Abstract
Context: Garcinia mangostana Linn. (Guttiferae) (GM) pericarp has been shown to exhibit good in vitro antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA); however, there is currently no available information regarding its in vivo antibacterial activity.
Objective: To examine in vivo antibacterial activity of G. mangostana extract against MRSA.
Materials and methods: GM pericarp was extracted by ethanol (GM-EtOH) and methanol (GM-MeOH). The crude extracts were examined for in vitro antibacterial activity against MRSA using broth microdilution assay. The in vivo antibacterial activity of 10% GM-EtOH against MRSA was determined in a tape stripping mouse model of superficial skin infection for 9 days by evaluating transepidermal water loss (TEWL) and performing colony counts from cultured swabs.
Results: GM-EtOH showed greater in vitro activity against MRSA than GM-MeOH in broth microdilution assay with minimum inhibitory concentration 17 versus 20 μg/mL and minimum bactericidal concentration 30 versus 35 μg/mL, respectively. The GM-EtOH (13.20 ± 0.49%) contained α-mangostin more than the GM-MeOH (9.83 ± 0.30%). In the tape stripping mouse model, 10% GM-EtOH reduced the number of MRSA colonies (0–1) recovered from infected wounds (>100 colonies) on the first day of treatment, restored TEWL to normal levels on the fourth day, and had completely healed the wounds by day 9.
Conclusion: GM-EtOH showed promising in vivo antibacterial activity against MRSA in a superficial skin infection model in mice. It is of interest to develop a topical formulation of GM-EtOH to further study its potential as a novel antibacterial agent.
Introduction
The fruit of Garcinia mangostana Linn. (Guttiferae) (GM), commonly called mangosteen, is a tropical plant known as the “queen of fruit” due to its pleasant taste and health benefits. The pericarp of mangosteen has been used for a long time in Ayurvedic medicine for the treatment of diarrhea, dysentery and abdominal pain (Balasubramanian & Rajagopalan Citation1988) and it has been reported to possess anti-inflammatory (Gopalakrishnan et al. Citation1980; Nakatani et al. Citation2002; Chen et al, Citation2008), antioxidant (Yoshikawa et al. Citation1994; Moongkarndi et al. Citation2004; Jung et al. Citation2006), anticancer (Ho et al. Citation2002; Matsumoto et al. Citation2003), antimalarial (Riscoe et al. Citation2005; Mahabusarakam et al. Citation2006), antiparasite (Keiser et al. Citation2012), antifungal (Sundaram et al. Citation1983; Gopalakrishnan et al. Citation1997) and antibacterial activities (Iinuma et al. Citation1996; Suksamrarn et al. Citation2003; Chomnawang et al. Citation2005; Rassameemasmaung et al. Citation2007). The pericarp of G. mangostana is a rich source of bioactive xanthones, namely α-, γ- and β-mangostin, garcinone E and gartanin (Gutierrez-Orozco & Failla Citation2013). Both α- and γ-mangostin have been shown to exhibit antibacterial activity against a range of pathogens including Propionibacterium acnes, Staphylococcus aureus, Staphylococcus epidermidis, Mycobacterium tuberculosis and Streptococcus mutans (Mahabusarakam et al. Citation1986; Iinuma et al. Citation1996; Suksamrarn et al. Citation2003; Chomnawang et al. Citation2009; Koh et al. Citation2013; Torrungruang et al. Citation2013).
S. aureus is a Gram positive coccoid bacteria that is a major cause of infections of the integument, soft tissue, bone and cardiovascular system (Lowy Citation1998). Superficial skin infections caused by S. aureus result in abscess wounds and a purulent exudate (Daum Citation2007). The drugs of choice for the treatment of S. aureus infection are β-lactam antibiotics, but there is an increasing incidence of S. aureus strains resistant to β-lactam compounds, so-called methicillin-resistant S. aureus (MRSA) (Haddadin et al. Citation2002). Hence, development of a new effective antibacterial compound against MRSA is required (Iinuma et al. Citation1996; Haddadin et al. Citation2002; Chomnawang et al. Citation2009).
Several in vitro studies have shown activity of G. mangostana pericarp extract against MRSA. Minimum inhibitory concentrations (MICs) have been reported in the range of 0.78–1.25 mg/mL and minimum bactericidal concentrations (MBCs) are 0.78–5 mg/mL (Sutabhaha et al. Citation1997; Voravuthikunchai & Kitpipit Citation2005; Chomnawang et al. Citation2009). To our knowledge, there is currently no available information on the activity of G. mangostana extract against MRSA in vivo. Therefore, the aim of this study was to examine the antibacterial effect of G. mangostana pericarp crude extract against MRSA in a superficial skin infection using the tape stripping mouse model.
Materials and methods
Chemicals and reagents
Folin-Ciocalteu reagent, quercetin, dimethylsulphoxide (DMSO), gallic acid, gentamicin, erythromycin and oxacillin were the products of Sigma-Aldrich Chemical (St. Louis, MO). Aluminium chloride and sodium acetate were supplied by Ajax Finechem Pty Ltd. (Auckland, New Zealand). Mueller Hinton agar (MHA), Mueller Hinton broth (MHB) and mannitol salt agar (MSA) were from Himedia (Mumbai, India). α- and γ-Mangostin were obtained from Chengdu Biopurify Phytochemicals Ltd. (Sichuan, China). Propylene glycol was a product of Srichand United Dispensary Co., Ltd. (Bangkok, Thailand). Other chemicals were obtained from commercial suppliers with high purity and quality.
Preparation of G. mangostana pericarp crude extract
Mangosteens were purchased from a fruit market in Khon Kaen province, Thailand in April 2014. Reference specimen (PANPB-GM 2014-001) was deposited at the Herbarium of the Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand. The fruits were washed and the pericarps were collected and dried in an oven at 50–60 °C prior to grinding. The powdered G. mangostana pericarp was subjected to Soxhlet extraction at 80–90 °C for 3 h using ethanol (GM-EtOH) and methanol (GM-MeOH) as solvents. The extracts were then filtered before rotary evaporation and freezing at −20 °C overnight. The frozen extracts were lyophilized using a freeze-dryer and kept at −20 °C for further study.
