Abstract
Context: Development of a reliable and selective anti-inflammatory agent of cyclooxygenase-2 (COX-2), induced or up-regulated by inflammatory/injury stimulus such as IL-1β, TNF-α and LPS in the various types of organs, tissues and cells, with low side effects is a long-standing medicinal chemistry problem with significant social implications.
Objective: To target druggable enzymome COX-2 by exploiting NSAIDs and genipin (GEP) in anti-inflammatory infection.
Materials and methods: The compound aspirin GEP ester (AGE) was designed by computer-assisted screening, synthesized in the esterification of the acylate derivative and the methylate derivative with Et3N, and evaluated with 20, 40 and 60 mg/kg from days 18 to 24 after immunization in collagen-induced arthritis (CIA) rats by the sequential enzymatic experiments, western-blot analysis and pathological observation methods.
Results: AGE exhibited higher binding affinity with COX-1 and displayed the lowest estimated free energy with COX-2 than other ligands built by hanging NSAIDs with GEP, and was characterized by 1H NMR, 13C NMR and HRMS. AGE was competed against COX-2 with molecule-dependent potencies and selectivity (IC50: 0.36 mM; selectivity index: 275) in the sequential enzymatic experiments and decreased the expression of COX-2 in peripheral blood lymphocytes of CIA rats. AGE (40 and 60 mg/kg) could significantly relieve the secondary hind paw swelling and arthritis index, along with observing AGE attenuated histopathological changes of fibroblast like synovial tissue (FLST) and mesenteric lymph node lymphocytes (MLNL) in CIA rats.
Discussion and conclusion: AGE pharmacophore reported herein may be an effective strategy to develop a novel anti-inflammatory agent and potential inhibitor of COX-2.
Introduction
Geniposide (GE, ), an iridoid glycoside, is the major active ingredient of Gardenia jasminoides J. Ellis (Rubiaceae) fruit, which has anti-inflammatory and other important therapeutic activities. Genipin (GEP, ) is the aglycone of GE by β-glucosidase metabolism that exerts the pharmacological effects in the body (Koo et al. Citation2004; Dai et al, Citation2014). The anti-inflammatory activity of GE has been demonstrated (Zhang et al. Citation2013; Shi et al. Citation2014; Song et al. Citation2014). However, most researchers consider the liver toxicity of GE to be related to the GE structure (Yamano et al. Citation1990; National Toxicology Program Citation2010; Wang et al. Citation2012). Some scholars have expressed the view that it should be modified to GEP or other metabolites (Yamano et al. Citation1988; Ozaki et al. Citation2002; Chang et al. Citation2005) and some literature confirms that GEP and GE in vivo animal models also have the anti-inflammatory, anti-allergic, immunosuppressive and other pharmacological effects after oral administration (Akao et al. Citation1994; Koo et al. Citation2006; Phatak Citation2015). Aglycone is a direct form of glycoside which exerts pharmacological effects in vivo (Zhao et al. Citation2015). However, GEP containing the structure of hemiacetal results in it being unstable in the acidity of the stomach. So, it is necessary to modify the structure of GEP to retain anti-inflammatory action.
Non-steroidal anti-inflammatory drugs (NSAIDs), which suppress the activities of cyclooxygenase (COX), commonly were used for the treatment of inflammation, pain and fever. Two distinct cyclooxygenase isoforms are characterized as COX-1 and COX-2. COX-1 is expressed ubiquitously and constitutively, and it plays a housekeeping role in processes such as gastrointestinal (GI) mucosa protection, while COX-2 is induced or up-regulated by inflammatory/injury stimuli such as IL-1β, TNF-α and LPS in the various types of organs, tissues and cells. Furthermore, COX-2 contributed to the production of prostaglandin E2 (PGE2) and prostaglandin I2 (PGI2), which closely associate with inflammation, fever and pain, evoking and sustaining systemic or peripheral inflammatory disease, but it is not involved in the COX-1 mediated GI tract events. However, non-selective COX inhibitors exhibit serious side-effects such as stomach bleeding and gastric ulcers because of their free carboxyl group which directly stimulates the GI mucosa and inhibits activities of COX-1 (Shanbhag & Crider Citation1992). Development of a reliable and selective anti-inflammatory agent with low side effects is a long-standing medicinal chemistry problem with significant social implications. Since COX-2 is an important rate-limiting enzyme for PGs production, inhibiting the activity that COX-2 is able to attenuate the levels of PGs that are involved in the inflammatory symptoms such as heat, swelling, flare and pain. Selective COX-2 inhibitors have been taken into account as a significant target of drug candidates treated of pyrexia, inflammation and pain (Daniel et al. Citation2004).
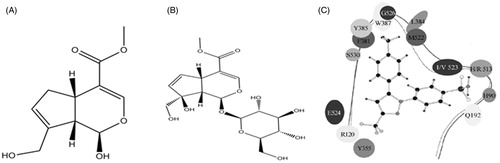
Docking simulation, an alternative way based on the principles of geometry complementary ligands, receptors and energy matched, was aimed to study the binding structure of a newly identified inhibitor with its receptor and has been successfully used in many applications, such as the study for novel covalent proteasome inhibitors (Li et al. Citation2014). COX-2 inhibitors might be as potential anti-inflammatory drugs and shed light on the detailed mechanisms of drug (Vanegas & Schaible Citation2001). Compositions and structural characteristics of the active pocket structure of COX-1 and COX-2 protein in the crystal are shown in . A ‘V’ type of binding pocket, composed of some hydrophobic residues and bifurcated in the number 523 residue of COX-1 and COX-2, forms a strong and narrow hydrophobic pocket. The left pocket of COX is defined as ‘L’ binding pocket, while the right is defined as ‘R’ and the difference of the binding pocket between of COX-1 and COX-2 is that the Val-523 volume of COX-1 is bigger than the Ile-523, resulting in that the ‘R’ volume of COX-1 is smaller than the COX-2. Non-selective inhibitors (NSAIDs) have similar performance, which are bound with the pockets of COX-1 and COX-2 at the same time, while the selective inhibitor of COX-2 (the celecoxib, the small molecular in ) is designed based on the different sizes of ‘R’ binding pocket of COX-1 and COX-2. The reason of celecoxib’s selectivity is that its large group (benzene sulphonic acid) can bind with the ‘R’ pocket of COX-2 rather than the ‘R’ pocket of COX-1.
