Abstract
Context: The aerial parts of Sphagneticola trilobata (L.) Pruski (Asteraceae) are popularly used to treat topical inflammation, but have not been fully investigated.
Objective: To identify polar compounds in S. trilobata extracts and develop a new topical phytomedicine based on the kaurenoic acid (KA) content while monitoring and demonstrating its topical anti-inflammatory activity.
Materials and methods: Ethanol spray-dried extract of S. trilobata was analysed by LC-MS while the KA content from semisolid was analysed by LC-UV. The extent of ear edema induced by applying 20 μL of croton oil (2.5%), arachidonic acid (AA; 2 mg/ear) and decanoylphorbol-13-acetate (TPA; 2.5 mg/ear) in mice was used to evaluate the biological activity of the semisolids, which were applied 30 min before the phlogistic agents.
Results: Eight phenylpropanoids and four oleanane-type triterpenoid saponins were identified, majority of them reported for the first time in this species, in addition to KA. The semisolid containing 1.0% of dried extract reduced the ear edema induced by croton oil [77.2 ± 4.5%; ID50 = 0.49 (0.28–0.87%)], TPA (81.5 ± 2.4%) and AA (39.1 ± 6.9%), with decreasing effect at higher KA concentrations. This was accompanied by neutrophil migration inhibition as investigated by biochemical and histological assays.
Discussion and conclusion: The anti-inflammatory effects were (at least in part) due to the interference in protein kinase C (PKC) activation, AA-cascade products and neutrophil migration inhibition, demonstrating the efficacy of the folk topical usage of this plant. The results support the development of a novel topical anti-inflammatory phytomedicine properly standardized to treat inflammatory dermatological diseases.
Introduction
Sphagneticola trilobata (L.) Pruski (Asteraceae) [syn. Acmella brasiliensis and Wedelia paludosa (Tropicos Citation1996)] is widespread along the Brazilian coast and in other Latin American countries (Kissmann & Groth Citation1992). In Brazil, it is popularly known as ‘pseudo-arnica’, ‘margaridão’ and ‘picão da praia’ (Corrêa Citation1984). This plant has been popularly used as a tincture to treat hematomas and general inflammatory processes, and also for the treatment of pain, muscle cramps, rheumatism, persistent wounds, ulcers, edemas, arthritis, joint pain, headaches, fevers and respiratory tract diseases (Roque et al. Citation1987; Maldini et al. Citation2009).
Phytochemical studies have shown the absence of alkaloids and the presence of steroids, terpene and flavonoid compounds as likely responsible for the effects described in folk medicine (Block et al. Citation1998a,Citationb; Carvalho et al. Citation2001). These compounds include kaurenoic acid (KA) (Roque et al. Citation1987; De Carli et al. Citation2009; Batista et al. Citation2010), luteolin and corepsin (Cechinel Filho Citation2000), stigmasterol and derivatives (Carvalho et al. Citation2001; Vieira et al. Citation2001) and paludolactone (Cechinel Filho et al. Citation2004). The published data demonstrate the presence of KA in all parts of the plant, however, high concentrations accumulate in the roots (Block et al. Citation1998b; Bresciani et al. Citation2000). The KA marker was quantified in S. trilobata spray-dried extracts by Fucina et al. (Citation2012); however, many peaks of the LC-UV chromatogram remain unidentified.
There are studies of the anti-inflammatory effects of fractions obtained from S. trilobata extracts (Block et al. Citation1998a,Citationb; Maldini et al. Citation2009), which predominantly showed less polar substances (extracted with hexane and dichloromethane).
Our research group, in addition to morpho-anatomical (Baccarin et al. Citation2009), phytochemical, efficacy (Block et al. Citation1998a.b; Cechinel Filho Citation2000; Bresciani et al. Citation2004) and oral safety studies (Bürger et al. Citation2005), has developed analytical methods to control the quality of S. trilobata hydroethanolic dried extracts (Fucina et al. Citation2012).
This work, therefore, focused on the additional chemical analysis of the spray-dried extract by LC-MS, the analytical LC-UV methodology validation to quantify KA extracted from the semisolid containing S. trilobata hydroalcoholic dried extract, and finally biomonitoring by the use of different ear-edema models in two different commercial vehicles (Steareth21® and Ceteth20®).
Materials and methods
Drugs and reagents
Methanol and acetonitrile LC grade (J.T. Baker, Phillipsburg, NJ); ultrapure water (Easy Pure equipment, Waltham, MA); polyoxyl 21 cetoestearyl ether or Steareth21® and Polyoxyl 20 cetyl ether or Ceteth20® (Lipo Chemicals, Paterson, NJ); cetearyl alcohol (Sasol, Hamburger, Germany); stearyl alcohol (Oxiteno, São Paulo, Brazil); caprylic/capric triglyceride (Croda, Yorkshire, UK); liquid petrolatum (Bahia, Brazil); cera alba (Koster Keunen Holland BV, Bladel, Netherlands); propylene glycol (Dow Chemical, Midland, MI); phenoxyethanol, methylparaben, ethylparaben, propylparaben and butylparaben (Chemyunion, Sorocaba, SP, Brazil); BHT (Sterlitamak, Russian Federation); Dexamethasone (Galena, Campinas, SP, Brazil). TMB, TPA, croton oil, arachidonic acid (AA), hematoxyline, eosine, EDTA, HTAB (Sigma Aldrich, St. Louis, MO), formaldehyde, ethanol 100%, H2O2, acetone, xylene (Vetec, RJ, Brazil), NaCl, Na2HPO4, KH2PO4 (Merck, Kenilworth, NJ). The chemical marker KA was isolated from S. trilobata roots with HPLC purity of >95% (Fucina et al. Citation2012). Other solvents and reagents were of analytical grade and were purchased from national suppliers and commercially available.
Plant material
A voucher specimen of S. trilobata was collected in Itajaí, in July 2007 (State of Santa Catarina, Brazil) and identified by Prof. Dr. Jimi Naoki Nakajima (Institute of Botany/Universidade Federal de Uberlândia-UFU, Uberlândia, Brazil). The plant sample was then deposited at the herbarium of the State University of Maringá (Maringá, Brazil) under number HUEM-24139.