Determination of chemical markers in the G. mangostana crude extract α- and γ-mangostin contents in the G. mangostana crude extract
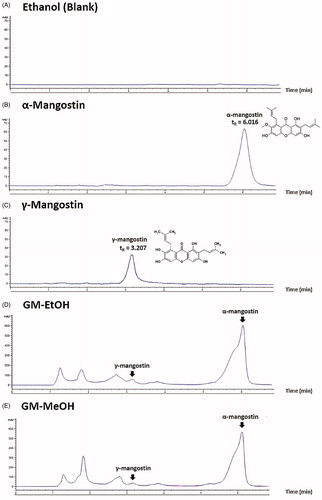
The α- and γ-mangostin contents was determined using high-performance liquid chromatography (HPLC). The G. mangostana extracts were diluted in ethanol and filtered through a 0.22-μm membrane before being subjected to HPLC using a C18 column (Hypersil ODS, 5 μm, 4.0 mm ×250 mm, Agilent Technologies, Santa Clara, CA). The isocratic mobile phase consisted of 70% acetonitrile in distilled water at a flow rate of 1 mL/min. The detection wavelength was set at UV 244 nm. The α- and γ-mangostin contents of the G. mangostana crude extracts were calculated from standard curves of either α- or γ-mangostin standards ().
Figure 1. HPLC chromatogram. (A) Ethanol solvent (blank), (B) α-mangostin standard solution (10 μg/mL) with retention time (tR) 6.016 min, (C) γ-mangostin standard solution (10 μg/mL) with retention time (tR) 3.207 min, (D) GM-EtOH, the ethanolic G. mangostana extract with the peak of α- and γ-mangostin and (E) GM-MeOH, the methanolic G. mangostana extract with the peak of α- and γ-mangostin.
Total phenolic content in the G. mangostana crude extract
The total phenolic content was determined using the Folin-Ciocalteu method (Chatuphonprasert & Jarukamjorn Citation2012). Briefly, a mixture of the G. mangostana extract with Folin-Ciocalteu reagent was spectrophotometrically measured at a wavelength of 700 nm. Total phenolic content was calculated as the weight unit equivalent to the dry weight of the gallic acid standard.
Total flavonoid content in the G. mangostana crude extract
Determination of the total flavonoid content was performed using the aluminium chloride colorimetric method (Chatuphonprasert & Jarukamjorn Citation2012) with some modifications. The G. mangostana extract was mixed with a reaction mixture of aluminium chloride–sodium acetate–distilled water (10:10:160) and incubated at room temperature for 30 min before spectrophotometry at a wavelength of 405 nm. Total flavonoid content was determined as the weight unit equivalent to the dry weight of the quercetin standard.
Anthocyanin pigment content in the G. mangostana crude extract
The anthocyanin pigment content was determined using the pH differential method of Wrolstad (Citation1993). The G. mangostana extract was adjusted to pH 1.0 and another to pH 4.5. The proportion of the G. mangostana extract absorbance at different wavelengths (500–540 nm) was calculated to the anthocyanin content using cyanidin-3-glucoside as the standard.
Percent contribution of tannin in the G. mangostana crude extract
The principle of polymeric tannin pigment resistance to bisulphite bleaching (Wrolstad Citation1993) was employed to analyse the tannin content in the G. mangostana extract. First, the maximal absorbance of G. mangostana extract at 500–540 nm was used to calculate total colour density. Bisulphate solution was then added to the sample and the maximal absorbance at 500–540 nm was used to calculate the polymeric colour fraction.
In vitro antibacterial activity of the ethanolic G. mangostana extract and α-mangostin
Preparation of bacterial suspension
The microorganisms employed in this study were methicillin-sensitive S. aureus (MSSA) ATCC 23235 and MRSA DMST 20651 (kind gift from Dr. Suttiwan Thammawat, Mahasarakham University, Thailand). To prepare inocula, bacteria were incubated on MHA plates at 37 °C for 12–18 h before colonies were selected and resuspended in 0.9% normal saline solution (NSS) to a concentration of 1 × 106 CFU/mL.
Agar well diffusion assay
Four hundred microlitres of a 1 × 106 CFU/mL bacterial inoculum (MSSA or MRSA) in NSS was evenly spread on a MHA plate before 6 mm wells were made in the agar using a Kochborer drill (No. 3). Fifty microlitre aliquots of the ethanolic G. mangostana extract (GM-EtOH, 5 mg/well), α-mangostin (0.66 mg/well, equivalent to α-mangostin content in the GM-EtOH), erythromycin (10 μg/well), gentamicin (15 μg/well) and control (DMSO) were loaded into the wells and the plates were incubated at 37 °C for 24 h. The diameters of the inhibition zones (mm) were measured and are presented as the average from four replicates.
Determination of MIC and MBC
MIC was determined by the broth microdilution method. Bacterial inoculum (2 mL) (1 × 106 CFU/mL) was mixed with 8 mL of MHB and 100-μL aliquots were pipetted into 96-well microplates. Then, 100-μL aliquots of the G. mangostana extracts, α-mangostin, erythromycin or gentamicin in MHB were added and serially diluted two-fold across the microplate. The microplates were incubated at 37 °C for 24 h and bacterial growth was measured as absorbance at 625 nm in a plate reader. The lowest concentration of the G. mangostana extract to inhibit the growth of the target organism was the MIC value. To examine MBC, all wells from the MIC value to higher concentrations were sampled onto MHA plates using a simple streak technique, and incubated at 37 °C for 24 h. The lowest concentration of the extract with no colonies of the target organism was the MBC value.
In vivo antibacterial effect of the ethanolic G. mangostana extract and α-mangostin
Preparation of bacterial suspension
MRSA DMST 20651 was incubated on MHA plates at 37 °C for 12–18 h. Colonies were directly suspended in 0.9% NSS to a concentration of 1 × 108 CFU/mL.
Preparation of antibacterial agents
The commercial topical antibiotics were purchased from a local drugstore namely 0.1% gentamicin cream (Skinfect®, Bangkok Lab and Cosmetic, Bangkok, Thailand) and 4% erythromycin gel (Erazit™, Zyg Pharma Pvt., Pithampur, India). Ten percent GM-EtOH, 1.32% α-mangostin (equivalent to α-mangostin in the 10% GM-EtOH) and 1.32% erythromycin were prepared in a 10% ethanol in propylene glycol.