The present study seeks to discover a new inhibitor of selective COX-2 and to control the inflammatory process by making use of previous report the key residues of COX crystalline form (Sasieni & Winnett Citation2003) and lead molecules were based on methylated GEP and NSAIDs scaffold. Then, the hit compound was synthesized and evaluated in bio-activity. Fortunately, the results obtained with computer-assisted in silico screening were satisfactory agreement with the evaluated bio-activity, and the hit compound represents a novel and promising lead structure for the development of anti-inflammatory drugs as COX-2 interaction disruptors. A highly significant discovery of a novel non-acid type COX-2 inhibitor is reported herein.
Materials and methods
Molecular docking studies
The choice of ligands
The structures of ligands, which are built by hanging NSAIDs with methylated GEP, were prepared in CS Chem Draw Ultra 8.0 (). Crystalline forms of COX-1 (ID: 1CX2) (Picot 1994) and COX-2 (ID: 1PRH) (Kurumbail et al. Citation1996) were downloaded from Protein Data Bank.
Table 1. Structure and docking results of the ligands with COX-1 and COX-2.
The pretreatment of ligands and receptors for docking
Polar hydrogens were in addition to the protein structures of COX-1 and COX-2, and ‘Gasteiger’ charges were assigned. Non-polar hydrogens were merged, and partial charges were included in their parent carbon atoms. Only the polarity hydrogens are optimized in the optimization process, while other atoms remain the same form in the entire optimization. Energy optimization was to be processed by the ‘Minimise’ molecular mechanics program, the ‘Powell’ method and the ‘Tripos’ standard force field (Zheng et al. Citation2010), then crystalline forms of COX-1 and COX-2 were charged with the ‘Kollman all-atom’ charge, energy convergence limited at 0.4% kJ/mol, and the dielectric constant was ɛ = 4rij, while the three-dimensional structure of COX-1 and COX-2 protein was not changed significantly after energy convergence. Compared with the optimization of enzyme molecules, the small molecules were optimized to plus hydrogenation for all and relied on the ‘Conj Grad’ optimized method, energy convergence limited at 0.2% kJ/mol, and the dielectric constant was ɛ = rij.
Molecular docking
We utilize the database built by our lab to dock with the COX-2 by the AutoDock tools 1.5.4. The protein molecules were accompanied by the Kollman all-atom charged, and small molecules were accompanied with the Gasteiger–Huckel charged in the process of docking. The parameters of the protein atomic volumes and the fragmental volumes were generated by the addsol module of autodock. The calculated parameters of the lattice were 80 × 80 × 80 lattice and 0.0375 nm distance. Lattice file was generated by autogrid program relying on the lattice centre of the crystal structure of COX-2 inhibitors. The method of docking was used with Lamarckian genetic algorithm (Fuhrmann et al. Citation2010), which allowed to handle a large number of degrees of freedom, and the part parameters were settled as follows: translational and rotational steps were 0.02 nm and 5°, respectively, and the maximum number of the energy calculation and the algebra of maximum genetic were 1.5 × 106 and 2.7 × 104, respectively, while other parameters were default values. The docked composite compounds were optimized by the molecular mechanics to avoid the irrational interatomic collisions. Then, we use the small molecules which have lower ability of binding free energy with COX-2 to dock with COX-1 at the same docking process and the parameters were settled as COX-2. Finally, we investigate the similarities and differences of the binding between small molecules and COX-1/2 proteins, which we examined.
Chemical syntheses
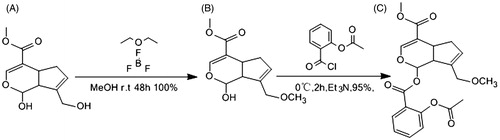
Methyl 7-(hydroxymethyl)-1-methoxy-1,4a,5,7a-tetrahydrocyclopenta[c] pyran 4-carboxylate (b)
A stirred solution of 1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-[c]pyran-4-carbo xylate (a) (3 g, 13.3 mmol) in dry methanol and boron trifluoride etherate as the catalyst was dropwise added at room temperature. The reaction mixture was stirred at room temperature for 24 h, permitted to stand overnight and quenched by the saturated sodium bicarbonate solution for 1 h. The mixture was extracted by ethyl acetate for a few times and then dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was uncrystallized from dry EtOH to afford 2.8 g of the title product (b) in 99% yield as a colourless oily liquid.
(4-Methoxy-7-propionyl-3a,4,7a-tetrahydro-1H-inden-3-yl)methyl 2-acetoxyb-enzoate (c)
A stirred solution of intermediate (b) in dry CH2Cl2 (pH 8, contain the triethylamine) was added dropwise intermediate acetyl salicylic chloride within the CH2Cl2 at 0 °C. Then, the reaction mixture was stirred at room temperature for 1 h and quenched by the saturated sodium bicarbonate solution. The mixture was extracted by ethyl acetate for a few times and then dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The crude product was purified by silica gel column chromatography (silica gel 100–200 mesh, petroleum ether–ethyl acetate = 6:1) to afford 2.1 g of the title product (c) (a colourless oily liquid) in 61% yield.
Bioassay
Animals
The male Sprague-Dawley rats (SD rats; 180–200 g; age, 5 months) were supplied by the Experimental Animal Center of Anhui Medical University (Anhui, China) and allowed to acclimatize for at least 1–2 weeks before experimentation, fed with a standard diet and allowed water ad libitum. All animal experiments were performed in compliance with the Animal Management Rules of the Ministry of Health of the People’s Republic of the Care and Used of Laboratory of our university.
