by the Institutional Animal Ethics Committee. The project proposal was approved by the Institutional Animal Ethics Committee (vide no. BBDNIIT/IAEC/005/2014).
Study design
For the different studies, male albino mice were divided into seven groups of five mice each (Johnson & Besselsen Citation2002). Group 1 serving as control received 0.5% aqueous carboxymethyl cellulose (CMC) suspension 10 mL/kg b.w., p.o.; group 2 received standard drug (Diazepam, 5 mg/kg, b.w., p.o. for anticonvulsant activity and Fluoxetine, 5 mg/kg, b.w., p.o. for antidepressant activity), groups 3, 4, 5 and 6 received crude extract, n-hexane fraction, dichloromethane fraction and aqueous fraction, respectively, at the doses of 100, 200, 400 mg/kg, b.w., p.o.; group 7 received the isolated compound at the doses of 25, 50, 100 mg/kg, b.w., p.o.
Anticonvulsant activity
Maximal electroshock-induced convulsions in mice
In the study, the male albino mice were divided into respective groups of five mice each. A drop of 0.9% saline was instilled in the eyes prior to the application of electrodes, in order to prevent the death of the animal. Seizures were induced by MES using the apparatus with corneal electrodes (Medicraft Electro-Convulsiometer). The intensity of the stimulus applied was 50 mA, 60 Hz for 0.20 s, via corneal electrodes, for each animal. This intensity was enough to produce a characteristic extensor tonus phase. The animals were observed for approximately 2–5 min and the characteristic phases such as flexion, extensor, clonus, stupor and recovery or deaths were recorded. Finally, the percentage inhibition of seizures, relative to the control, was calculated at various doses of the extract and fractions. All the anticonvulsant agents have the ability to shorten or abolish the extensor phase of convulsions (Kulkarni Citation1999; Vogel Citation2002; Ya’u et al. Citation2008; Hegde et al. Citation2009; Tripathi et al. Citation2011; Bhandari et al. Citation2013).
Isoniazid induced convulsions in mice
For the study, the male albino mice were divided into respective groups of five mice each. After 60 min, seizures were induced in the animals by the chemical convulsant, isoniazid (300 mg/kg, b.w., p.o.). During the next 120 min the occurrence of tonic-clonic seizures and death were recorded. All the anticonvulsants have the ability to shorten or abolish the tonus-clonus phase of convulsions (Coyer & Nicholson Citation1976; Vogel Citation2002).
Antidepressant activity
Forced swim test (FST) in mice
For the antidepressant activity studies, the male albino mice were divided into six groups of five mice each. The study was started 60 min after the per oral (p.o.) administration of vehicle/test/standard drugs, which were suspended in 0.5% aqueous CMC. Mice were forced to swim inside a vertical cylinder individually (height: 40 cm; diameter: 18 cm, containing 15 cm of water maintained at 25 °C). The mice placed in the cylinders for the first time were initially highly active, vigorously swimming in circles, trying to climb the wall or diving to the bottom. After sometime, the activity began to subside and immobility reached a plateau, where the mice remained immobile for approximately 80% of the time. The total duration of immobility was measured during a 5-min test. An animal was judged to be immobile when it ceased struggling and remained floating motionless in water, making only those movements as were necessary to keep its head above water (Vogel Citation2002; Pawar et al. Citation2012).
Tail suspension test (TST) in mice
The male albino mice were divided into six groups of five mice each for the test. The study was started 60 min after the per oral (p.o.) administration of vehicle/test/standard drugs, which were suspended in 0.5% aqueous CMC. The mice were suspended on the edge of a shelf, 58 cm above a table top, by adhesive tape placed ∼1 cm from the tip of the tail. The duration of immobility was recorded for a period of 5 min. The mice were considered immobile when they did not show any body movement, hung passively and completely motionless for at least 1 min (Vogel Citation2002; Pawar et al. Citation2012).