The tape stripping mouse model
Adult male ICR mice (7 weeks old) were obtained from the National Laboratory Animal Center, Mahidol University, Nakhon Pathom, Thailand. All animals were housed in polysulphone cages with wood chip bedding in the Northeast Laboratory Animal Center, Khon Kaen University, Khon Kaen, Thailand, under the supervision of a certified laboratory veterinarian. Commercial regular diet and water were supplied ad libitum. The research protocol was approved by the Animal Ethics Committee of the Northeast Laboratory Animal Center (AEKKU-NELAC001/2558).
The mice were anaesthetized by intraperitoneal injection of pentobarbital sodium (Nembutal®, Libourne, France) at a dose of approximately 50–80 μg/kg and the back was shaved with a sterile blade. Small pieces of elastic bandage tape were applied to and stripped from a 2 × 2 cm2 dorsal area 10–20 times, until the skin glistened and reddened with no bleeding. The degree of skin damage was standardized to a transepidermal water loss (TEWL, DermaLab®Combo, Hadsund, Denmark) of 70–75 g/m2 h before 10 μL MRSA (1 × 108 CFU/mL) was applied. A one-hundred microlitre aliquot of each topical agent, 10% GM-EtOH in a 10% ethanol in propylene glycol, 10% ethanol in propylene glycol (Base), 4% commercial erythromycin gel and 0.1% commercial gentamicin cream, was applied to the wound surface (n = 10) at 24 h intervals. The control (no MRSA) and the no treatment control (MRSA infected) groups were simply left untreated. The TEWL was measured every other day for 9 days and the wounds were swabbed daily for culture on MSA plates and determination of the number of S. aureus colonies.
Moreover, in vivo antibacterial activity of α-mangostin was further examined. The tape stripping model was performed as previous examination. A one hundred microlitre aliquot of each topical preparation, 10% GM-EtOH, 1.32% α-mangostin (Alpha-MGS) or 1.32% erythromycin in 10% ethanol in propylene glycol, was applied to the wound surface everyday (n = 10) at 24 h intervals. The TEWL was measured every other day for 9 days (three replicates) and the wounds were swabbed daily for culture on oxacillin-contained mannitol salt agar plates (Oxa-MSA, oxacillin 6 μg/mL MSA) (Isenberg Citation1998) and determination of the number of MRSA colonies.
Statistics
The results were analysed by one-way analysis of variance (ANOVA) followed by LSD post hoc test (SPSS ver. 17.0, Chicago, IL). p ≤ 0.05 was considered statistically significant.
Results
Contents of chemical markers in the G. mangostana crude extract
There were only small differences in the phenolic and flavonoid content of the extracts (). Total phenolic content (as gallic acid equivalents) was 62.88 ± 3.68 mg/g for GM-EtOH and 69.42 ± 5.10 mg/g for GM-MeOH and total flavonoid content (as quercetin equivalents) was 36.95 ± 0.03 mg/g for GM-EtOH and 33.18 ± 3.83 mg/g for GM-MeOH. The GM-EtOH extract had a higher anthocyanin content (as cyaniding-3-glucoside equivalents) than GM-MeOH (98.20 ± 22.04 versus 11.70 ± 0.69 mg/g) but the percentages of tannin contribution were similar (64.56 ± 1.08 versus 71.49 ± 1.57%, respectively).
Table 1. Contents of chemical markers in the G. mangostana crude extract.
The G. mangostana extracts were examined for α- and γ-mangostin contents using HPLC (). The quantitative analysis was validated with a linearity range of 1–100 μg/mL for α-mangostin (R2 = 0.9999) and 10–100 μg/mL for γ-mangostin (R2 = 0.9997). The coefficients of variation within the linearity range of α-mangostin were 1.07 ± 0.38% (within-day) and 1.51 ± 0.50% (between-day), and γ-mangostin were 1.20 ± 0.48% (within-day) and 0.75 ± 0.13% (between-day). The accuracy of α- and γ-mangostin was 100.42 ± 0.23% and 97.74 ± 0.63%, respectively. The retention time of α-mangostin was 6.016 min () and γ-mangostin was 3.207 min (). The GM-EtOH extract had a higher α-mangostin content than the GM-MeOH extract (13.20 ± 0.49 versus 9.83 ± 0.30% dry weight, respectively), whereas the γ-mangostin content was similar (2.34 ± 1.01 and 2.96 ± 0.28% dry weight, respectively; ). The 10% GM-EtOH in a 10% ethanol in propylene glycol preparation that was used for the in vivo experiments was also analysed for its α- and γ-mangostin contents and the % recoveries were 92.17 ± 6.30 and 92.39 ± 8.04%, respectively.
In vitro antibacterial activity of G. mangostana crude extract and α-mangostin against MSSA and MRSA
In the agar diffusion assay, the in vitro antibacterial activity of the GM-EtOH and GM-MeOH extracts was examined in comparison with two standard antibiotics, gentamicin and erythromycin, at the standard doses for antibiotic susceptibility testing, and its active constituent, α-mangostin. The GM-EtOH (5 mg), GM-MeOH (5 mg) and α-mangostin (0.66 mg, the equivalent content to the GM-EtOH) produced inhibition zones against both MSSA (12.75 ± 1.04, 13.25 ± 0.65 and 11.17 ± 0.29 mm, respectively) and MRSA (13.17 ± 1.15, 12.50 ± 0.00 and 10.00 ± 0.00 mm, respectively; ), while gentamicin (10 μg) and erythromycin (15 μg) did inhibition zones against MSSA only (16.50 ± 2.29 and 22.83 ± 0.29, respectively; ). Neither gentamicin nor erythromycin produced inhibition zone against MRSA. For the broth microdilution assay, the GM-EtOH produced lower MIC values compared to GM-MeOH against both MSSA (14 and 17 μg/mL, respectively) and MRSA (17 and 20 μg/mL, respectively). MBC values were 25 μg/mL for both extracts against MSSA but the GM-EtOH extract produced a lower MBC against MRSA compared to GM-MeOH (30 versus 35 μg/mL; ). MIC values of α-mangostin were 6.25 μg/mL against both MSSA and MRSA and better than either GM-EtOH or GM-MeOH extracts, while those of MBC against MSSA and MRSA (100 μg/mL) were weaker than both extracts. These findings suggested to select the GM-EtOH for the in vivo wound healing study and its antibacterial activity based on its higher α-mangostin content () and superior in vitro antibacterial activity against MRSA ().