Induction of CIA and drug administration
Freund’s complete adjuvant (FCA) was prepared by suspending heat-killed BCG in liquid paraffin at 10 mg/mL. Briefly, the collagen-induced arthritis (CIA) rat model was immunized on day 0 by a single intradermal injection with 100 μL of FCA into the right hind footpad (Xu et al. Citation2014). Before the onset of arthritis, all rats were divided into nine groups randomly (six animals in each group): Group I, normal control; Group II, CIA model; Groups III–V, CIA rats intragastrically administered with aspirin GEP ester (AGE) (20, 40, 60 mg/kg); Group VI, aspirin (ASP, 40 mg/kg); Group VII, GE (40 mg/kg); Group VIII, celecoxib (30 mg/kg) from days 18 to 24 after immunizations. While in groups of normal and CIA model, rats were given an equal volume of water at the same time. At the end of the experiment, the animals were anaesthetized, and blood was collected from the abdominal aorta.
Arthritis assessment
The rats were assessed daily for signs of arthritis by two independent observers who were blinded to the experimental design. The volume of the non-injected hind paw was measured by using a YLS-7A volume meter (Shandong Academy of Medical Sciences Equipment Station, P. R. China). The preinjected values of paw volumes were measured before immunization and were considered as the baseline. The increasing paw volume was calculated and expressed in millilitres. The paws were examined and graded for severity and swelling loci using a four-point scale: 0 = no swelling; 1 = swelling of finger joints; 2 = swelling of phalanx joint and digits; 3 = severe swelling of the entire paws; 4 = deformity or ankylosis. Maximum arthritis score per rat was 12 including three secondary arthritis paws.
Histopathological examination
After the animals were sacrificed, the synoviums of rats were excised and taken from the knee joints after the skin, superficial muscle and tissues were removed. The samples were fixed with 10% paraformaldehyde in phosphate-buffered saline (PBS) for 24 h and then dehydrated, processed and embedded in paraffin wax for histological analysis. A series of paraffin sections (5 μm) were stained with haematoxylin and eosin (HE, 200× and evaluated with a microscope.
Cell proliferation inhibition assay by methyl thiazolyl tetrazolium assay (MTT)
Peripheral blood was removed in sterile condition, and peripheral blood lymphocytes (PBLs) were isolated by routine method. Then, the cells were cultured in triplicate in a concentration of 1 × 1010 cell/L in 100 μL Dulbecco modified eagle medium (DMEM) containing 10% defined foetal bovine serum (FBS). First, the cells were stimulated with 5 mg/L concanavalin A (ConA) and 5% of CO2 for 48 h at 37 °C. A 10 μL sample of MTT (5 g/L) was added before the end of stimulation for 4 h and then the cultures were stimulated for 4 h continuously. After incubation, the cultures were centrifuged (760g, 10 min) and the supernatants were discarded. The 150 μL of dimethyl sulphoxide (DMSO) was added to each well and the absorbance (A) was examined at 490 nm using a MSS ELISA Microwell Reader (Thermo Scientific Co., USA).
Western blot
Frozen cells of PBL were lysed in buffer and the lysates were separated by 10% sodium dodecyl-sulphate–polyacrylamide gel electrophoresis. The proteins were electrophoretically transferred to nitrocellulose filter (NC) membranes (Millipore, Bedford, MA). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline/Tween 20 at room temperature for 3 h. Primary antibodies specific for COX-1 and COX-2 were incubated with the membranes overnight at 4 °C. Membranes were washed with PBS and incubated with the secondary antibody horseradish peroxidase-conjugated goat anti-mouse (1:1000) or goat anti-mouse (1:5000) IgG. Immunoreactive proteins were detected with 3,3′-diaminobenzidine tetrahydrochloride. All the experiments were performed three times and the results were reproducible.
The specifical enzyme experiments in vitro
A COX fluorescent inhibitor screening assay kit, consisting of ovine COX-1 and human recombinant COX-2 enzymes, determined COX-1 and COX-2 inhibition activities of tested compound AGE (Bansal et al. Citation2014). The stock solutions of tested compound AGE were prepared with DMSO. Ten millilitres of test solutions (0.01, 0.1, 1, 10, 50, and 100 mM) were added to 960 mL supplied buffer solution (0.1 M Tris–HCl pH 8.0 containing 5 mM EDTA and 2 mM phenol) with either COX-1 or COX-2 (10 mL) enzyme in the presence of 10 mL heme and 10 mL fluorometric substrate 10-acetyl-3,7-dihydroxyphenoxazine (ADHP). Then, 10 mL of arachidonic acid solution (100 mM) was added to the solutions, which were kept at 37 °C for 5 min, and the COX reaction was stopped by the addition of 50 mL of 1 M HCl after 2 min. The resorufin containing of fluorescence and produced by the reaction of PGG2 and ADHP were measured at an excitation wavelength at 535 nm and an emission wavelength at 590 nm. The strength of the fluorescence is proportional to the amount of resorufin, which is proportional to the number of PGE2 present in the well during the incubation. The IC50 values of tested AGE and reference drug celecoxib to inhibit the COX-1 and COX-2 isozymes were calculated from the response curve of inhibitory concentration.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM), in triplicate. One-way ANOVA test and Student’s t-test (SPSS17.0 Software Products, Chicago, IL) were used to determine significant differences between groups. The histopathological analysis was analysed by Ridit procedure. In all tests, p value <0.01 was considered statistically significant.
