Abstract
Context: Rheumatoid arthritis is a chronic, autoimmune and systemic inflammatory disease, which targets synovial joints leading to joint destruction mediated in part by migration of inflammatory cells into the synovial tissue.
Objective: The present study evaluates the anti-rheumatic effect of a methanol extract of Ananas comosus (L.) Merr. (Bromeliaceae) peel in rats.
Materials and methods: Anti-rheumatic activity of crude extract of peels of A. comosus in complete Freund’s induced arthritis model in rats was studied at doses of 50, 100, 250 and 500 mg/kg b.w. for 21 days. Parameters such as paw size, levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), C-reactive proteins (CRP) and prostaglandins (PGE2) were analysed.
Results: Oral administration of the extract significantly reduced the swelling in the paw of rats (EC50 65.1 ± 2.95 mg/kg b.w.) with a maximal inhibition of 77.01 ± 10.53% on 21st day at 500 mg/kg b.w. The extract also significantly reduced the levels of SOD, CAT and GPx in liver (EC50 26.84 ± 16.37, 68.37 ± 19.22, 106.54 ± 34.81 mg/kg b.w., respectively), kidney (EC50 261.75 ± 81.5, 176.38 ± 8.08, 14.32 ± 6.64, mg/kg b.w., respectively) and spleen (EC50 152.14 ± 39.57, 83.97 ± 14.6, 47.1 ± 10.45 mg/kg b.w., respectively); and CRP (EC50 36.37 ± 12.4 mg/kg b.w.) and PGE2 (EC50 191.06 ± 71.54 mg/kg b.w.) in tissue homogenate and serum, respectively, at 500 mg/kg b.w. as compared to arthritic control group.
Discussion and conclusion: These results suggest that A. comosus fruit peel extract exerts anti-rheumatic activity.
Introduction
Rheumatoid arthritis (RA) is a systemic, chronic and autoimmune inflammatory disease that leads to joint destruction, caused partly by the migration of inflammatory cells into the synovial tissue. It affects approximately 1% of the adult population globally each year (Wei et al. Citation2014). The common indications of RA are pain, stiffness, fatigue, sleep disturbances and fever. Pathogenesis of RA is complicated with pathological changes at multiple targets. Progression of RA is associated with release of proinflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 from monocytes, synovial fibroblasts and macrophages. Inflammatory mediators such as superoxide and hydrogen peroxide are also produced by activated polymorphonuclear leucocytes and macrophages. Superoxide radicals cause cellular disruption through peroxidation of membrane lipids (Devi et al. Citation2007). However, activated oxygen intermediates together with secondarily formed hydroxyl radicals (OH) and antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), cause damage to the membrane lipids, proteins, DNA, hyaluronic acid and cartilage. Additionally, the levels of inflammatory markers such as C-reactive proteins (CRP) and specifically, prostaglandins (PGE2) produced from arachidonic acid by cyclic oxidase isozymes (COX2), also rise during the inflammatory process causing tissue damage (Ricciotti & Fitzgerald Citation2011). Currently, the development of natural anti-inflammatory drugs for prevention or treatment of inflammatory diseases is receiving increasing attention. Medicinal plants are the main sources of chemical substances with potential therapeutic effects and many of compounds from plants have already been characterized.
Ananas comosus (L.) Merr. (pineapple) is a tropical plant native to South America. A. comosus is by far the most economically important plant in the Bromeliaceae family with an edible fruit (Coppens d’Eeckenbrugge & Leal Citation2003). Ananas comosus is reported to possess several medicinal properties such as antidiabetic (Weidong et al. Citation2005), antitumour (Kalra et al. Citation2008), antioxidant, hepatoprotective (Dougnon et al. Citation2009), platelet aggregation (Bhattacharyya Citation2008), anti-helmintic (Gillian et al. Citation2004), anti-inflammatory (Eric et al. Citation2005) and immunomodulatory (Engwerda et al. Citation2001) activities. Various pharmacologically active phytochemicals present in this plant are ananasate, β-sitosterol, chlorogenic acid, rutin, naringenin (Makoto et al. Citation2007), bromelain (Eric et al. Citation2005) glycosides, flavonoids (Chao et al. Citation2007) and neurotransmitters, such as serotonin (Feldman & Lee Citation1985), dopamine, adrenaline and non-adrenaline (Odjakova & Hadjiivanova Citation1997). Bromelain extracted from A. comosus stem belongs to a family of sulphhydryl proteolytic enzymes and is reported to possess anti-inflammatory properties. In a clinical study, bromelain showed decrease in swelling and pain in 72% of rheumatoid arthritis (RA) patients (Cohen & Goldman Citation1964).
Bromelain, to the best of our knowledge, is the only component studied for its anti-rheumatic activity with specific inhibitory activities against Cox-2 and PGE2 expression (Gaspani et al. Citation2002). Considering the commercial use of A. comosus as a cash crop and the fact that bromelain is extracted from the stem of the plant, we evaluated the anti-rheumatic activity of the fruit peel, an inedible part that is discarded as waste. We studied the effect of methanol extract of fruit peel (MEFP) of A. comosus on complete Freund’s adjuvant (CFA) induced arthritis in a rat model to understand its possible mechanism of action.