Table 2. Inhibition zone, MIC and MBC values of the G. mangostana crude extract against MSSA and MRSA.
In vivo wound healing and antibacterial activity of the ethanolic G. mangostana extract and α-mangostin in the tape stripping mouse model
Wound healing
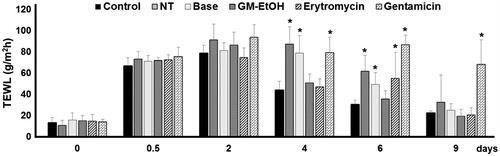
We used TEWL as an indicator of skin barrier function in the tape stripping mouse model of superficial skin infection. The TEWL values were determined before initiation of the wound (day 0), after initiation of the wound (day 0.5), and on days 2, 4, 6 and 9 of treatment (). Baseline TEWL was approximately 10–20 g/m2 h after shaving for all groups. After tape stripping for 10–20 times (day 0.5), skin damage was observed and TEWL ranged from 70 to 90 g/m2 h. There was no difference in TEWL values 2 days after treatment for any groups. On day 4, the control (no MRSA) group showed a marked reduction in TEWL, indicating wound healing as did the GM-EtOH extract and erythromycin topical treated MRSA-infected groups. In contrast, the no treatment control, base and gentamicin treatment groups had significantly higher TEWL values on day 4 compared to the no MRSA control. On day 6, the no MRSA control, no treatment, base and GM-EtOH treatment groups all showed a reduction in TEWL from day 4, but the GM-EtOH group was the only treatment to not have a significantly higher TEWL than the no MRSA control. On day 9, only the gentamicin group showed significantly higher TEWL compared to the no MRSA control. Despite TEWL levels returning to baseline on day 9 for most treatment groups, the MRSA-infected mice with high TEWL levels for 6 days exhibited a worsening of the wound-appearance with purulent lesions noted on the last day of observation (day 9). The gentamicin-treated MRSA-infected mice showed the highest TEWL values (63–117 g/m2 h) for the duration of the experiment and these were associated with incurable purulent wounds throughout the period of observation.
Figure 2. Effects of GM-EtOH on TEWL in the tape stripping model in mice. TEWL was measured on the back of the mice as described in the method (n = 9–10). Control, non-infected mice with the tape stripping induced wound; NT, MRSA-infected wound in mice with no treatment; Base, MRSA-infected wound in mice treated with 100 μL of a 10% ethanol in propylene glycol solution; GM-EtOH, MRSA-infected wound in mice treated with 100 μL of a 10% GM-EtOH in a 10% ethanol in propylene glycol solution; Erythromycin, MRSA-infected wound in mice treated with 100 μL of a 4% commercial erythromycin gel; Gentamicin, MRSA-infected wound in mice treated with 100 μL of a 0.1% commercial gentamicin cream. *p < 0.001 versus control on the same day using one-way ANOVA followed by LSD post hoc test.
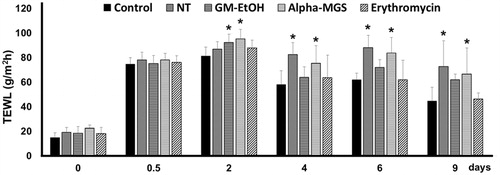
To examine the wound healing property of α-mangostin, the tape stripping mouse model of superficial skin infection was carried out. TEWL was measured to indicate skin barrier function of the wounds before initiation of the wound (day 0), after initiation of the wound (day 0.5), and on days 2, 4, 6 and 9 of treatment (). After shaving, TEWL was in a range of 15–20 g/m2 h for all groups, and it was increased to 75–80 g/m2 h after tape stripping. On day 4, TEWL values of the no treatment (NT) and the α-mangostin topical treated MRSA-infected groups were significantly higher than the no MRSA control, and the TEWL levels of these two groups were persistently high till day 9. Consistent to the previous independent experiment, TEWL values of the GM-EtOH and the erythromycin topical treated MRSA-infected groups were returned to the levels similar to that of the control (no MRSA) group on day 4.
Figure 3. Effects of GM-EtOH and α-mangostin on TEWL in the tape stripping model in mice. TEWL was measured on the back of the mice as described in the method (n = 7–10). Control, non-infected mice with the tape stripping induced wound; NT, MRSA-infected wound in mice with no treatment; GM-EtOH, MRSA-infected wound in mice treated with 100 μL of a 10% GM-EtOH in a 10% ethanol in propylene glycol solution; Alpha-MGS, MRSA-infected wound in mice treated with 100 μL of a 1.32% α-mangostin in a 10% ethanol in propylene glycol solution; Erythromycin, MRSA-infected wound in mice treated with 100 μL of a 1.32% erythromycin in a 10% ethanol in propylene glycol solution. *p < 0.001 versus control on the same day using one-way ANOVA followed by LSD post hoc test.
Antibacterial activity
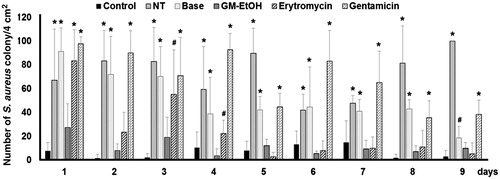
To determine the antibacterial effect of the treatments, wounds were swabbed daily and colony counts were performed on the MSA plates. Only yellow pigmented colonies representing S. aureus were counted. The no treatment (NT) group had high number of S. aureus colonies from the wounds on the starting day (day 1) and persistence along the period of study (9 days, ). The treatment of GM-EtOH ultimately reduced the number of S. aureus colonies to the level comparable to those of the control (without MRSA infection) group since the first to the last day of application. The erythromycin treated MRSA-infected group showed the constant reduction in the number of S. aureus colonies at the fifth day. The base (10% ethanol in propylene glycol) and the gentamicin treated MRSA-infected group did not exhibit any efficient reduction in number of S. aureus colonies throughout the course of the study compared with the NT and control groups, respectively.