Results
Structure–activity relations of COX-2 inhibitors
The docking studies of COX and GEP are coupling with NSAIDs
The docking results revealed that there were four coupled complexes (AGE, 1c, 2a, 3a) exhibited lower binding energy after docking with the ‘R’ binding pocket of COX-2, which is composed of Phe-518, His-90, Arg-513, Val-523 and Ser-353 residues, as shown in . The released energy was relatively lower with the COX-2 and could achieve below the −103.5 kJ/mol, reflecting that they can combine with the COX-2 enzyme better. Thus, the four coupled complexes may have inhibitory ability of COX-2, but do they have the ability to selectively inhibit COX-2? The results revealed that only the coupled complex AGE has the lower binding energy interacting with the ‘R’ binding pocket of COX-1 and the energy difference was about 41 kJ/mol, so it is a description of conjugates may effectively inhibit COX-2 enzyme as shown in .
The binding mode of AGE interacted with the COX-2
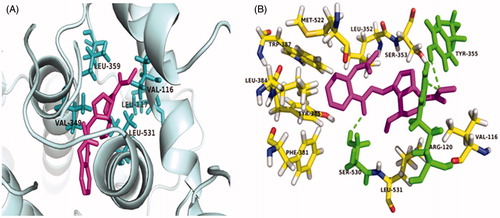
The study on the docking of AGE and COX-2 enzyme displays that it occupy the ‘R’ site of COX-2 enzyme binding pocket, rather than the ‘L’ site of COX-2 enzyme binding pocket. Carboxyl group substituted hydroxyl of AGE was placed in the hydrophobic pocket, formed by val-116, Leu-117, Val-349, Leu-359, Leu-531 residues of COX-2 (). Furthermore, the ester carbonyl group in AGE displayed hydrophobic interaction with Tyr-355 residue of the COX-2, while phenyl ring of oxadiazole moiety showed hydrophobic interaction with Arg-120 residue of the target enzyme ().
Chemistry
Novel COX-2 inhibitor (4-methoxy-7-propionyl-3a,4,7a-tetrahydro-1H-inden-3-yl) methyl 2 acetoxybenzoate derivative (compound AGE) was synthesized as shown in . First, the methylation reaction for 1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-[c]pyran-4-carboxylate and methanol was performed with boron (tri)fluoride etherate to prepare methyl 7-(hydroxymethyl)-1-methoxy-1, 4a, 5, 7a-tetrahydrocyclopenta[c]pyran-4-carboxy-late (b) (Moon et al. Citation1998,Citation2001). Second, 2-(chlorocabonyl) phenyl acetate was acidulated with two chloro sulphoxide in CH2Cl2/DMF condition to form acetyl salicylic chloride (Cai et al. Citation2012). Third, the esterification of the acylate derivative and the methylate derivative was performed with Et3N to form compound (c) as a sole product AGE. The structure of compound AGE was characterized by 1H NMR, 13C NMR and HRMS ().
1H NMR (600 MHz, CDCl3) δ 8.00 (d, J = 7.2 Hz, 1H), 7.53 (t, J = 7.7 Hz,1H), 7.41 (s, 1H), 7.08 (d, J = 7.8 Hz, 2H), 5.98 (s, 1H), 5.07 (d, J = 3.4 Hz,1H), 4.95 (s,2H), 3.70 (s, 3H), 3.53 (s, 3H), 3.22–3.18 (m, 1H), 3.11–3.07 (m, 1H), 2.30 (s, 3H), 2.08 (d, J = 8.2 Hz, 2H).
13C NMR (151 MHz, CDCl3) δ 169.91, 167.95, 164.49, 152.38, 150.92, 150.76, 138.10, 136.21, 131.22, 126.24, 124.04, 124.43, 111.59, 111.01, 77.23, 63.30, 57.34, 56.53, 51.46, 35.54, 33.88, 21.25, 0.19.
HRMS: C21H21O8Na, [M + Na]+ 425.1202.
Biological evaluations
Effect of AGE on secondary arthritis in CIA rats
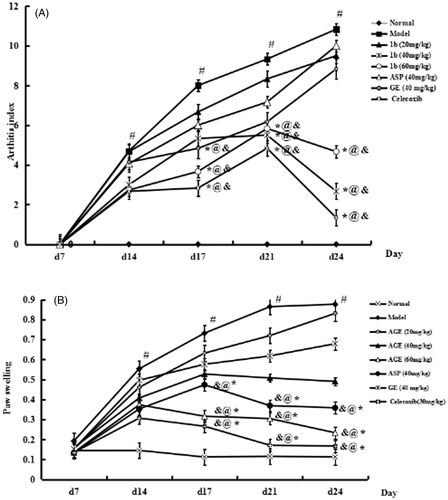
The paw swelling and arthritis scores were examined. The onset and peak of the inflammatory reaction occurred on day 14 and days 18–21 after FCA injection triggered, respectively. The model, which accompanied with paw swelling, pain and polyarthritis, was a remarkable secondary inflammatory response. As expected, the administration of AGE (40 and 60 mg/kg) suppressed significantly in inhibiting the paw swelling of CIA rats (p < 0.01), while there was no significant in reducing paw oedema with AGE at 20 mg/kg (p > 0.05) (). In addition, AGE (40, 60 mg/kg) suppressed significantly in inhibiting the paw swelling of group VI ASP and group VII GE. The arthritis scores were reduced by AGE administration () as same as the paw swelling assay. These observations indicated that AGE (40, 60 mg/kg) significantly inhibited the joint inflammation of CIA rats, and there was no significance in inhibiting the joint inflammation with AGE at 20 mg/kg (p > 0.05) (). In addition, AGE (40, 60 mg/kg) suppressed significantly in inhibiting the joint inflammation of group VI ASP and group VII GE.
Figure 4. Effects of AGE on secondary arthritis (A) and paw swelling (B) in CIA rats. Rats were immunized on day 0 by a single intradermal injection into the left hind paw with 0.1 mL of FCA for each rat. Group I, normal control; Group II, CIA model; Group III–V, CIA rats intragastrically administered with AGE (20, 40, 60 mg/kg, respectively); Group VI, aspirin (ASP, 40 mg/kg); Group VII, GE (40 mg/kg); Group VIII, celecoxib (30 mg/kg) were given intragastrically to CIA rats from days 18 to 24 after immunization. Data are expressed as mean ± SD (n = 6). #p < 0.01 versus normal control group. *p < 0.01versus CIA model group, @p < 0.05 versus ASP group, &p < 0.05 versus GE group.