Neurotoxicity studies
For this study, the male albino mice were divided into 17 groups of 5 mice each. The mice underwent a pre-test on the rota-rod apparatus (Medicraft Rotarod Apparatus, Lucknow, India) and they were trained to stay on the accelerating rota-rod that rotated at a rate of 10 rpm. Only those animals which demonstrated their ability to remain on the revolving rod for at least 1 min were used for the test. The study was started 60 min after the oral administration of vehicle/test/standard drugs, which were suspended in 0.5% aqueous CMC. Neurotoxicity was indicated by the inability of the animal to maintain equilibrium on the rod, for at least 3 min, in each of the trails (Vogel Citation2002; Alam et al. Citation2010; Vijayalakshmi et al. Citation2011).
Molecular docking studies
The isolated compound, betulin, was computationally docked into GABAA receptor protein crystal structure (PDB ID: 4COF), using GLIDE module (version 6.6) of Schrodinger Inc. (New York, NY) to obtain the binding mode of the ligand. The protein crystal structures used for the molecular docking were retrieved from the Protein Data Bank (PDB), and subsequently optimized and minimized with the ‘protein preparation wizard’ workflow. The ligands were built using Maestro 10.1 build panel and prepared by LigPrep, version 3.3 (Schrödinger, NY) application, that uses optimized potential liquid simulations 2005 force field, and it gave the corresponding energy minima 3D conformers of the ligands. The default settings were used for all the other parameters. All ligand atoms, but no protein atoms, were allowed to move during the calculations. To validate the docking protocol, a re-docking experiment was performed in which the ligand conformation of the co-crystal ligand was extracted from the crystal structure of the corresponding GABAA receptor protein/ligand complex and docked back into its native binding pocket. GLIDE was able to perfectly reproduce the experimental position of the ligand, confirming the ability of the method to accurately predict the binding conformation.
In silico prediction of pharmacokinetic properties
Nearly 40% of the drug candidates fail in clinical trials due to poor ADME (absorption, distribution, metabolism and excretion) properties. The ability to detect the problematic candidates early can dramatically reduce the amount of time and resources, and streamline the overall development process. QikProp, version 4.3, Schrödinger, LLC, New York, NY, 2015 program was used for in silico prediction of the pharmacokinetic properties of the isolated compound, betulin.
Statistical analysis
All the values of the experimental results are expressed as mean ± SEM and the statistical significance between the groups was calculated by one-way analysis of variance, followed by Dunnett’s multiple comparison test (p < 0.01 was considered statistically significant). Statistical analysis was carried out using Graph Pad Instat 3.0 (Graph Pad Software, San Diego, CA).
Results
Extraction, isolation and characterization
In the present study, MAE technique has been successfully employed to extract and isolate the desired phyto-constituents from the stem bark of S. asper, with noteworthy advantages over conventional extraction procedures, such as lesser time consumption, minimum solvent requirement, good yield and green approach. The preliminary phyto-profile was established and the percentage yields of the crude extract and the various fractions were calculated and are presented in .
Table 1. Preliminary phytoprofile and percentage yield of crude extract and various fractions of the stem bark of S. asper Lour.
After the microwave-facilitated extraction of the powered S. asper stem bark, the crude extract and the different fractions were examined for the presence of phyto-constituents such as alkaloids, steroids, glycosides, proteins, phenolic compounds, amino acids and carbohydrates, as shown in .
Table 2. Results of phytochemical tests of crude extract and various fractions of the stem bark of S. asper.
The most active, n-hexane fraction, was analysed and subjected to column chromatography, for the isolation and separation of phyto-constituents responsible for the activity, based on literature expediency. The isolated compound was characterized on the basis of phyto-chemical tests, physical properties, TLC and HPTLC techniques, spectral studies (UV, IR, 1H NMR, 13C NMR and mass spectroscopy) and elemental analysis, as given in and . These characterization studies confirmed that the isolated compound was the bioactive non-sterol, betulin (C30H50O2, mol. wt. 442.72).