Figure 4. The effect of GM-EtOH on** the number of S. aureus colonies. MRSA was swabbed from the wound of the mouse back to culture on the MSA plate and number of colonies were counted at 24 h after the incubation (n = 9-10). Control, non-infected mice with the tape stripping induced wound; NT, MRSA-infected wound in mice with no treatment; Base, MRSA-infected wound in mice treated with 100 μL of a 10% ethanol in propylene glycol solution; GM-EtOH, MRSA-infected wound in mice treated with 100 μL of a 10% GM-EtOH in a 10% ethanol in propylene glycol solution; Erythromycin, MRSA-infected wound in mice treated with 100 μL of a 4% commercial erythromycin gel; Gentamicin, MRSA-infected wound in mice treated with 100 μL of a 0.1% commercial gentamicin cream. *p < 0.001 versus control and #p < 0.001 versus GM-EtOH on the same day using one-way ANOVA followed by LSD post hoc test.
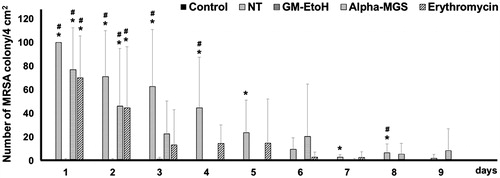
To quantify the exact number of MRSA colonies that were present on the wound after the treatments, a more specific selective media, oxacillin-contained mannitol salt agar (Oxa-MSA), which might restrict the growth of MSSA, was further considered to examine antibacterial activity of GM-EtOH and α-mangostin. The wounds were swabbed daily and cultured on Oxa-MSA plates where only MRSA were grown, and the number of MRSA colonies was counted. The treatment of GM-EtOH consistently reduced the number of MRSA colonies to the level comparable to those of the control (without MRSA infection) group since the first to the last day of application (). The no treatment (NT) group showed high number of MRSA colonies from the wounds on the starting of the application and significantly differed from the control (without MRSA infection) group for 5 days, while the α-mangostin and the erythromycin treated MRSA-infected group showed the constant reduction in the number of MRSA colonies to the comparable level as the control (without MRSA infection) group at the third day.
Figure 5. The effect of GM-EtOH and α-mangostin in the number of MRSA colonies. MRSA was swabbed from the wound of the mouse back and diluted to 2 mL of NSS before culturing on the MSA containing oxacillin (6 μg/mL) plate. The number of colonies was counted at 24 h after the incubation (n = 7–10). Control, non-infected mice with the tape stripping induced wound; NT, MRSA-infected wound in mice with no treatment; GM-EtOH, MRSA-infected wound in mice treated with 100 μL of a 10% GM-EtOH in a 10% ethanol in propylene glycol solution; Alpha-MGS, MRSA-infected wound in mice treated with 100 μL of a 1.32% α-mangostin in a 10% ethanol in propylene glycol solution; Erythromycin, MRSA-infected wound in mice treated with 100 μL of a 1.32% erythromycin in a 10% ethanol in propylene glycol solution. *p < 0.05 versus control and #p < 0.05 versus GM-EtOH on the same day using one-way ANOVA followed by LSD post hoc test.
Photographs of the mouse skin wounds on the last day (day 9) of observation are shown in and . The non-infected wound (control) was completely healed within the period of observation (). The MRSA-infected skin without treatment (NT) or treated with the base only resulted in purulent wounds on day 9 (), which was consistent with the delayed return of TEWL values to baseline () and the high number of colonies from their swabs ().
Figure 6. The effect of GM-EtOH on the wound-appearance. The photograph of the wound was recorded daily (n = 9–10) after the treatments. The figure showed the wound-appearance on the last day of observation (the ninth day). Control, non-infected mice with the tape stripping induced wound; NT, MRSA-infected wound in mice with no treatment; Base, MRSA-infected wound in mice treated with 100 μL of a 10% ethanol in propylene glycol solution; GM-EtOH, MRSA-infected wound in mice treated with 100 μL of a 10% GM-EtOH in a 10% ethanol in propylene glycol solution; Erythromycin, MRSA-infected wound in mice treated with 100 μL of a 4% commercial erythromycin gel; Gentamicin, MRSA-infected wound in mice treated with 100 μL of a 0.1% commercial gentamicin cream.
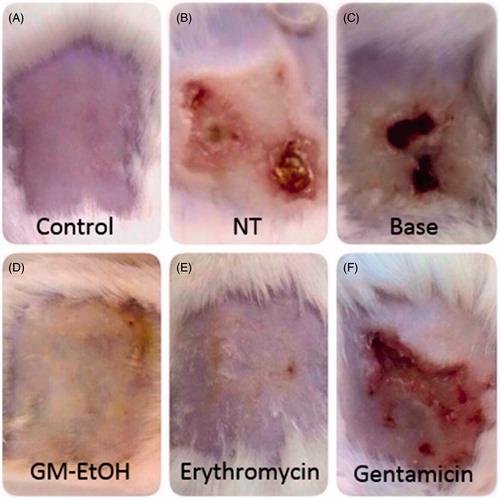
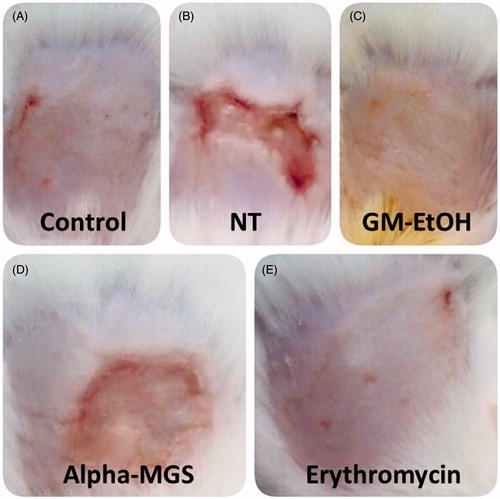
Figure 7. The effect of GM-EtOH and α-mangostin on the wound-appearance. The photograph of the wound was recorded daily (n = 7–10) after the treatments. The figure showed the wound-appearance on the last day of observation (the ninth day). Control, non-infected mice with the tape stripping induced wound; NT, MRSA-infected wound in mice with no treatment; GM-EtOH, MRSA-infected wound in mice treated with 100 μL of a 10% GM-EtOH in a 10% ethanol in propylene glycol solution; Alpha-MGS, MRSA-infected wound in mice treated with 100 μL of a 1.32% α-mangostin in a 10% ethanol in propylene glycol solution; Erythromycin, MRSA-infected wound in mice treated with 100 μL of a 1.32% erythromycin in a 10% ethanol in propylene glycol solution.