The relationship between dose and time of the inhibitory effect of AGE on the proliferation of PBL in CIA rats
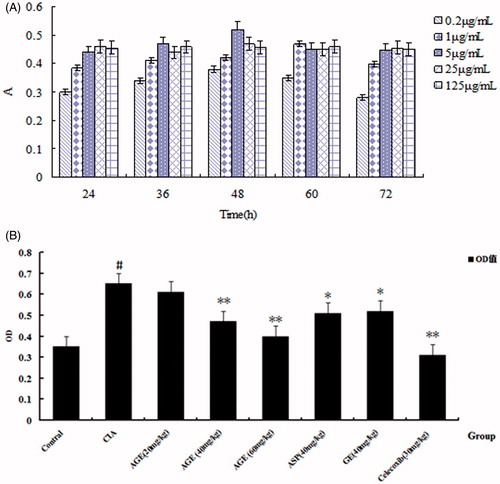
With different concentrations of AGE (2.5, 5, 10, 20, 40, 80, 160 μM) affecting on PBL of CIA rats at different time (24, 36, 48, 60, 72 h) in vitro, the results suggested that AGE effecting with 48 h is more significant on the proliferation of PBL. Considering the inhibitory effects AGE (80, 160 μM) were not significant and the concentration was higher, so we study the effect of AGE at the five concentrations (2.5, 5, 10, 20, 40 μM) with 48 h in vitro at the following ().
Figure 5. (A) Proliferation of PBL treated of AGE in different concentrations of Con A at various time points. With different concentrations of AGE (2.5, 5, 10, 20, 40, 80,160 μM) effecting on PBL of CIA rats at different time (24,36,48,60,72 h) in vitro, the results suggest that AGE effecting with 48 h is more significant on the proliferation of PBL. (B) Effects of AGE on the proliferation of PBL induced by Con A. #p < 0.01 versus control group;*p < 0.05 versus CIA group; **p < 0.01 versus CIA group.
Effect of AGE on the lymphocytes proliferation of PBL in CIA rats
The PBL of CIA rats was stimulated with Con A (5 μg/mL) and was cultured for 48 h at different concentrations of AGE (2.5, 5, 10, 20, 40 μM). MTT method was utilized to detect AGE proliferation in vitro effect on PBL in CIA rats. As shown in , the model group significantly promoted the proliferation of PBL, and different concentrations of AGE showed varying degrees inhibition of proliferation in response to CIA-PBL hyperfunction in vitro, and the inhibition ability with the concentration of AGE has a related trend. The results showed that AGE could inhibit the proliferation ability on PBL in CIA rats ().
Effects of AGE on histopathological changes of MLNs and FLS in CIA rats
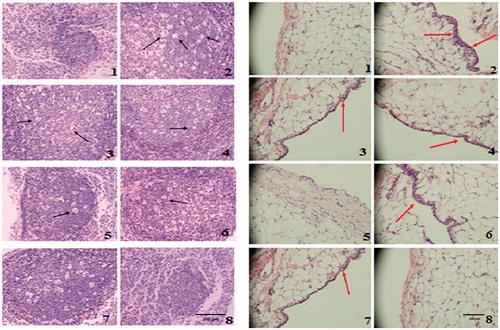
Rats were sacrificed on day 24 after immunization and subjected to histopathological examination. The suppressive effects of AGE in CIA rats were further supported by histological analysis of MLNs () and FLS (). The MLN of CIA rats showed the increased in the number of inflammatory cells. Treatment with AGE at 60 mg/kg revealed a marked decrease in hyperplasia of lymphatic follicle of CIA rats (). Massive mononuclear cell infiltration of the synovial tissue and synovial hyperplasia was observed in FLS of CIA rats. The AGE-treated groups (40, 60 mg/kg) exhibited a significant reduction in synovial hyperplasia and synovial inflammatory cell infiltration compared with the CIA model group ().
Figure 6. (A) Histopathological examination of MLN in CIA rats. (1) In normal rats, fewer and smaller lymphatic follicles and no inflammatory cells were observed in MLN. (2) In CIA rats, hyperplastic lymphatic follicle and the infiltration of inflammatory cells were observed. (3), (6) and (7) In CIA rats treated with AGE (40, 60 mg/kg) and celecoxib (30 mg/kg) show little lymphatic follicles hyperplasia and cells infiltration. (4), (5) and (8) In CIA rats treated with AGE (20 mg/kg), ASP (40 mg/kg), GE (40 mg/kg), lymphatic follicles hyperplasia and inflammatory cells infiltration were significantly reduced (original magnification ×200). (B) Effects of AGE on histopathological changes in the FLS of CIA rats (original magnification ×200). (1) Normal control, (2) CIA model, (3) CIA + AGE (20 mg/kg), (4) CIA + AGE (40 mg/kg). (5) CIA + AGE (60 mg/kg), (6) CIA + ASP (40 mg/kg), (7) CIA + GE (70 mg/kg), (8) CIA + celecoxib (30 mg/kg). Arrowheads indicate synovial inflammatory cell infiltration.