Figure 2. Spectral characterization of the isolated compound: (a) UV spectrum; (b) IR spectrum; (c) mass spectrum; (d) 1H NMR spectrum and (e) 13C NMR spectrum.
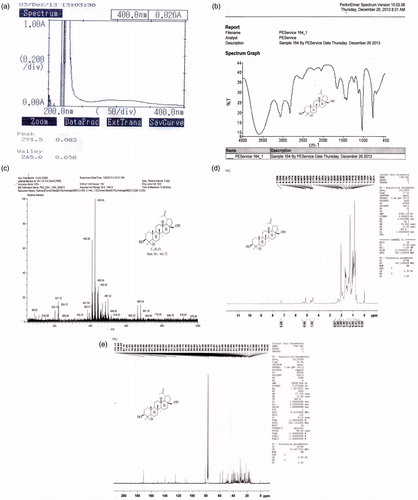
Table 3. Observed characterization details and predicted computational data of the isolated non-sterol triterpenoid, betulin.
HPTLC profiling
The calibration curve of the working standard of betulin was linear in the range of 10–100 μg/mL, as shown in . The 3D spectra (all tracks bring out together and are suggestive of similarities between the test tracks and the standard tracks) scanned at 295 nm are shown in , which expound the presence of the biomarker in the plant extract, n-hexane fraction and isolated compound. From the regression equation, y = 4.06x + 5160, the quantity of betulin present in the plant extract, n-hexane fraction and isolated compound was calculated and it was found to be maximum in the isolated compound, as presented in . The fingerprints and HPTLC chromatogram of the bioactive compound present in the crude extract, n-hexane fraction and isolated compound of the S. asper are depicted in and . These results illustrate that the HPTLC method developed for the identification and quantification of betulin in the plant extract, n-hexane fraction and isolated compound from S. asper was simple, precise, accurate, specific and sensitive ().
Figure 3. (a) Calibration curve of standard betulin working standard solution; (b) 3D spectra of betulin working standard solutions (tracks 1–5), crude extract (track 6), n-hexane fraction (track 7) and isolated compound (track 8) scanned at 295 nm wavelength.
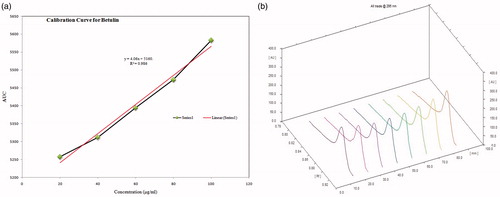
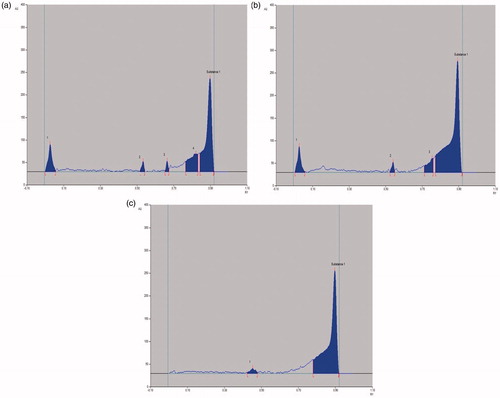
Figure 5. HPTLC chromatogram of (a) crude extract; (b) n-hexane fraction and (c) isolated compound, against standard biomarker, betulin.
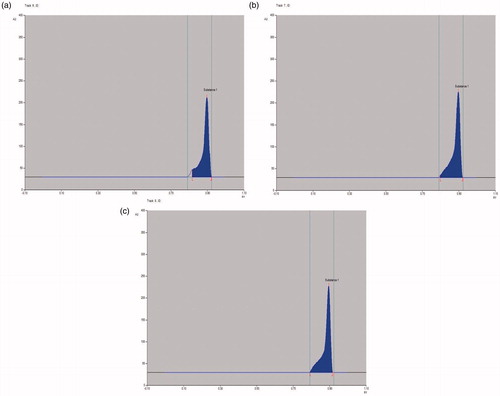
Table 4. Data showing quantity of betulin in crude extract, n-hexane fraction and isolated compound of S. asper.