The dried extract was prepared in a pilot scale (three batches of 1 kg) at Centroflora (Botucatu-SP, Brazil). The batches were composed of dried aerial parts to solvent (ethanol 60% v/v) in a 1:9 ratio, subjected to dynamic maceration for 8 h at room temperature and then filtered. The solution was concentrated in a Bernhauer concentrator at 70 °C under vacuum (20–25 bar, at 4.00 kgf cm²), to obtain approximately 25% of total solids (concentrated extract). The dried extract was obtained and the concentrate was mixed with 15% (w/w total solids of concentrate) of colloidal silicon dioxide and then dried in an industrial spray dryer (GEA-Niro, Søborg, Denmark) with inlet and outlet temperatures of 150–170 °C and 70–80 °C, respectively. Batches 1, 2 and 3 were analysed by a validated HPLC-UV method presenting 6.3, 5.9 and 6.1 mg/g of KA, respectively (Fucina et al. Citation2012).
Semisolid formulations
Semisolid formulations were prepared containing 0.5, 1, 2, 3 and 5% of S. trilobata hydroalcoholic dried extract (6.3 mg/g of KA) using two emulsifying agents, polyoxyl 21 cetoestearyl ether (Steareth21®) or polyoxyl 20 cetyl ether (Ceteth20®) as vehicles. In addition, formulations containing 0.0025–0.015% of isolated KA were also prepared.
Steareth21® was composed by Steareth21® (1.5%), cetearyl alcohol (7.0%), liquid petrolatum (3.0%), cera alba (5.0%), propylene glycol (15.0%), Phenonip® (0.5%), disodium EDTA (0.5%), BHT (0.1%) and water (qsp 100.0%). Ceteth20® formulation contained Ceteth20® (2.0%), cetearyl alcohol (6.0%), stearyl alcohol (3.0%), caprylic/capric triglyceride (5.0%), liquid petrolatum (5.0%), propylene glycol (8.0%), disodium EDTA (0.1%), BHT (0.1%), Phenonip® (0.5%) and water (qsp 100.0%).
For both formulations, the contents were divided into hydrophilic and lipophilic components. Both phases were heated to approximately 70 °C, over a water bath. The aqueous phase was then added to the oil phase and stirred until the product reached room temperature.
The dried extract (or isolated KA) was levigated with propylene glycol (5%) and incorporated into the formulations. The semisolids were packed in aluminium tubes with a seal and polypropylene cover.
Instrumentation and chromatographic conditions
LC-MSn analysis
For the compounds’ structure elucidation, an LC-MSn methodology was developed. The analysis was performed using an UFLC (Shimadzu, Japan) consisting of two LC10AD solvent pumps, an SLC 10A system controller and a 20 μL loop with a Rheodyne model 7125 injector (Cotati, CA) with a DAD (SPD-M20A) and Mass Spectrometer (Amazon SL, Billa Rica). The mobile phase (flow 1.0 mL/min) consists of 0.1% acetic acid (A) and acetonitrile with 0.1% acetic acid (B) with the following gradient: 0.0–20.0 min (10–60% B); 20.0–40.0 min (60–100% B); 40.0–45.0 min (100% B); 45.0–47.00 min (100–10% B); 47.0–55.0 min (10% B). The column used was a C18 XB – Kinetex (Phenomonex® −250 mm ×4.6 mm × 5 μm). All the experiments were conducted using an ion trap mass spectrometer instrument (Bruker® model HTC Ion Trap MS). Ions were generated using an electrospray ionization interface (ESI) and detected in the negative ion mode. Argon was used as a collision gas.
LC-UV analysis
Agilent HPLC1200 series (Santa Clara, CA) consists of a binary pump G1311A and an Agilent G1315D photodiode array detector. The samples were monitored at 210 and 338 nm. An auto-sampler and software Chemstation CS were used. The injections (20 μL each) were carried out in a C18 Luna 250 × 4.6 mm (5 μm) (Phenomenex, Torrance, CA) and conditioned in a Shimadzu CTO-10A column oven, equilibrated at 30 °C). The mobile phase consisted of gradient A (acetonitrile) and B (acidified water pH 3.0 with phosphoric acid) of 15:85 (A:B) (0 min); 20:80 (5 min); 22:78 (8 min); 25:75 (12 min); 30:70 (20 min); 60:40 (22 min); 90:10 (25 min). This composition was maintained for 40 min before returning to the initial conditions for an additional 10 min at a flow rate of 1.0 mL/min.
This methodology, previously developed and validated for S. trilobata spray-dried extract analysis, proved to be linear over a range of 4.5–30 μg/mL for KA, accurate (recovery of 99.0%), precise (the RSD % values for the intra- and inter-day precision studies were <2.0 and 8.0% for inter-laboratorial study), selective, stability-indicative and robust (Fucina et al. Citation2012).
Semisolid extraction and analytical validation
The semisolid (10 g of placebo or phytomedicine at 1% of spray-dried extract) was added to a volumetric flask of 50.0 mL, followed by the addition of 12.5 mL of dichloromethane and 30.0 mL of methanol. The volumetric flask was sonicated for 30 min, and then the volume was made up to the mark with methanol. This solution was transferred to a centrifuge tube, which was stored in an ice bath for 30 min. The solution was then centrifuged at 3500 rpm for 15 min. The supernatant was filtered using filter paper, and the resulting filtrate was re-filtered through a modified 0.45 μm PTFE membrane and injected into the HPLC. These sample solution contained 2.0 mg/mL of standardized spray-dried extract.
The KA was assayed by external quantification. A blank was performed using the semisolid components without the dried extract to analyze the influence of the matrix on the marker recovery. The standard solution was prepared at 15 μg/mL of KA in methanol followed by dilution with methanol: dichloromethane (75:25 v/v).
The semisolid containing 0.5, 1.0 or 1.5% of dried extract was subjected to a liquid–liquid extraction method and validated following the ICH guidelines (Citation2005). The selectivity was evaluated by comparing the chromatogram of the blank (methanol:dichlorometane 75:25 v/v), the mobile phase, the sample solution and the placebo solution to detect any co-elution interference. The resolution between the KA peak and the neighbouring peaks was calculated, as well as the peak purity by a PDA detector.