Effects of AGE on expression of COX-1, COX-2 and β-actin in CIA rats
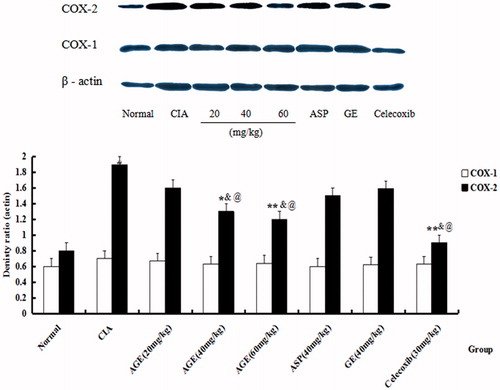
The protein expression of COX-1, COX-2 and β-actin of PBL in CIA rats was estimated on day 24 after immunization. It was found that the expression of COX-2 was heightened in CIA rats when compared with normal group (p < 0.01). The administration of AGE (40, 60 mg/kg) markedly decreased that of COX-2 (p < 0.01), and there was no significant in reducing expression with AGE at 20 mg/kg (p > 0.05), while the protein levels of total COX-1 had no change (). In addition, AGE (40, 60 mg/kg) suppressed significantly in decreasing expression of COX-2 than group VI ASP and group VII GE ().
Figure 7. Effects of AGE on expression of COX-1, COX-2 and β-actin in CIA rats. The protein expression of COX-1, COX-2 and β-actin of PBL in CIA rats were estimated on day 24 after immunization. It was found that the reducing expression of COX-2 was heightened in CIA rats when compared with normal group. #p < 0.05 versus normal control group. *p < 0.05 versus CIA model group, **p < 0.01 versus CIA model group, @p < 0.05 versus ASP group, &p < 0.05 versus GE group.
In vitro COX inhibition assay
The ability of the tested drug AGE and reference drug celecoxib to inhibit the COX-1 and COX-2 isozymes was evaluated using colorimetric COX (ovine) inhibitor screening assay and the results, which were expressed as IC50 (concentration exhibiting 50% enzyme inhibition), obtained are listed in . The selectivity index (SI), which was calculated by IC50 (COX-1)/IC50 (COX-2), of celecoxib and diclofenac against COX-1 and COX-2 was found to be 393.4 and 1.15, respectively. The results kept pace with that celecoxib have selectivity against COX-1 and COX-2, while diclofenac has not. Compared with the IC50 (99 mM) of AGE inhibited COX-1, the IC50 (0.36 mM) of AGE inhibited COX-2 is more than 200 multiples. It indicated that the compound AGE has more selectivity with COX-2 than COX-1.
Table 2. The inhibitory rate of AGE, celecoxib and diclofenac on PBL.
Discussion
In the present study, the computer-assisted rational designed compound AGE competed against and interfered with the sequential enzymatic experiments in vitro as COX-2 inhibitors with molecule-dependent potencies and selectivity. As shown in , the docking results revealed that four coupled complexes (AGE, 1c, 2a, 3a) docked with the ‘R’ binding pocket of COX-2 with below the −103.5 kJ/mol and only the coupled complex AGE docked with COX-1 with lower energy (41 kJ/mol), indicating that the compound AGE may effectively inhibit COX-2 enzyme. Besides, the docked results of AGE with COX-2 enzyme display that it occupy the ‘R’ site of COX-2 enzyme binding pocket, which is composed of Phe-518, His-90, Arg-513, Val-523 and Ser-353 residues, rather than the ‘L’ site of COX-2 enzyme binding pocket. The complex AGE, carboxyl group of ASP substituted hydroxyl of methylated GEP, was placed in the hydrophobic pocket by val-116, Leu-117, Val-349, Leu-359, Leu-531 residues of COX-2 (). Furthermore, carbonyl of ester in AGE displayed hydrophobic interaction with Tyr-355 residue of the COX-2, while phenyl ring of iridoid aglycone showed hydrophobic interaction with Arg-120 residue of the target enzyme ().
As structure–activity relationship (SAR) study of COX-2 inhibitor with computer-assisted rational designed, the compound AGE was estimated as IC50 against COX-2 over IC50 against COX-1 in the PBL with the sequential enzymatic experiments in vitro as COX-2 inhibitors. Hence, as the specific enzyme experiments in vitro, the inhibitory activities of the compound AGE were determined on TXB2 production to investigate COX-1 inhibition, and on 6-keto-prostaglandin F1a (6-keto-PGF1a, spontaneously degraded stable form of PGI2) expressing COX-2 induced by IL-1β in the PBL, respectively. As shown in , the ability of the tested drug and reference drug celecoxib, competed against and interfered with the COX-1 and COX-2 isozymes by colorimetric COX (ovine) inhibitor screening assay, is expressed as IC50. The SI of celecoxib and diclofenac against effects of COX-1 and COX-2 was found to be 376 and 0.28, respectively. The results indicated that celecoxib has selectivity against effects of COX-1 and COX-2, while diclofenac has not this function. Compared with the IC50 (99 mM) of AGE inhibited COX-1, the IC50 (0.36 mM) of AGE inhibited COX-2 is more than 200 multiples and indicated that the compound AGE has more selectivity with COX-2 than COX-1. The molecular characteristics of the inhibitors described above would be significant to determine pharmacophore-recognition/interaction mode between respective compounds and respective isozymes in terms of orientation, conformation and potency. Moreover, the novel non-acid type COX-2 inhibitors with the present findings might be useful to elucidate detailed mechanisms underlying COX-2 selective potent inhibitory activity in vitro for further COX-2 inhibitor study. As well, in the present SAR study, the significant various substituent-effects towards potent and selective COX-2 inhibition clarified in vitro might be useful for designing further COX-2 inhibitors.