Table 5. Data showing Rf range and area under the curve of standard betulin (Tracks 1–5), crude extract (Track 6), n-hexane fraction (Track 7) and isolated compound (Track 8).
Pharmacological evaluation
The results of anticonvulsant activity in MES and isoniazid induced convulsion models are presented in . In the MES model, the crude extract and the various fractions (n-hexane, dichloromethane and aqueous fraction), at the dose of 100, 200, 400 mg/kg, showed 27.03–87.84% inhibition and the isolated compound, at the dose of 25, 50, 100 mg/kg, showed 44.60–85.14% inhibition of seizures as compared to the control. n-Hexane fraction, at the dose of 400 mg/kg, and isolated compound, at the dose of 100 mg/kg, showed maximum activity in the MES model (87.84% and 85.14% inhibition of seizure, respectively). Furthermore, crude extract and various fractions also prevented the mortality in mice. In isoniazid induced convulsion model, the crude extract as well as the n-hexane, dichloromethane and aqueous fractions, at the dose of 100, 200 and 400 mg/kg, showed 17.07–88.85% and the isolated compound, at the dose of 25, 50, 100 mg/kg, showed 43.90–83.18% inhibition of seizures as compared to the control. In isoniazid induced convulsion model, n-hexane fraction at the dose of 400 mg/kg, and isolated compound, at the dose of 100 mg/kg, showed maximum activity (88.85% and 83.18% inhibition of seizure, respectively). The anticonvulsant activity observed in both the models were found be in a dose-dependent manner.
Table 6. Data showing anticonvulsant, antidepressant and neurotoxicity studies of the crude extract, various fractions and isolated compound from S. asper Lour.
The antidepressant effect of crude extract and various fractions of S. asper was studied by observing the per cent reduction in immobility in the two models, FST and TST. In this study, the n-hexane fraction and dichloromethane fraction significantly reduced the duration of immobility at the dose of 400 mg/kg, b.w., p.o., as compared to the control ().
Activity of the drugs interfering with motor impairment was assessed for the crude extract, different fractions and the isolated compound by the rota-rod test. It was observed that administration of the test compounds at various doses did not produce any significant effect on the motor coordination and, therefore, lack the symptoms of neurotoxicity ().
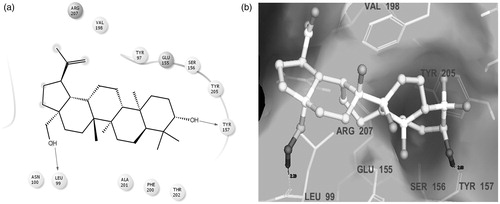
Molecular docking studies were performed to gain some insight into the binding mode of the isolated compound, betulin, in the typical binding pocket of GABAA receptor protein. The stable ligand–receptor complex conformation and the non-covalent interactions of betulin with amino acid residues have been depicted in . It may be inferred from the docking studies that Leu99 and Tyr157 are the key interacting residues at the binding site of GABAA receptor protein 4COF, making crucial H-bond contacts with the –OH groups of the non-sterol triterpenoid-betulin. Also, favourable in silico ADME performance was observed for the isolated betulin with a great correlation to the CNS related parameters ().