Linearity was evaluated for the analytical curves using two different procedures. In the first procedure, KA standard solutions (150 μg/mL) were used at dilutions in the range of 4.5–52.5 μg/mL. The influence of the formulation in the linearity of KA was analysed by spiking a sample solution (submitted to extraction method as described above) with increasing volumes of a concentrated KA standard solution (at 150 μg/mL), corresponding to 30–350% of the KA theoretical value (15 μg/mL). A 27-point analytical curve was plotted and statistically evaluated for each solution.
The accuracy of the method was evaluated by the extraction of the semisolid containing 0.5, 1.0 and 1.5% of S. trilobata dried extract. The samples, in triplicate for each level, were prepared as described above and the KA recovery was calculated.
The precision of the method was evaluated by repeating the extraction method described above with the semisolid formulation at 1.0% of dried extract, in sextuplicate, on the same day and by the same analyst. These studies were repeated on an additional day by a second analyst. The average marker recovery and RSD percentage were then calculated.
Pharmacological studies
Male Swiss mice (20–28 g) were used throughout this study. The animals were housed under conditions of optimum light, temperature and humidity (12 light–dark cycle, 22 ± 1 °C), with food and water provided ad libitum. Swiss mice were obtained from the vivarium of University of Itajaí Valley (UNIVALI, Itajaí, Santa Catarina, Brazil). The in vivo experiments were conducted by a blinded observer to the treatments, and all the procedures used in this study followed the ‘Principles of Laboratory Animal Care’ of NIH publication No. 85–23 and were approved by the Animal Ethics Committee (protocol numbers 432/08 UNIVALI). The number of animals and the intensity of noxious stimuli used were the minimum necessary to demonstrate consistent effects.
Ear thickness enhance induced by phlogistic agents was used as indicative of edema. Both ears were evaluated using a digital micrometer (Great, MT-045B), and the difference between the left and right ears (in micrometer) was used to express the experimental results. The micrometer was applied near the ear tip, just distal to the cartilaginous ridges.
The inflammation was induced in the mouse’s right ear 30 min after the treatment with the semisolids. The croton oil diluted in acetone (2.5%, 20 μL/ear) was topically administered on the back surface of the right ear (Swingle et al. Citation1981). The ear-thickness evaluation was performed 4 h after the edema induction, as described previously. The mice were then euthanized, and the ear samples were collected to perform myeloperoxidase (MPO) activity assays.
With the aim of evaluating the involvement of the eicosanoid system or PKC activation in the anti-inflammatory effect of S. trilobata, ear edema was induced by the topical administration of AA (2 mg/ear) or 12-O-tetradecanoyl phorbol-13-acetate (TPA; 2.5 mg/ear), respectively. Firstly, mice received the semisolids on the front surface of the ear and 30 min after the treatment they received AA or TPA, diluted in acetone (20 μL/ear) on the back surface. The ear thickness was evaluated using a micrometer, as described previously, 2 and 4 h after the AA and TPA applications, respectively.
Neutrophil recruitment was also indirectly assessed by means of ear tissue MPO activity, according to the method described previously (Cunha et al. Citation2005). The animals were treated with the S. trilobata semisolid formulation (1%) 30 min before the administration of croton oil, as described above. The mice were euthanized 6 h after the ear-edema induction, and the right ear tissue (samples of 6 mm diameters) was collected. These samples were homogenized at 5% (w/v) in EDTA/NaCl buffer (pH 4.7) and centrifuged at 10,000 rpm for 15 min at 4 °C. The pellet was re-suspended in 0.5% hexadecyltrimethyl ammonium bromide buffer (HTAB; pH 5.4), and the samples were frozen and thawed three times in liquid nitrogen. After thawing, the samples were centrifuged again (10,000 rpm, 15 min, 4 °C), and 25 μL of the supernatant was used for the MPO assays. The enzymatic reaction was assessed with 1.6 mM tetramethylbenzidine (TMB), 80 mM NaPO4, and 0.3 mM hydrogen peroxide (H2O2). The absorbance was measured at 650 nm, and the results were expressed as the optic density (OD) per milligram of tissue.
Ear samples were also collected 6 h after the croton oil administration and immediately submerged into formaldehyde solution (3.7%) for a 24 h fixation. The samples were subjected to a dehydrated protocol using ethanol in increasing concentrations (70–100%) and then to a diaphanization protocol with xylene. The samples were embedded in paraffin and slides were prepared in sufficient numbers using a non-serial sectioning method. The slices were cut on a rotary microtome (American Optical 820®) with 4 mm disposable razors, sectioned at 5 μm and stained with haematoxylin-eosin (HE) (Brancoft & Cook Citation1988). A representative area was selected for qualitative light microscopic analysis of the inflammatory cellular response with a 40x objective.
Statistical analysis
The results are presented as the mean ± SEM of five to eight animals for each experimental group. The percentages of inhibition are reported as the mean ± SEM of inhibition values for each animal based on the control group value. The statistical analysis between these values was performed by one-way ANOVA followed by the Newman-Keuls post hoc test. Similar statistical analyses were carried out for the MPO activity results. Statistical analysis of the data presented in bar graphs was performed by two-way ANOVA, followed by Bonferroni’s post hoc test. The p values of less than 0.05 (p < 0.05) were considered indicative of significance.
Results
Chemical studies
Structural analyses were performed based on the mass spectra along with the ultraviolet absorption spectra of the substances analysed. Their chemical structures were elucidated by comparing the spectral data with those reported previously. These data are summarized in . shows the UV chromatogram (280 nm) and the total ion chromatogram of the spray-dried extract of S. trilobata acquired in the negative ion mode.
Figure 1. Chromatograms of the analysis of Sphagneticola trilobata extracts, with detection at 280 nm (A) and ESIMS (negative mode) (B).