The treatments of MRSA-infected mice with GM-EtOH () or erythromycin () resulted in completely healed wounds on day 9. Treatment with gentamicin did not appear to improve the condition of the wound on MRSA-infected mice () with the wounds remaining purulent with corresponding high TEWL values (75.67 ± 13.14 g/m2 h in day 9, ) and large number of bacterial colonies from the swab ().
The mouse skin wounds on the last day (day 9) of observation after the treatments of GM-EtOH and α-mangostin were further compared (). The non-infected wound (control) was completely healed within the period of observation () as the previous independent examinations. Likewise, the treatments of MRSA-infected mice with GM-EtOH () or erythromycin () resulted in completely healed wounds on day 9. The MRSA-infected skin without treatment (NT) resulted in purulent wounds on day 9 (), which was consistent with the delayed return of TEWL values to baseline () and the high number of MRSA colonies from their swabs (). The treatment with α-mangostin improved condition of the wounds on MRSA-infected mice (), however, the wounds were not completely healed with remaining mild purulent on the last day of observation (day 9) and corresponding high TEWL values (66.67 ± 21.08 g/m2 h in day 9, ).
Discussion
Despite a major cause of nosocomial infection, endocarditis, osteomyelitis, pyoarthrosis, Staphylococcal scalded skin syndrome (Brewer et al. Citation2008) and toxic shock syndrome (Iwatsuki et al. Citation2006), S. aureus is a common cause of superficial skin infections (Chiller et al. Citation2001). The increasing incidence of multidrug-resistant S. aureus strains (Lowy Citation1998) is resulting in the treatment of S. aureus infection becoming more difficult and complicated (Price et al. Citation1998; Cohen & Kurzrock Citation2004; Iyer & Jones Citation2004). The resistance to methicillin was related to mecA gene encoded penicillin-binding protein 2a (PBP2a), which had low binding affinity for β-lactams antibiotics (Henze & Berger-Bächi Citation1995; Chambers Citation1997). Thus, there is a great need for the development of new compounds that are active against antibiotic-resistant strains, in particular, MRSA. Previous reports have indicated that a compound isolated from mangosteen pericarb, α-mangostin, has potent antibacterial activity against MRSA, however, to our knowledge there has been no in vivo study of its effectiveness.
In the present study, α-mangostin in the GM-EtOH was 13.20 ± 0.49% dry weight. Corresponding with the study of Pothitirat, Chomnawang, Supabphol, et al. (Citation2009) that reported the content of α-mangostin at 13.63 ± 0.06% dry weight in the ethanolic extract of mature fruit, whereas that contained in the young fruit extract was 8.07 ± 0.11% dry weight. The content of α-mangostin in the G. mangostana extract varied by several factors, such as stage of maturity, cultivated area, harvesting period and extraction method. G. mangostana from South Thailand had little higher α-mangostin than those of the Eastern (17.64 ± 3.89 versus 16.43 ± 3.69% w/w in the extract and 4.85 ± 0.83 versus 4.35 ± 0.60% w/w in the dried powder, respectively) (Pothitirat, Chomnawang and Gritsanapan Citation2009).
Voravuthikunchai and Kitpipit (Citation2005) reported that the MIC of ethanolic G. mangostana extract against MRSA (PSU 0201-PSU 0235) was 0.05–0.4 mg/mL with the MBC of 0.1–0.4 mg/mL. We presently found the lower MIC and MBC values of the GM-EtOH against MRSA (DMST 20651) of 17 and 30 μg/mL, respectively. In addition, MIC and MBC values against several strains of MRSA were reported. Sutabhaha et al. (Citation1997) demonstrated MIC and MBC of petroleum ether G. mangostana extract against S. aureus ATCC 25923 of 3.12 and 6.25 μg/mL, respectively, while both values against MRSA (a local strain of Thailand) were of 0.78 μg/mL. Chomnawang et al. (Citation2009) reported antibacterial screening of ethanolic G. mangostana extract against 16 clinical MRSA strains but only the susceptibility values against MRSA T1301 were considered effective with MIC of 0.039 mg/mL and MBC of 0.312 mg/mL. The antibacterial effect of G. mangostana varied upon several factors, i.e., bacterial strain, stage of maturity, cultivated area, and harvesting period including solvent, and method of extraction.
Previous studies have reported that α-mangostin possessed antibacterial activity (Mahabusarakam et al. Citation1986; Suksamrarn et al. Citation2003). In our hands, the GM-EtOH and GM-MeOH extracts showed higher MIC values against MSSA and MRSA compared to the equivalent dose of pure α-mangostin (); however, MBC values against MSSA and MRSA of either GM-EtOH or GM-MeOH were lower than those of α-mangostin. Furthermore, the ranges of MIC and MBC of α-mangostin against either MSSA or MRSA were markedly different than those values of the extracts. These observations possibly referred to bacteriostatic property of α-mangostin. Hence, to get to the bactericidal level, α-mangostin should be applied to the wound more than once a day. In addition, the in vitro and the in vivo findings on antibacterial activity were inconsistence. The GM-EtOH effectively reduced the number of MRSA colonies to the level comparable to those of the control (without MRSA infection) group since the first day of application () and resulted in completely healed wounds on the last day of observation (day 9), while the α-mangostin showed the constant reduction in the number of MRSA colonies to the comparable level as the control group at the third day and the wounds with remaining mild purulent on the last day of observation (). These observations might be explained by the existence of other constituents in the GM-EtOH extract that additionally possess antibacterial properties, such as γ- and β-mangostin (Suksamrarn et al. Citation2003; Koh et al. Citation2013). These might additionally underline that only in vitro examination is insufficient to assure in vivo antibacterial activity.