Discussion
The results of the study indicated that the n-hexane fraction of S. asper was active in all the experimentally induced laboratory seizure models. The anticonvulsant effect of S. asper was compared with that produced by the GABAA agonist diazepam (Chen et al. Citation2012), a potent antiepileptic drug highly effective in preventing convulsions induced by maximal electroshock and isoniazid. Since inhibition of the MES test predicts activity against the generalized tonic‐clonic seizures, the activity against MES induced seizures suggests that the n-hexane fraction of stem bark of S. asper was useful in suppressing generalized tonic‐clonic seizures by regulating GABA mediated synaptic inhibition (Castel-Branco et al. Citation2009). However, isoniazid exerts its convulsive effect by inhibiting GABA synthesis. It is a potent glutamic acid decarboxylase inhibitor (enzyme involved in GABA synthesis) and thus increases the brain monoamine content and inhibits GABA synthesis, respectively, thereby leading to CNS excitation and convulsions. Suppression of isoniazid induced seizures confirms the GABA enhancing activity of the plant extract (Taylor Citation1976). Thus, it is evident from the present investigation that the isolated compound betulin was able to ameliorate the epileptic seizures induced in the laboratory animals. There are numerous molecular mechanisms through which drugs can block seizure spread and/or elevate seizure threshold. However, betulin inhibits the progression and precipitation of seizures by suppressing clonic-tonic seizures and delaying the onset of seizures. Probable mechanism of action of betulin intuited its binding to the GABAA-receptor sites in mice brain to potentiate the GABAA-receptor functions (Muceniece et al. Citation2008). A dose-dependent attenuation of epileptic seizures was exhibited by betulin, at doses of 50 and 100 mg/kg, b.w., p.o. Moreover, n-hexane and dichlormethane fractions of stem bark of S. asper were found to possess the most significant antidepressant effect at a dose of 200 mg/kg, b.w., p.o., in the FST and TST models, respectively. The molecular mechanism of antidepressant activity of these types of compounds is still unclear; however, it may influence the monoamine concentration in the brain.
Unlike earlier reports (Muceniece et al. Citation2008), this study has been focused on the scientific justification of the ethno-medicinal use of S. asper in neuro-pharmacological disorders. The anticonvulsant and antidepressant effects observed in the model experiments scientifically corroborate the use of this plant for the treatment of convulsive and depressive disorders, using traditional system of medicine. This is the first report on these activities of this plant. Also, an environmentally benign and faster extraction procedure using microwave irradiation, along with the use of docking calculations to predict the binding interactions (with the GABA receptors to establish GABArgic mechanism of anticonvulsant action) of the isolated compound betulin, was employed in this study for the first time.
Conclusions
A successful attempt to isolate and characterize the anticonvulsant principle from S. asper, in particular from the stem bark, was pursued. The study also validates the ethno-pharmacological potential of S. asper in the treatment of neurological disorders, such as epilepsy and depression. Although, it is evident that S. asper contains several important bioactive compounds but most of the compounds have not been properly evaluated for their biological activities. Hence, isolation of some pure phytopharmaceuticals is important as they can be used as lead molecules or pharmacophores for synthesizing novel agents, having good therapeutic activity. The results obtained from the study reveal a definite role of S. asper crude extract, different fractions and the isolated compound in suppressing seizures with a noticeable effect in the depressive behaviour of the tested mice. It is also clear from the study that per cent inhibition of seizures at variable doses of betulin vary significantly. The acceptable potency and lack of acute neurotoxicity promise the development of some potential neuro-pharmacological agents, of natural origin, from S. asper.
Acknowledgements
We express our sincere gratitude to National Botanical Research Institute (NBRI), Lucknow, India for the identification and authentication of the plant material. We also express our sincere gratitude to Central Drug Research Institute (CDRI), Lucknow, India for providing the library and sophisticated analytical instrument facilities.
Disclosure statement
The authors declare no conflict of interest.
References
- Alam O, Mullick P, Verma SP, Gilani SJ, Khan SA, Siddiqui N, Ahsan W. 2010. Synthesis, anticonvulsant and toxicity screening of newer pyrimidine semicarbazone derivatives. Eur J Med Chem. 45:2467–2472.
- Bai X, Qui A, Guan J. 2007. Optimization of microwave-assisted extraction of antihepatotoxic triterpenoid from Actinidia deliciosa root and Its comparison with conventional extraction methods. Food Technol Biotechnol. 45:174–180.
- Basuri TS. 2011. Analgesic activity of stem sark extracts of Sterblus asper. Int J Pharm Pharm Sci. 3:219–220.