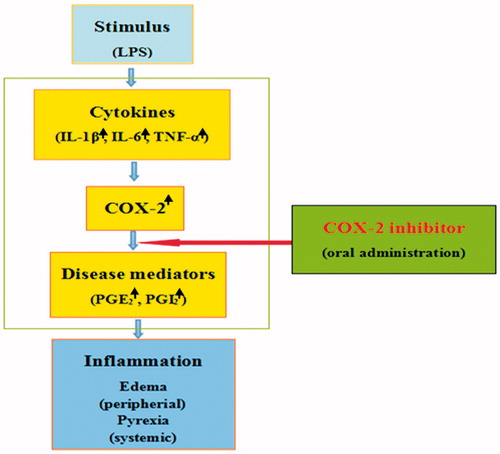
Inflammation is a basic pathological process to the tissue or the microcirculation caused by the body’s injury and various inflammatory stimuli. The inflammatory reaction is mediated by a variety of mediators, and the PG plays an important role in the inflammatory reaction. COX is a kind of double functional enzyme, which is a key rate-limiting enzyme for PG production cascades. The mechanisms of both anti-inflammation effects and side-effects of traditional COX inhibitors are associated with the existence of two COX isoforms. Thus, while COX-1 is expressed ubiquitously and constitutively, and it serves a housekeeping role in processes such as GI mucosa protection, COX-2 is absent or exhibits a low level of expression in most tissues, and is induced and highly up-regulated in the peripheral tissues such as macrophages, monocytes, synoviocytes, blood, liver and lung, as well as in the nerve systems such as peripheral nerves, spinal cord or brain. And then, COX-2 action-derived inflammatory-signals such as PGs are released, thereby causing pyrexia and oedema as systemic- and/or peripheral-inflammatory symptoms, but it is not involved in the COX-1-mediated GI tract events. Furthermore, in the acute, subacute and chronic inflammation model, selective inhibition of COX-2 activity has obvious anti-inflammatory effect (). COX-2 may be as rheumatoid arthritis (RA) therapeutic effect of potential target.
Figure 8. The mechanisms of orally potent anti-pyretic effect in the LPS-induced fever model and anti-oedematous effect in the carrageenan-induced oedema-formation model for COX-2 inhibitor.
RA is a chronic, inflammatory and systemic autoimmune disease, which is characterized by joint swelling and pain, joint stiffness, deformity and serious functional damage. CIA could act as an experimental model in our study to demonstrate the effects of compound AGE on human RA because of its similarity to human RA in both clinical and histopathological features. As shown in , the present study showed that compound AGE (40, 60 mg/kg) significantly relieved the secondary hind paw swelling and arthritis index in CIA rats. In addition, AGE (40, 60 mg/kg) suppressed significantly in inhibiting the paw swelling and arthritis index of group VI ASP and group VII GE. The compound AGE at dose (more than 20 mg/kg) markedly reduced the secondary hind paw swelling and polyarthritis index, indicating a protective effect on FCA-induced injury in vivo, while AGE at low dose (less than 20 mg/kg) did not show a significant inhibitory effect on the secondary hind paw swelling and arthritis index in CIA rats. This failure may be accounted for several possibilities such as, low dose of compound AGE could not be high enough for attenuating the secondary hind paw swelling and arthritis index, or the treatment time for CIA rats with AGE was too short to see improvement.
CIA was closely related to the hyperfunction of immune function. As shown in , the model group significantly promoted the proliferation of PBL, and different concentrations of AGE showed varying degrees inhibition of proliferation in response to CIA-PBL hyperfunction in vitro, and the inhibition ability with the concentration of AGE has a related trend by MTT. Compound AGE could reduce the proliferation of PBL in CIA rats, suggesting that AGE could get the immune function back to normal levels by acting on immune cells, so as to relieve CIA. Interestingly, as shown in , the MLN of CIA rats showed increase in the number of inflammatory cells. Treatment with AGE at 100 mg/kg revealed a marked decrease in hyperplasia of lymphatic follicle of CIA rats. The proliferation of MLNL was inhibited by CIA in the interestinal mucosal immune system, while AGE could restore and even enhance the proliferation activity of MLNL. This hinted that the effect of AGE on CIA may also be played by the mucosal immune tolerance mechanisms. Scilicet, compound AGE stimulated the mucosal immune system and then the intestinal mucosal immunity was enhanced, but the immune response of lesions was suppressed. In addition, compound AGE (40, 60 mg/kg) improved histological status of FLS in CIA rats indicated that AGE could alleviate the extent of arthritis in CIA rats with FCA-induced. Besides, the protein expression of COX-1, COX-2 and β-actin of PBL in CIA rats was estimated on day 24 after immunization. It was found that the expression of COX-2 was heightened in CIA rats when compared with normal group (p < 0.01). The administration of AGE (40, 60 mg/kg) markedly decreased that of COX-2 (p < 0.01), and there was no significant in reducing expression with AGE at 20 mg/kg (p > 0.05), while the protein levels of total COX-1 had no change (). In addition, AGE (40, 60 mg/kg) suppressed significantly in decreasing expression of COX-2 than group VI ASP and group VII GE, and there was no significance in reducing expression of COX-1 with delivery groups ().
In conclusion, a (4-methoxy-7-propionyl-3a,4,7a-tetrahydro-1H-inden-3-yl) methyl 2-acetoxybenzoate derivative AGE, which is obtained from the computer-aided design of virtual screening, was synthesized and evaluated in bio-activity. Molecular docking analysis of AGE docked with COX-1 was exhibited higher binding affinity than other ligands, while AGE docked with COX-2 was displayed the lowest estimated free energy, among all the docked compounds. Results of in vitro COX assay indicated that AGE showed considerable COX-2 selectivity than COX-1. The compound AGE was significantly relieved the secondary hind paw swelling and arthritis index, along with observing that AGE attenuated histopathological changes of FLST and MLNL in CIA rats. Taken together, AGE pharmacophore reported herein would be an effective strategy to develop the novel non-steroidal anti-inflammatory agents. Thus, AGE seemed to be the best candidate for being further evaluation of its pharmacokinetics profile and for its ulcerogenic activity in vivo and toxicity model to validate the safety of anti-inflammatory potential.
Funding information
This project was financially supported by the National Natural Science Foundation of China (Nos 81473400, 81073122), Anhui Provincial Natural Science Research Project in Colleges and Universities (KJ2009A045Z), the youth Natural Science Foundation of Anhui University of Chinese Medicine (qn201308) and Science and technology projects in Anhui Province (13Z04013).