Gentamicin and erythromycin, two antibacterial agents that act by inhibiting protein synthesis in the bacteria, were selected as the comparison drugs for this study as they are commonly found in topical antibacterial preparations and can be purchased easily at a relatively low price from drugstores. Although some strains of MRSA have been reported to be susceptible to gentamicin (Riley et al. Citation1995; Turnidge & Bell Citation1999; Nimmo et al. Citation2000), in microdilution method, as expected, gentamicin and erythromycin were less susceptible ()
The tape stripping mouse model represents an abrasion-wound similar to that possibly occurring in daily life (Dai et al. Citation2011). This model is performed easily with an uncomplicated procedure and is suitable for the superficial S. aureus skin infection (Kugelberg et al. Citation2005). The infection is established by using the tape stripping technique to disrupt the epidermal layer until a TEWL of 70–75 g/m2 h is achieved and inoculating with bacteria at 1 × 108 CFU/mL (Kugelberg et al. Citation2005). The wounds and the number of recovered MRSA from MRSA infected-mice with no treatment (NT) were persistent until the last day of the study (). The base treatment slightly decreased the number of colonies, but the wound was not better improved (). These observations supported that the base did not exert antibacterial effect. The wound of gentamicin-treated group appeared worsely with deficient healing (). These observations partly resulted from inappropriate formulation type of the gentamicin cream for the treatment of this superficial MRSA infection.
For culturing S. aureus from the wound, MSA was employed due to its selective characteristic media to S. aureus (Flournoy et al. Citation1990; Smyth & Kahlmeter Citation2005). Only S. aureus grows on the MSA with mannitol fermentation and produces yellow zone surrounding the colony to facilitate for S. aureus colony-counting (Kateete et al. Citation2010). Furthermore, oxacillin (6 μg/mL) was added into MSA (Oxa-MSA) to restrict the growth of MSSA to quantify the exact number of MRSA colonies on the wound (Isenberg Citation1998).
The 10% ethanol solution was additionally mixed with GM-EtOH to increase solubility of the extract before further mixing with propylene glycol. Propylene glycol was applied as a co-solvent for GM-EtOH because it was expected to be a componential agent in a topical formulation of GM-EtOH for further development.
Erythromycin is commonly ineffective against MRSA, but it might be active in some strains of MRSA (Riley et al. Citation1995). Although erythromycin shows less inhibitory property against MRSA in the in vitro study of the present study (), corresponding to the previous study (Rayner & Munckhof Citation2005), manifestation of the wound after the treatment of erythromycin was almost completely healed ( and ), comparable to the control ( and ). Erythromycin decreased the TEWL values as GM-EtOH did ( and ), and showed the satisfied wound-healing effect for the MRSA superficial infection by the tape stripping in mice, albeit its effect in the number of MRSA colonies was weaker than GM-EtOH ( and ). These controversial outcomes of erythromycin between in vitro and in vivo might result from its low water solubility property to compatible with the water phase of MHA to show the antibacterial efficiency. On the other hand, the commercial erythromycin product tested in the present study was present as a gel which is suitable for the treatment of an abrasion wound. Moreover, erythromycin might diffuse through the bacterial membrane due to the co-solvent effect in this commercial preparation.
The GM-EtOH exhibited the strongest antibacterial activity for the superficial MRSA infection in mice. The appearance of the wound was completely healed ( and ), comparable to the control ( and ). Moreover, the GM-EtOH properly decreased the number of colonies in the wound ( and ).
This is the first time to simultaneously report the efficiency of GM-EtOH against MRSA in the in vitro agar well diffusion followed by broth microdilution assay and revealed the antibacterial activity of G. mangostana against the superficial MRSA infection in the tape stripping mouse model representing the abrasion skin infection. Therefore, it is worthwhile for further study on efficacy of G. mangostana in other skin infection models and its molecular mechanism against MRSA including the antibacterial activity against other bacterial strains. These findings suggested G. mangostana as a promising antibacterial candidate for the superficial MRSA infection.
Conclusions
The present study ultimately assured superior antibacterial activity of GM-EtOH against MRSA in the in vitro agar well diffusion and microdilution examinations, and in the superficial MRSA infection in mice than a commercial antibiotic erythromycin. Therefore, GM-EtOH is a promising antibacterial candidate to be of interest to further develop as an alternative topical formulation against MRSA.
Funding information
Nitima Tatiya-aphiradee sincerely thanks the Graduate School of Khon Kaen University, Thailand, for a scholarship (571H117)
Acknowledgments
Dr. Suttiwan Thammawat, Mahasarakham University, Thailand, for kindly provided MSSA ATCC 23235 and MRSA DMST 20651 cultures. Dr. Glenn Borlace is kindly acknowledged for English editing and critical evaluation of the manuscript preparation.
Disclosure statement
The authors have no conflict of interest.
References
- Balasubramanian K, Rajagopalan K. 1988. Novel xanthones from Garcinia mangostana, structures of BR-xanthone-A and BR-xanthone-B. Phytochemistry. 27:1552–1554.
- Brewer JD, Hundley MD, Meves A, Hargreaves J, Hargreaves MT, Pittelkow MR, et al. 2008. Staphylococcal scalded skin syndrome and toxic shock syndrome after tooth extraction. J Am Acad Dermatol. 59:342–346.
- Chambers HF. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 10:781–791.
- Chatuphonprasert W, Jarukamjorn K. 2012. Impact of six fruits – banana, guava, mangosteen, pineapple, ripe mango and ripe papaya – on murine hepatic cytochrome P450 activities. J Appl Toxicol. 32:994–1001.
- Chen LG, Yang LL, Wang CC. 2008. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 46:688–693.
- Chiller K, Selkin BA, Murakawa GJ. 2001. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc. 6:170–174.
- Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. 2005. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J Ethnopharmacol. 101:330–333.
- Chomnawang MT, Surassmo S, Wongsariya K, Bunyapraphatsara N. 2009. Antibacterial activity of Thai medicinal plants against methicillin-resistant Staphylococcus aureus. Fitoterapia. 80:102–104.
- Cohen PR, Kurzrock R. 2004. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: an emerging clinical problem. J Am Acad Dermatol. 50:277–280.
- Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR, et al. 2011. Animal models of external traumatic wound infections. Virulence. 2:296–315.
- Daum RS. 2007. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 357:380–390.
- Flournoy DJ, Wongpradit S, Silberg SL. 1990. Screening media for detection of methicillin-resistant Staphylococcus aureus from non-sterile body sites. Med Microbiol Immunol. 179:25–30.
- Gopalakrishnan C, Shankaranarayanan D, Kameswaran L, Nazimudeen SK. 1980. Effect of mangostin, a xanthone from Garcinia mangostana Linn. in immunopathological & inflammatory reactions. Indian J Exp Biol. 18:843–846.