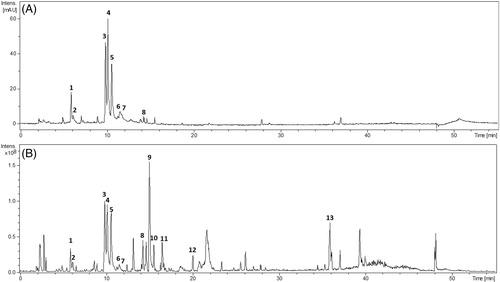
Table 1. Chemical constituents from Sphagneticola trilobata extract by UFLC-DAD-MS.
The chromatographic peaks 1–7 were identified as chlorogenic acids, assigned as 5-O-caffeoylquinic acid (1), 3-O-caffeoylquinic acid (2), 4,5-di-O-caffeoylquinic acid (3), 3,5-di-O-caffeoylquinic acid (4), 3,4-di-O-caffeoylquinic acid (5), 3-O-feruloyl-4-O-caffeoyl quinic acid (6), and tri-O-caffeoylquinic acid (7). The chromatographic peak 8 was assigned as ethyl caffeate (8). Their fragmentation patterns and UV absorption are in agreement with the literature (Clifford et al. Citation2003; Jaiswal & Kuhnert Citation2010; Barcia et al. Citation2014). Recently, 5-O-caffeoylquinic acid and 4,5-di-O-cafeoliquínico were identified in S. trilobata (Chagas-Paula et al. Citation2015), so, to our best knowledge, most phenylpropanoids were first described in the specie.
Chromatographic peaks 9–12 were identified as oleanane-type triterpenoid saponins based on their fragmentation patterns. Additionally, no UV absorption was observed, which is consistent with the proposed structural properties, due to the absence of a conjugated system. This class of compound has been previously reported to be present in this genus (Matos & Tomassini Citation1983; Li et al. Citation2012).
Compounds 11 and 12 presented the same pseudomolecular ions ([M-H] = m/z 793). Compound 11 produced a MS² base peak at m/z 631 by the loss of one hexose residue (loss of 162 Da) and a peak at m/z 455 by the loss of a glucuronic acid (loss of 338 Da). The product ion spectrum for the compound 12 showed an ion at m/z 455 (loss of hexose and glucuronic acid) suggesting the same oleanolic acid aglycone, and an intense ion at m/z 613 corresponding to the loss of one hexose unit (loss of 180 Da).
The compound 9 showed m/z 955 [M − H]– in the MS spectrum, which has a difference of 162 mass units compared to that of the compounds 11 and 12, indicating an additional hexose. The MS² fragmentation showed the ions m/z 793 and 613, indicating the loss of two hexose units. The MS³ spectrum on the fragmentation of the ion m/z 793 showed the same pattern of fragmentation for compound 12, where the ions m/z 613 and 455 were also observed. Therefore, the data indicated that this compound is an oleanane-type triterpenoid saponin containing two hexose units and one glucuronic acid unit.
The compound 10 produced the ion m/z 971 [M-H]– in the MS spectrum, which is 16 a.m.u. higher than that of compound 9, indicating a dihydroxylated aglycone. The MS² spectrum showed ions at m/z 953 (loss of water), 795 (loss of glucuronic acid), 647 (loss of two hexose units), 471 (dihydroxylated aglycone) and 323, corresponding to a dissaccharide unit. The fragment ion at m/z 795 is indicative of a terminal glucuronic acid unit. Thus, it is suggested to be an oleanane-type triterpenoid saponin with two hydroxyl groups on aglycone, and it has a position functionalized with a dissaccharide unit and another one with a glucuronic acid unit. Further investigations by NMR methods are required to identify the hexose units and to define the attachment points of the substituents to the saponin aglycone. Peak 13 m/z 301 [M-H]– was identified as KA by comparison with an authentic standard.
Development of semisolid formulations
Considering the topical use of S. trilobata in folk medicine (Corrêa Citation1984) and pharmacological studies regarding the anti-inflammatory activity, semisolids containing 0.5–5.0% of hydroethanolic dried extract and 0.0025–0.015% of isolated KA were developed. The semisolid containing the extract showed a slightly greenish colour, depending on the concentration of the extract, with a homogeneous and glossy aspect and an odour characteristic of vegetal dried extracts. The formulations were found to be stable in a preliminary stability study of its physical aspects (data not shown).
Assay of KA in formulations and analytical validation (LC-UV)
Several solvents and extraction strategies of semisolids were applied. The use of methanol:dichloromethane, followed by sonication, low temperature, centrifugation and filtration allowed an average recovery of 97.6% of KA from the semisolid phytomedicine () during the accuracy determination. The chromatographical conditions were the same as those used to quantify the spray-dried extract of S. trilobata by Fucina et al. (Citation2012).
Table 2. Summary of the extraction method validation by LC-UV.
The extraction solvent and placebo did not interfere in the marker (KA) elution, ensuring the selectivity of the HPLC method (). The chromatogram was similar to those observed for spray-dried extracts of S. trilobata (Fucina et al. Citation2012) with an additional peak of solvent (, at 18 min), but without interfering with the peak marker (KA) and the other peaks that eluted earlier (12–15 min), which are attributed to the presence of more polar compounds (named peaks 1, 2, 3) present in the extract. These compounds are probably chlorogenic acids, as shown in , which, in the LC-UV chromatographic system (), appear to be co-eluting (peaks 1–3) and may represent potential polar markers to extract, apart from KA, which is the major nonpolar compound.
Figure 2. Chromatograms of Standard solution of KA at 15 μg/mL (A); sample solution of Steareth21® with 1% of S. trilobata dried extract (B); placebo of A (C); solvent (methanol:dichlorometane 75:25 v/v) (D), at 210 nm.
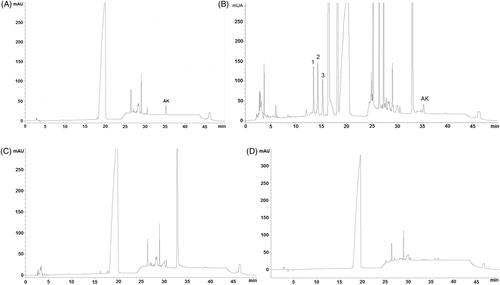
The matrix components of the semisolid and herbal extracts did not interfere with the linearity of the method in the tested range of KA, as shown by the similar angular coefficients for both analytical curves (with or without matrix spiking) ().