- Bhandari S, Tripathi AC, Saraf SK. 2013. Synthesis and anticonvulsant activity of some 2-pyrazoline derivatives. Med Chem Res. 22:5290–5296.
- Castel-Branco MM, Alves GL, Figueiredo IV, Falcao AC, Caramona MM. 2009. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find Exp Clin Pharmacol. 31:101–106.
- Chen H, Li J, Wu Q, Niu XT, Tang MT, Guan XL, Li J, Yang RY, Deng SP, Su XJ. 2012. Anti-HBV activities of Streblus asper and constituents of its roots. Fitoterapia. 83:643–649.
- Coyer JR, Nicholson DP. 1976. Isoniazid-induced convulsions. South Med J. 69:294–297.
- Das AK, Mandal V, Mandal SC. 2012. Design of experiment approach for the process optimisation of microwave-assisted extraction of lupeol from Ficus racemosa leaves using response surface methodology. Phytochem Anal. 24:230–247.
- Delazar A, Nahar L, Hamedeyazdan S, Sarker SD. 2012. Microwave-assisted extraction in natural products isolation. Methods Mol Biol. 864:89–115.
- Fan JP, Zhang RF, Zhu JH. 2010. Optimization of microwave-assisted extraction of total triterpenoid in Diospyros kaki leaves using response surface methodology. Asian J Chem. 22:3487–3500.
- Gaitonde BB, Vaz AX, Patel JR. 1964. Chemical and pharmacological study of root bark of Streblus asper Linn. Indian J Med Sci. 18:191–199.
- Hegde K, Thakker SP, Joshi AB, Shastry CS, Chandrashekhar KS. 2009. Anticonvulsant activity of Carissa carandas Linn. root extract in experimental mice. Trop J Pharm Res. 8:117–125.
- Hridya VK, Godson PS, Chandrasekar N. 2012. Chromatographic identification of two biologically important triterpenoids from the chloroform extract of Rhizophora mucronata. Acta Chromatogr. 241:123–129.
- Ibrahim NM, Mat I, Lim V, Ahmad R. 2013. Antioxidant activity and phenolic content of Streblus asper leaves from various drying methods. Antioxidants. 2:156–166.
- Johnson PD, Besselsen DG. 2002. Practical aspects of experimental design in animal research. Ilar J. 43:202–206.
- Khan I, Sangwan PL, Dhar JK, Koul S. 2012. Simultaneous quantification of five marker compounds of Betula utilis stem bark using a validated high-performance thin-layer chromatography method. J Sep Sci. 35:392–399.
- Khare CP. 2004. Encyclopedia of Indian medicinal plants. Berlin: Springer-Verlag.
- Kim HG, Oh MS. 2012. Natural products as potential anticonvulsants: caffeoylquinic acids. Arch Pharm Res. 35:389–392.
- Kulkarni SK. 1999. Hand book of experimental pharmacology. 3rd ed. Chandigrah: Vallabh Prakashan.
- Kumar RBS, Kar B, Dolai N, Bala A, Haldar PK. 2012. Evaluation of antihyperglycemic and antioxidant properties of Streblus asper Lour against streptozotocin-induced diabetes in rats. Asian Pac J Trop Med. 2:139–143.
- Muceniece R, Saleniece K, Rumaks J, Krigere L, Dzirkale Z, Mezhapuke R, Zharkova O, Klusa V. 2008. Betulin binds to gamma-aminobutyric acid receptors and exerts anticonvulsant action in mice. Pharmacol Biochem Behav. 90:712–716.
- Mukherjee K, Roy LN. 1983. Chemical examination of Streblus asper leaves. Int J Crude Drug Res. 21:189–190.
- Nadkarni AK. 2002. K. M. Nadkarni’s Indian materia medica. Vol. 1, 3rd ed. Mumbai: Popular Prakashan.
- Pandey R, Verma RK, Gupta MM. 2007. High-performance thin-layer chromatographic method for quantitative determination of 4alpha-methyl-24beta-ethyl-5alpha-cholesta-14,25-dien-3beta-ol, 24beta-ethylcholesta-5, 9(11),22E-trien-3beta-ol, and betulinic acid in Clerodendrum inerme. J Sep Sci. 30:2086–2091.