- Gopalakrishnan G, Banumathi B, Suresh G. 1997. Evaluation of the antifungal activity of natural xanthones from Garcinia mangostana and their synthetic derivatives. J Nat Prod. 60:519–524.
- Gutierrez-Orozco F, Failla ML. 2013. Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients. 5:3163–3183.
- Haddadin AS, Fappiano SA, Lipsett PA. 2002. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad Med J. 78:385–392.
- Henze UU, Berger-Bächi B. 1995. Staphylococcus aureus penicillin-binding protein 4 and intrinsic beta-lactam resistance. Antimicrob Agents Chemother. 39:2415–2422.
- Ho CK, Huang YL, Chen CC. 2002. Garcinone E, a xanthone derivative, has potent cytotoxic effect against hepatocellular carcinoma cell lines. Planta Med. 68:975–979.
- Iinuma M, Tosa H, Tanaka T, Asai F, Kobayashl Y, Shimano R, Miyauchi KI. 1996. Antibacterial activity of xanthones from guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol. 48:861–865.
- Isenberg HD. 1998. Essential procedures for clinical microbiology. Washington, DC: ASM Press.
- Iwatsuki K, Yamasaki O, Morizane S, Oono T. 2006. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 42:203–214.
- Iyer S, Jones DH. 2004. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 50:854–858.
- Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. 2006. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 54:2077–2082.
- Kateete DP, Kimani CN, Katabazi FA, Okeng A, Okee MS, Nanteza A, Joloba ML, Najjuka FC. 2010. Identification of Staphylococcus aureus: DNase and mannitol salt agar improve the efficiency of the tube coagulase test. Ann Clin Microbiol Antimicrob. 9:23.
- Keiser J, Vargas M, Winter R. 2012. Anthelminthic properties of mangostin and mangostin diacetate. Parasitol Int. 61:369–371.
- Koh JJ, Qiu S, Zou H, Lakshminarayanan R, Li J, Zhou X, Tang C, Saraswathi P, Verma C, Tan DT, et al. 2013. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Biophys Acta Biomembr. 1828:834–844.
- Kugelberg E, Norström T, Petersen TK, Duvold T, Andersson DI, Hughes D, et al. 2005. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob Agents Chemother. 49:3435–3441.
- Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med. 339:520–532.
- Mahabusarakam W, Kuaha K, Wilairat P, Taylor WC. 2006. Prenylated xanthones as potential antiplasmodial substances. Planta Med. 72:912–916.
- Mahabusarakam W, Wiriyachitra P, Phongpichit S. 1986. Antimicrobial activities of chemical constituents from Garcinia mangostana Linn. J Sci Soc Thailand. 12:239–242.
- Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y. 2003. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod. 66:1124–1127.
- Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N, et al. 2004. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol. 90:161–166.
- Nakatani K, Nakahata N, Arakawa T, Yasuda H, Ohizumi Y. 2002. Inhibition of cyclooxygenase and prostaglandin E2 synthesis by gamma-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem Pharmacol. 63:73–9.
- Nimmo GR, Schooneveldt J, O'Kane G, McCall B, Vickery A. 2000. Community acquisition of gentamicin-sensitive methicillin-resistant Staphylococcus aureus in southeast Queensland, Australia. J Clin Microbiol. 38:3926–31.
- Pothitirat W, Chomnawang MT, Gritsanapan W. 2009. Anti-acne inducing bacteria activity and α-mangostin content of Garcinia mangostana fruit rind extracts from different provenience. Songklanakarin J Sci Technol. 31:41–47.
- Pothitirat W, Chomnawang MT, Supabphol R, Gritsanapan W. 2009. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia. 80:442–447.
- Price MF, McBride ME, Wolf JE Jr. 1998. Prevalence of methicillin-resistant Staphylococcus aureus in a dermatology outpatient population. South Med J. 91:369–371.
- Rassameemasmaung S, Sirikulsathean A, Amornchat C, Hirunrat K, Rojanapanthu P, Gritsanapan W. 2007. Effects of herbal mouthwash containing the pericarp extract of Garcinia mangostana L. on halitosis, plaque and papillary bleeding index. J Int Acad Periodontol. 9:19–25.
- Rayner C, Munckhof WJ. 2005. Antibiotics currently used in the treatment of infections caused by Staphylococcus aureus. Intern Med J. 35:S3–S16.
- Riley TV, Pearman JW, Rouse IL. 1995. Changing epidemiology of methicillin-resistant Staphylococcus aureus in Western Australia. Med J Aust. 163:412–414.
- Riscoe M, Kelly JX, Winter R. 2005. Xanthones as antimalarial agents: discovery, mode of action, and optimization. Curr Med Chem. 12:2539–2549.
- Smyth RW, Kahlmeter G. 2005. Mannitol salt agar-cefoxitin combination as a screening medium for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 43:3797–3799.
- Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, Suksamrarn A. 2003. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem Pharm Bull. 51:857–859.
- Sundaram BM, Gopalakrishnan C, Subramanian S, Shankaranarayanan D, Kameswaran L. 1983. Antimicrobial activities of Garcinia mangostana. Planta Med. 48:59–60.
- Sutabhaha B, Darntrakoon U, Furuya T, Nagumo T. 1997. The inhibitory activities of mangosteen’s pericarb extract on methicillin-resistant Staphylococcus aureus. Bull Chiang Mai Assoc Med Sci. 30:40–46.
- Torrungruang K, Vichienroj P, Chutimaworapan S. 2013. Antibacterial activity of mangosteen pericarp extract against cariogenic Streptococcus mutans. CU Dent J. 30:1–10.
- Turnidge JD, Bell JM. 1999. Methicillin-resistant Staphylococcal aureus evolution in Australia over 35 years. Microb Drug Resist. 6:223–229.
- Voravuthikunchai SP, Kitpipit L. 2005. Activity of medicinal plant extracts against hospital isolates of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 11:510–512.
- Wrolstad RE. 1993. Colour and pigment analyses in fruit products. Station Bulletin, 624. Corvallis, OR: Agricultural Experiment Station, Oregon State University.
- Yoshikawa M, Harada E, Miki A, Sukamoto K, Liang SQ, Yamahara J, Murakami N. 1994. Antioxidant constituents from the fruit hulls of mangosteen (Garcinia mangostana L.) originating in Vietnam. Yakugaku Zasshi. 114:129–133.