The semisolid extraction methodology was accurate and precise for marker quantification in semisolids, when evaluated in three levels of spray-dried extracts (0.5, 1.0 and 1.5%), as shown by the mean recovery of 97.6% and RSD <2.0% (). Therefore, this methodology is suitable for the quality control of these semisolid formulations.
Pharmacological studies
Topical administration of croton oil was able to significantly induce ear edema, as indicated by the large increase in right ear thickness (). Both semisolids used as placebos, Ceteth20® and Steareth21®, slightly reduced the ear edema induced by croton oil, probably due to physical and/or chemical interference in croton oil absorption. However, mice topically treated with the semisolids containing S. trilobata dried extract (0.5–5%), presented a significant decrease in ear edema, with a maximum inhibition of 77.2 ± 4.5% for the Steareth21® semisolid formulation at 1.0% of extract (p < 0.01; ). This formulation exhibited an ID50 of 0.49 (0.28–0.87%), with decreasing effect at higher concentrations. Significant results were also obtained with animals treated with dexamethasone (inhibition of 84.9 ± 2.0%).
Figure 3. Effects of semisolids containing S. trilobata dried extract (0.5–5.0%) prepared in (A) Ceteth20® or (B) Steareth21® basis, (C) isolated KA (0.0025–0.015%), dexamethasone (0.5%) or placebo on ear edema induced by croton oil (2.5%) in mice. Each group represents the mean of five to seven animals, and the vertical bars indicate the S.E.M. Significant differences from control values are indicated as *p < 0.05 and **p < 0.01 (one-way ANOVA followed by Newman-Keuls post hoc test).
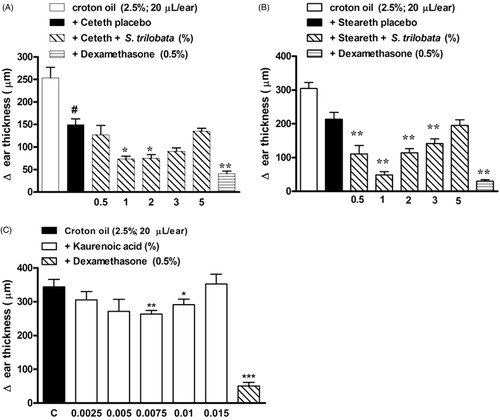
In order to compare the biological effect of these formulations with the isolated marker, semisolids containing isolated KA (0.0025–0.015%) were also submitted to the same pharmacological model (). The best doses of KA were 75–100 μg/g (or 0.0075–0.01%) with maximum inhibition of 23.3 ± 3.1% (), since the increase to 0.015% showed a significant decrease in biological effect.
According to the pharmacological evaluation and the physical stability of both semisolids (data not shown), Steareth21® was chosen because it presented better conditions for the development and validation of the analytical methodology, and for the subsequent pre-clinical analysis.
The next step was to investigate the possible mechanism involved in the anti-edematogenic effect of the S. trilobata hydroethanolic dried extract. In order to evaluate the role of protein kinase C (PKC), ear edema was induced by topical administration of TPA. The data presented in demonstrate that TPA produced a prominent ear-thickness increase (p < 0.001) at both evaluation time points (4 and 6 h). The Steareth21® semisolid containing S. trilobata (1%) reduced the ear edema induced by TPA, with inhibition values of 81.5 ± 2.4% and 69.8 ± 5.0% for the 4 and 6 h time points, respectively (). Similar inhibition values were also observed with mice treated with dexamethasone (0.5%; 88.3 ± 1.5%).
Figure 4. Effects of semisolids containing S. trilobata dried extract (1.0%), dexamethasone (0.5%) or placebo (Steareth21®) on ear edema induced by (A) TPA (2.5 mg/ear) or (B) AA (2 mg/ear) in mice. Each group represents the mean of five to seven animals, and the vertical bars indicate the S.E.M. Significant differences from control values are indicated as *p < 0.05, **p < 0.01 and ***p < 0.001 (one-way ANOVA followed by the Newman–Keuls post hoc test).
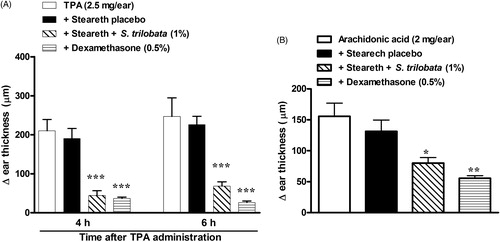
Topical treatment with semisolids containing S. trilobata (1.0%) or dexamethasone (0.5%), when administered 30 min before AA instillation, significantly interfered with edema formation, with inhibitions of 39.1 ± 6.9% and 57.6 ± 3.1%, respectively ().
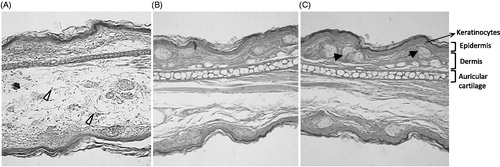
demonstrates that the anti-edematogenic effect of semisolids containing S. trilobata against croton oil-induced inflammation was accompanied by a reduction in neutrophil migration, with inhibition values of 44.4 ± 5.8% versus 70.9 ± 10.2% for the dexamethasone treated group, as can also be observed by histological evaluation (). Histological examination of the ear tissue showed important parameters of inflammation that indicate prominent inflammatory signals in control animals, including epidermal hyperplasia, vasodilatation and massive leukocyte influx (). On the other hand, in the animals treated with S. trilobata or dexamethasone, these inflammatory parameters were visibly reduced ().
Figure 5. MPO activity in mice treated with semisolids containing S. trilobata dried extract (1.0%), dexamethasone (0.5%) or placebo (Steareth21®) and subjected to croton oil-induced ear edema (2.5%). Each group represents the mean of four to five animals, and the vertical bars indicate the S.E.M. Significant differences from control values are indicated by ***p < 0.001. Significant differences from the naïve group is indicated by #p < 0.001 (one-way ANOVA followed by the Newman–Keuls post hoc test).