- Pawar VS, Akhade A, Baokar S, Shivakumar H. 2012. Antidepressant-like effects of Acorus calamus in forced swim and tail suspension test in mice. Asian Pac J Trop Med. 1:S17–S19.
- Phutdhawong W, Donchai A, Korth J, Pyne SG, Picha P, Ngamkham J, Buddhasukh D. 2004. The component and anticancer activity of the volatile oil from Streblus asper. Flavour Fragr J. 19:445–447.
- Prakesh K, Deepak D, Khare A, Khare MP. 1992. A pregnane glycoside from Streblus asper. Phytochemistry. 31:1056–1057.
- Rao DS, Penmatsa T, Kumar AK, Reddy MN, Gautam NS, Gautam NR. 2014. Antibacterial activity of aqueous extracts of Indian chewing sticks on dental plaque: an in vitro study. J Pharm Bioallied Sci. 6:S140–S145.
- Rastogi S, Kulshreshtha DK, Rawat AKS. 2006. Streblus asper Lour (Shakhotaka): a review of its chemical, pharmacological and ethnomedicinal properties. ECAM Adv Acc. 3:1–6.
- Rout KK, Singh RK, Mishra SK. 2011. Simultaneous quantification of two bioactive lupane triterpenoids from Diospyros melanoxylon stem bark. J Planar Chromatogr. 24:376–380.
- Saxena VK, Chaturvedi SK. 1985. Cardiac glycosides from the roots of Streblus asper. Planta Med. 51:343–344.
- Sripanidkulchai B, Junlatat J, Wara-aswapati N, Hormdee D. 2009. Anti-inflammatory effect of Streblus asper leaf extract in rats and its modulation on inflammation-associated genes expression in RAW 264.7 macrophage cells. J Ethnopharmacol. 124:566–570
- Taylor BA. 1976. Genetic analysis of susceptibility to isoniazid-induced seizures in mice. Genetics. 83:373–377.
- The wealth of India: raw material. 1998. Vol. 4. New Delhi: Council of Scientific and Industrial Research.
- Tripathi AC, Gupta R, Saraf SK. 2011. Phytochemical investigation characterisation and anticonvulsant activity of Ricinus communis seeds in mice. Nat Prod Res. 25:1881–1884.
- Verma SC, Subhani S, Vashishth E, Singh R, Pant P, Mangal AK, Padhi MM, Dhiman KS. 2015. Development of HPTLC-UV method for comparative phytochemical study of stem bark versus small branches of Streblus asper Lour. World J Pharm Res. 4:503–512.
- Vijayalakshmi A, Ravichandiran V, Anbu J, Velraj M, Jayakumari S. 2011. Anticonvulsant and neurotoxicity profile of the rhizome of Smilax china Linn. in mice. Indian J Pharmacol. 43:27–30.
- Vishnoi NK. 2007. Advanced practical organic chemistry. 2nd ed. Delhi: Vikas Publishing House.
- Vogel HG. 2002. Psychotropic and neurotropic activity. In: Drug discovery and evaluation. 2nd ed. Berlin, Heidelberg: Springer Verlag
- Warrier PK, Nambiar VPK, Ramankutty C, Sala AV 1996. Indian medicinal plants: a compendium of 500 species. Vol. 5. Hyderabad: Sangam Books.
- Wongkham S, Laupattarakasaem P, Pienthaweechai K, Areejitranusorn P, Wongkham C, Techanitiswad T. 2001. Antimicrobial activity of Streblus asper leaf extract. Phytother Res 15:119–121.
- Ya’u J, Yaro AH, Abubakar MS, Anuka JA, Hussaini IM. 2008. Anticonvulsant activity of Carissa edulis (Vahl) (Apocynaceae) root bark extract. J Ethnopharmacol. 120:255–258.