Disclosure statement
This work was supported by the Key Laboratory of Modernized Chinese Medicine in Anhui Province. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akao T, Kobashi K, Aburada M. 1994. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol Pharm Bull. 17:1573–1576.
- Bansal S, Bala M, Suthar SK, Choudharyd S, Bhattacharyaa S, Bhardwaje V, Singlaf S, Josephc A. 2014. Design and synthesis of novel 2-phenyl-5-(1,3-diphenyl-1H-pyrazol-4-yl)-1,3,4-oxadiazoles as selective COX-2 inhibitors with potent anti-inflammatory activity. Eur J Med Chem. 80:167–174.
- Cai J, Duan Y, Yu J, Chen J, Chao M, Ji M. 2012. Bone-targeting glycol and NSAIDS ester prodrugs of rhein: synthesis, hydroxyapatite affinity, stability, anti-inflammatory, ulcerogenicity index and pharmacokinetics studies. Eur J Med Chem. 188:409–419.
- Chang WL, Wang HY, Shian L, Lai JH, Lin HC. 2005. Immunosuppressive iridoids from the fruits of Gardenia jasminoides. J Nat Prod. 68:1683–1685.
- Dai MM, Wu H, Li H, Chen J, Chen JY, Hu SL, Shen C. 2014. Effects and mechanisms of geniposide on rats with adjuvant arthritis. Int Immunopharmacol. 20:46–53.
- Daniel LS, Regina MB, Timothy H. 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 56:387–437.
- Fuhrmann J, Rurainski A, Lenhof HP, Neumann D. 2010. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J Comput Chem. 31:1911–1918.
- Koo HJ, Lim KH, Jung HJ, Park EH. 2006. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 103:496–500.
- Koo HJ, Song YS, Kim HJ. 2004. Anti-inflammatory effects of genipin, an active principle of Gardenia. Eur J Pharmacol. 495:201–208.
- Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, et al. 1996. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 384:644–648.
- Li A, Sun H, Du L, Wu X, Cao J, You Q, Li Y. 2014. Discovery of novel covalent proteasome inhibitors through a combination of pharmacophore screening, covalent docking, and molecular dynamics simulations. J Mol Model. 20:2515–2528.
- Moon SH, Chol HJ, Lee SJ, Chung JU, Kim JH, Chung DH, Park MS, Cho IK, Chol KH. 1998. Novel genipin derivative having anti hepatitis B virus activity. World Intellectual Property Organization Patent No. 17663. 04, 30.
- Moon SH, Choi HJ, Lee SJ, Chung JU, Ha JR, Lee KJ, Oh SW, Jeong KW. 2001. Genipin derivative having liver protection activity. United State Patent No. 6262083. 07, 17.
- National Toxicology Program. 2010. Toxicology and carcinogenesis studies of isoeugenol (CAS No. 97-54-1) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 551:1–178.
- Ozaki A, Kitano M, Furusawa N, Yamaguchi H, Kuroda K. 2002. Genotoxicity of gardenia yellow and its components. Food Chem. Toxicol. 40:1603–1610.
- Phatak RS. 2015. Phytochemistry, pharmacological activities and intellectual property landscape of Gardenia jasminoides Ellis. Pharmacognosy J. 7:254–265.
- Sasieni PD, Winnett A. 2003. Martingale difference residuals as a diagnostic tool for the Cox model. Biometrika. 90:899–912.
- Shanbhag VR, Crider AM. 1992. Ester and amide prodrugs of ibuprofen and naproxen: synthesis, anti-inflammatory activity, and gastrointestinal toxicity. J Pharm Sci. 81:149–154.
- Shi Q, Cao J, Fang L, Zhao H, Liu Z, Ran J, Zheng X, Li X, Zhou Y, Ge D, et al. 2014. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-κB, MAPK and AP-1 signaling pathways in macrophages. Int Immunopharmacol. 20:298–306.
- Song X, Zhang W, Wang T, Jiang H, Zhang Z, Fu Y, Yang Z, Cao Y, Zhang N. 2014. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation. 37:1588–1598.
- Vanegas H, Schaible HG. 2001. Prostaglandins and cyclooxygenases [correction of cyclooxygenases] in the spinal cord. Prog Neurobiol. 64:327–363.
- Wang QS, Xiang YZ, Cui YL, Lin KM, Zhang XF. 2012. Dietary blue pigments derived from genipin, attenuate inflammation by inhibiting LPS-Induced iNOS and COX-2 expression via the NF-κB Inactivation. PLoS ONE. 7:e34122.
- Xu F, Li YH, Li S, Ma Y, Zhao N, Liu Y, Qian N, Zhao H, Li Y. 2014. Complete Freund’s adjuvant-induced acute inflammatory pain could be attenuated by triptolide via inhibiting spinal glia activation in rats. J Surg Res. 188:174–182.
- Yamano T, Tsujimoto Y, Noda T. 1988. Hepatotoxicity of gardenia yellow color in rats. Toxicol Lett. 44:177–182.
- Yamano T, Tsujimoto Y, Noda T, Shimizu M, Ohmori M, Morita S, Yamada A. 1990. Hepatotoxicity of geniposide in rats. Food Chem Toxicol. 28:515–519.
- Zhang HY, Liu H, Yang M, Wei SF. 2013. Antithrombotic activities of aqueous extract from Gardenia jasminoides and its main constituent. Pharm Biol. 51:221–225.
- Zhao M, Du L, Tao J, Qian D, Guo J, Jiang S, Shang E, Duan J, Wu C. 2015. Ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry for rapid analysis of the metabolites of morroniside produced by human intestinal bacteria. Chromatogr B Analyt Technol Biomed Life Sci. 976–977:61–67.
- Zheng YY, Wang DC, Feng HT. 2010. Study on molecular docking of constituents of Sinomenium acutum with cyclooxygenase. Chem Res. 21:43–47.