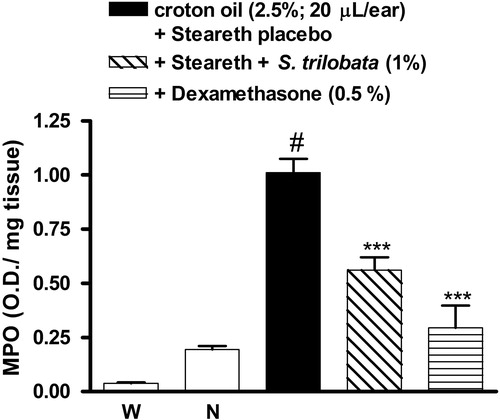
Figure 6. Representative pictures of histological slides of mouse ears treated with semisolids containing (A) placebo (Steareth21®), (B) S. trilobata dried extract (0.5%) or (C) dexamethasone (0.5%) and/or subjected to croton oil-induced ear edema (2.5%). The samples were stained with HE and photographed at 200× of augment. Sebaceous gland (black arrows) and inflammatory cells migration (white arrows).
Discussion
Medicinal plants have been identified as a safe and effective alternative for the treatment of dermatological inflammatory diseases, replacing conventional drugs such as corticosteroids and nonsteroidal anti-inflammatory drugs (Bralley et al. Citation2008; Maldini et al. Citation2009; Cesca et al. Citation2012). In this study, we identified polar compounds in S. trilobata hydroethanolic extract and also developed a new topical phytomedicine based on KA content with significant anti-inflammatory effects.
The choice of KA as a marker was based on its previously reported biological activity (De Carli et al. Citation2009). However, the relationship between dose and the effect of this marker had not yet been reported. In addition, the extraction of KA from semisolid phytomedicine was a difficult task due to the nonpolar characteristics of the compound and the characteristics of the semisolid components. The liquid–liquid extraction strategy proved to be accurate, precise and robust. Moreover, the HPLC-UV analysis was shown to be selective to the components of hydroethanolic S. trilobata. In addition to HPLC analysis (Batista et al. Citation2005), other techniques such as GC-FID (Bresciani et al. Citation2000) have been employed to quantify KA from herbal matrices. Despite the complexity of the plant matrix and semisolid formulation of S. trilobata hydroethanolic spray-dried extract, the developed and validated extraction methodology was shown to be suitable for the formulation quality control.
It is well known that many plants used worldwide in traditional medicine contain triterpenes saponins, which can often account for their therapeutic action, including anti-inflammatory properties (Navarro et al. Citation2001; Li et al. Citation2002). In addition to KA, the major apolar compound, there are others () that might contribute to the biological effects described in this study as phenylpropanoid derivatives that have been found to play crucial roles in plant biochemistry and physiology (Solecka Citation1997), also associated with anti-inflammatory effects (Korkina et al. Citation2011; Domínguez et al. Citation2011; Ma et al. Citation2013).
There are important challenges for the topical therapeutic use of herbal medicines: (i) the results reported in other routes of administration (oral or systemic) are not always observed when applying the product topically; (ii) the scale up of the phytomedicine requires the use of a safe solvent, such as ethanol:water, and the extent and amount of extracted compounds with this kind of solvent must be known; (iii) the need of reliable analytical methods for the extraction and assay of active markers, usually present in low concentration, from incorporated extracts into the semisolid formulations; (iv) due to the synergistic effect on biological activity between the different components or classes of compounds commonly found in plant extracts, it is recommended to obtain the most knowledge possible of the active compounds present in the standardized extract.
The skin is the most important organ that protects the body against microorganism infections. Dermatological inflammation can be induced by pathogens, mechanical and thermal noxious stimuli, chemical products, UV radiation, autoimmune response and psychic disorders (Dawid-Pać Citation2013). It involves several components, such as inflammatory cell migration, cell proliferation, release of inflammatory mediators, changes in vascular tonus, cell necrosis and apoptosis and tissue repair (Pasparakis et al. Citation2014). In fact, topical corticosteroid remains the first-line therapy to treat inflammatory skin disorders, although the disadvantages include a reduction in epidermal renewal and interference with the resolution process (Nguyen & Zuniga Citation2013).
This study demonstrated the significant topical effect of a semisolid containing S. trilobata hydroethanolic spray-dried extract in croton oil-induced ear edema in mice. Croton oil is a sticky liquid obtained from Croton tiglium L. (Euphorbiaceae) seeds that, when in contact with the epidermis, produces an expressive inflammatory reaction accompanied by redness, edema and epidermal hyperplasia. The croton oil-induced ear-edema model is mainly used to investigate topical anti-inflammatory activity (Tonelli et al. Citation1965). The inflammatory response induced by topical application of croton oil includes edema (that peaks 6 h after its application), leukocyte infiltration and cell proliferation, production of AA metabolites (prostanoids and leukotriene), cytokines and other pro-inflammatory mediators (Patrick et al. Citation1987).
It is important to note that all comparisons were made between animals treated with the extract or compound and the group that received the placebo semisolid formulation (). The highest anti-inflammatory effect was showed by the phytomedicine based on Steareth21® containing 1.0% of S. trilobata spray-dryed extract (which has 6.3 mg of KA per g of extract). Therefore, this semisolid presents approximately 60 μg of KA per g of semisolid, near to the best concentration range of isolated KA in regard to the anti-inflammatory effect (), and similar to that previously demonstrated effective concentration (De Carli et al. Citation2009). Choi et al. (Citation2011) found that KA was able to reduce, in a dose-dependent manner, nitric oxide (NO) and PGE2 production, COX2 and iNOS expression and the translocation of nuclear factor-κB (NF-κB) in RAW264.7 macrophages stimulated with lipopolysaccharide. Furthermore, the compound presented an anti-edematogenic effect when administered systemically in mice subjected to the carrageenan-induced paw-edema model.
There is evidence indicate that PKC is a crucial intracellular protein involved in many diseases, including cancer, neuropathic pain and dermatological diseases such as psoriasis (Mochly-Rosen et al. Citation2012). The PKC activation culminates in an increase of PLA2 activation, AA formation and consequently enhances eicosanoid synthesis. This hypothesis was investigated using a model of ear edema induced by TPA, which is a potent PKC activator. The semisolid containing the S. trilobata hydroethanolic dried extract also reduced the edema resulting in PKC activation, suggesting that the modulation of PKC phosphorylation could be important for the extract’s topical anti-inflammatory effects.
The extract was also able to topically interfere with the edema induced by AA application. As cited above, PKC activation culminates in PLA2 activation and consequently AA formation. This suggests a possible interference in the inflammatory effects produced by the AA metabolites, such as leukotrienes and prostanoids. Together, these activities could result in chemokine release and subsequent neutrophil migration, as supported by the drastic reduction in leukocyte infiltration (indirectly evaluated by the decrease of MPO activity) in animals treated with the extract.
However, a reduction of anti-inflammatory effects was observed to the semisolids containing spray-dried extract concentrations greater than 3% () and also to 0.015% of isolated KA in the semisolid (). Thus, the increases in extract concentration (or KA) in the semisolid show a decrease in the biological effect (). Previous studies have demonstrated the topical anti-inflammatory activity of the S. trilobata chloroform extract (Maldini et al. Citation2009) containing an unknown amount of KA and also to a semisolid containing 100 μg/g of isolated KA (De Carli et al. Citation2009). In this concentration (100 μg/g or 0.01%) the authors did not observe reactivity in the agarose overlay test, which is related with absence of potential cutaneous irritation (De Carli et al. Citation2009), despite report of the cytotoxicity of high KA levels (Ghisalberti Citation1997).
These results indicate the need of maintaining the KA content under control in the phytomedicine in order to achieve the optimal biological effect.
Conclusion
Taken together, our data demonstrates, for the first time, that topic application of S. trilobata hydroalcoholic dried extract presented important anti-inflammatory effects, including inhibition of plasma extravasation and leukocyte migration. These data suggest that the anti-inflammatory effects observed with the S. trilobata extract depend, at least in part, on the presence and concentration of KA among other constituents. The mechanisms by which this medicinal extract exerts its anti-inflammatory effects are partially unclear, and require further investigation; however, they appear to involve interference in PKC activation and/or AA-metabolite production. S. trilobata could constitute a new and attractive phytotherapic alternative for the management of inflammatory skin conditions in humans.
Funding information
This research was supported by Laboratório Farmacêutico Elofar (Florianópolis, SC, Brazil), Laboratório de Produção e Análise de Medicamentos da UNIVALI (UNIVALI-LAPAM; Itajaí, Brazil), Conselho Nacional de Ciência e Tecnologia (CNPq) and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) for their financial support.
Acknowledgements
We also thank Prof. Dr. João Luís Callegari Lopes (NPPNS – FCFRP-USP) for assistance in the assessment of LC-MS data.
Disclosure statement
The authors report no declarations of interest.
References
- Baccarin T, Czepula AI, Ferreira RA, Lucinda-Silva RM. 2009. Análise morfoanatômica das partes aéreas de Wedelia paludosa DC. (Acmela brasiliensis, Sphagneticola trilobata), Asteraceae. Braz J Pharmacog. 19:612–616.
- Barcia MT, Pertuzatti PB, Rodrigues D, Gomez-Alonso S, Hermosin-Gutierrez I, Godoy HT. 2014. Occurrence of low molecular weight phenolics in Vitis vinifera red 393 grape cultivars and their winemaking by-products from São Paulo (Brazil). Food Res Int. 62:500–513.
- Batista R, Braga FC, Oliveira AB. 2005. Quantitative determination by HPLC of ent-kaurenoic and grandiflorenic acids in aerial parts of Wedelia paludosa D.C. Rev Bras Farmacogn. 15:119–125.
- Batista R, Garcia PA, Castro MA, Del Corral JM, Feliciano AS, de Oliveira AB. 2010. iso-Kaurenoic acid from Wedelia paludosa D.C. Ann Acad Bras Cienc. 82:823–831.
- Block LC, Santos AR, Souza MM, Scheidt C, Yunes RA, Santos MA, Monache FD, Filho VC. 1998a. Chemical and pharmacological examination of antinociceptive constituents of Wedelia paludosa. J Ethnopharmacol. 61:85–89.
- Block LC, Scheidt C, Quintão NL, Santos AR, Cechinel-Filho V. 1998b. Phytochemical and pharmacological analysis of different parts of Wedelia paludosa DC. (Compositae). Pharmazie. 53:716–718.
- Bralley EE, Greenspan P, Hargrove JL, Wicker L, Hartle DK. 2008. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J Inflamm. 5:1–7.
- Brancoft JD, Cook CH. 1988. Manual of histological techniques. New York (NY): Churchill Livingstone.
- Bresciani LFV, Cechinel Filho V, Yunes RA. 2000. Comparative study of different parts of Wedelia paludosa by gas chromatography. Nat Prod Lett. 14:247–254.
- Bresciani LFV, Yunes RA, Bürger C, De Oliveira LE, Bóf KL, Cechinel-Filho V. 2004. Seasonal variation of kaurenoic acid, a hypoglycaemic diterpene present in Wedelia paludosa (Acmela brasiliensis) (Asteraceae). Z Naturforsch C. 59:229–232.
- Bürger C, Fischer DR, Cordenunzzi DA, Batschauer AP, Cechinel Filho V, Soares AR. 2005. Acute and subacute toxicity of the hydroalcoholic extract from Wedelia paludosa (Acmela brasiliensis) (Asteraceae) in mice. J Pharm Pharm Sci. 19:370–373.
- Carvalho GJA, Carvalho MG, Ferreira DT, Faria TJ, Braz-Filho R. 2001. Diterpenos, triterpenos e esteróides das flores de Wedelia paludosa. Quím Nova. 24:24–6.
- Cechinel Filho V. 2000. Principais avanços e perspectivas na área de produtos naturais ativos: estudos desenvolvidos no NIQFAR/UNIVALI. Quím Nova. 23:680–685.
- Cechinel Filho V, Block LC, Yunes RA, Delle Monache F. 2004. Paludolactone: a new eudesmanolide lactone from Wedelia paludosa DC. (Acmela brasiliensis). Nat Prod Res. 18:447–451.
- Cesca TG, Faqueti LG, Rocha LW, Meyre-Silva C, de Souza MM, Quintão NL, Silva RM, Filho VC, Bresolin TM. 2012. Antinociceptive, anti-inflammatory and wound healing features in animal models treated with a semisolid herbal medicine based on Aleurites moluccana L. Willd. Euforbiaceae standardized leaf extract: semisolid herbal. J Ethnopharmacol. 143:355–362.
- Chagas-Paula DA, Oliveira TB, Faleiro DPV, Oliveira RB, Costa FB. 2015. Outstanding anti-inflammatory potential of selected asteraceae species through the potent dual inhibition of cyclooxygenase-1 and 5-lipoxygenase. Planta Med. 81:1296–1307.
- Choi RJ, Shi EM, Jung HA, Choi JS, Kim YS. 2011. Inhibitory effects of kaurenoic acid from Aralia continentalis on LPS-induced inflammatory response in RAW264.7 macrophages. Phytomedicine. 18:677–682.
- Clifford MN, Johnston KL, Knight S, Kuhnert N. 2003. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J Agric Food Chem. 51:2900–2911.
- Corrêa PM. 1984. Dicionário de plantas úteis do Brasil e das exóticas cultivadas. Rio de Janeiro, Brasil: Imprensa Nacional.
- Cunha TM, Verri WA, Jr Silva JS, Poole S, Cunha FQ, Ferreira SH. 2005. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 102:1755–1760.
- Dawid-Pać R. 2013. Medicinal plants used in treatment of inflammatory skin diseases. Postepy Dermatol Alergol. 30:170–177.
- De Carli RBG, Siqueira PRA, Kaiser ML, Freitas RA, De Souza MM, Cechinel-Filho V, Lucinda-Silva RM. 2009. Topical anti-inflammatory effect of creams containing kaurenoic acid isolated from Wedelia paludosa in mice. Lat Am J Pharm. 28:594–598.
- Domínguez M, Avila JG, Nieto A, Céspedes CL. 2011. Anti-inflammatory activity of Penstemon gentianoides and Penstemon campanulatus. Pharm Biol. 49:118–124.
- Fucina G, Block LC, Baccarin T, Ribeiro TR, Quintão NL, Filho VC, Silva RM, Bresolin TM. 2012. Development and validation of a stability indicative HPLC-PDA method for kaurenoic acid in spray dried extracts of Sphagneticola trilobata (L.) Pruski, Asteraceae. Talanta. 101:530–536.
- Ghisalberti EL. 1997. The biological activity of naturally occurring kaurane diterpenes. Fitoterapia. 68:303–325.
- ICH International Comission on Harmonization (2005) Validation of Analytical Procedures: Text and Methodology – ICH Harmonized Tripartite Guideline, Geneva.
- Jaiswal R, Kuhnert N. 2010. Hierarchical scheme for liquid chromatography/multi-stage spectrometric identification of 3,4,5-triacyl chlorogenic acids in green Robusta coffee beans. Rapid Commun Mass Spectrom. 24:2283–2294.
- Kissmann KG, Groth D. (1992). Plantas infectantes e nocivas. Rio de Janeiro: BASF.
- Korkina L, Kostyuk V, De Luca C, Pastore S. 2011. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini Rev Med Chem. 11:823–835.
- Li DW, Lee EB, Kang SS, Hyun JE, Whang WK. 2002. Activity-guided isolation of saponins from Kalopanax pictus with anti-inflammatory activity. Chem Pharm Bull. 50:900–903.
- Li X, Wang Y, Shi Q, Sauriol F. 2012. A new 30-noroleanane saponin from Wedelia chinensis. Helv Chim Acta. 95:1395–1400.
- Ma X, Liang J, Zheng C, Hu C, Zhao X, Rahman K, Qin L. 2013. Phenylpropanoids from Podocarpium podocarpum. Pharm Biol. 51:1021–1025.
- Maldini M, Sosa S, Montoro P, Giangaspero A, Balick MJ, Pizza C, Della Loggia R. 2009. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifólia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcok. J Ethnopharmacol. 212:430–433.
- Matos MEO, Tomassini TCB. 1983. Wedelin, a saponin from Wedelia scaberrima. J Nat Prod. 46:836–840.
- Mochly-Rosen D, Das K, Grimes KV. 2012. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 11:937–957.
- Navarro P, Giner RM, Recio MC, Máñez S,Cerdá-Nicolás M, Ríos JL. 2001. In vivo anti-inflammatory activity of saponins from Bupleurum rotundifolium. Life Sci. 68:1199–1206.
- Nguyen T, Zuniga R. 2013. Skin conditions: new drugs for managing skin disorders. FP Essent. 407:11–16.
- Pasparakis M, Haase I, Nestle FO. 2014. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 14:289–301.
- Patrick E, Burkhalter A, Maibach HI. 1987. Recent investigations of mechanisms of chemically induced skin irritation in laboratory mice. J Invest Dermatol. 88:24–31.
- Roque NF, Gianella TL, Giesbrecht AM, Barbosa RCSC. 1987. Kaurenes diterpenes from Wedelia paludosa. Rev Lat Am Quim. 18:110–111.
- Solecka DA. 1997. Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol Plant. 19:257–268.
- Swingle KF, Reiter MJ, Schwartzmiller DH. 1981. Comparison of croton oil and cantharidin induced inflammations of the mouse ear and their modification by topically applied drugs. Arch Int Pharmacodyn Ther. 254:168–176.
- Tonelli G, Thibault L, Ringler I. 1965. A bio-assay for the concomitant assessment of the antiphlogistic and thymolytic activities of topically applied corticoids. Endocrinology. 77:625–634.
- Tropicos (1996). Missouri Botanical Garden, Sphagneticola trilobata. Available from: http://www.tropicos.org/Name/2743714. Accessed on 10 March 2015.
- Vieira HS, Takahashi JA, Boaventura MA. 2001. Constituents from aerial parts of Wedelia paludosa. Fitoterapia. 72:854–856.