Materials and methods
Plant material and extraction
The whole plant of A. comosus was collected in September 2011 from Ratnagiri district, Maharashtra, India. The plant material was authenticated by Blatter Herbarium, St. Xavier’s College, Mumbai and a voucher specimen has been deposited with accession number OS-1a. The fruit peel of A. comosus was air-dried in shade and ground into fine powder using a grinder. Powdered fruit peel (50 g) was extracted with 300 mL of methanol for 48 h in a Soxhlet apparatus. The MEFP obtained was then concentrated to dryness under vacuum at 60 °C using a rotary evaporator and the yield was calculated. The methanol content in the extract was determined by gas chromatography at Geo-Chem Laboratories Pvt Ltd., Mumbai, India. The extract was suspended in water for in vivo studies.
Phytochemical component analysis of MEFP
Qualitative phytochemical analysis
The MEFP of A. comosus was analysed for the presence of various phytoconstituents such as carbohydrates, proteins, triterpenes, phytosterol, cardiac glycosides, anthroquinone glycosides, flavonoids, alkaloids and tannins using standard methods (Chandana & Prabhukaran Citation2014).
Quantitative analysis of flavonoids
The total flavonoid content in the MEFP was estimated using the AlCl3 method with quercetin as a standard (Chang et al. Citation2002). Briefly, to 0.5 mL of the extract, 1.5 mL methanol, 0.1 mL aluminium chloride, 0.1 mL potassium acetate solution and 2.8 mL distilled water were added and mixed well. The absorbance was measured at 415 nm.
Quantitative analysis of tannins
The total tannin content in MEFP was estimated by modified Prussian Blue method with gallic acid as a standard (Graham Citation1992). Briefly, 0.1 mL of the extract was added to 6.9 mL of distilled water, 1 mL of 0.008 M potassium ferric cyanide, 1 mL of 0.2 M ferric chloride in 0.1 M HCl and mixed well. The absorbance was measured at 700 nm.
Thin layer chromatography (TLC) analysis of MEFP
Thin layer chromatography (TLC) of MEFP of A. Comosus was carried out on the G60 F254 silica plates (Merck KGaA, Darmstadt, Germany). Briefly, 5 μL of the sample was spotted on the plate and the chromatogram was developed using the solvent system toluene:ethylacetate:methanol:formic acid (4:6:4:0.2). The chromatograms were derivatized with 2-aminoethyl diphenylborinate (NP reagent) and vanillin sulphuric acid reagent (1% vanillin in 95% ethanolic sulphuric acid) for detecting flavonoids and tannins, respectively.
Experimental animals
Male Wistar rats weighing 100–150 g were purchased from Bharat Serum and Vaccines, Mumbai, India. The animals were kept in cages and maintained under standard housing conditions (24–27 °C with 12:12 light:dark cycles). Food was provided in the form of dry pellets (Nutrivet Life Science, Pune, India) and water ad libitum. The animals were acclimatized for a week under laboratory conditions before commencement of experiments.
The use of animals in the study was approved (CPCSEA/IAEC/SOS/P-36/2012) by the Institutional Animal Ethics Committee (IAEC) and all the experiments were carried out according to the guidelines of the Committee for the Purpose of Control on Supervision and Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India.
Acute toxicity studies
The acute toxicity studies with MEFP of A. comosus were conducted as per the OECD Guideline 423. The rats were fasted for 12 h prior to initiation of the experiment. Briefly, MEFP was administered orally to the rats as a single dose of 2000 mg/kg body weight. The rats were observed continuously for behavioural changes for the first 4 h then observed daily for 14 days for any toxic symptoms and/or mortality. Parameters such as breathing (palpitation), eye movement, eye colour, writhing effect, vomiting, diarrhea, abnormal body movements and texture and colour of fur were monitored.
Evaluation of anti-rheumatic activity of MEFP
CFA induced arthritis in rats
Experiments were carried out as per the method previously described by Pearson and Wood (Citation1959). Briefly, 0.1 mL of CFA (Sigma-Aldrich, MO) containing 10 mg/mL of heat killed Mycobacterium tuberculosis was injected into the left hind paw of the rats. The MEFP at doses of 50, 100, 250 and 500 mg/kg b.w. was administered orally to each rat; the control group received distilled water (0.1 mL/kg b.w.); and reference group received prednisolone (10 mg/kg b.w.) daily for 21days. Treatment was initiated from day 1 after FCA injection and the oedema on the left hind paw was evaluated daily using a digital plethysmometer. After 21 days, the animals were sacrificed by cervical dislocation and the blood was collected by cardiac puncture. The liver, kidney and spleen were immediately removed and washed with ice cold 10 mM phosphate buffered saline (PBS). The tissues were cut into pieces and homogenized using ice cold 10 mM PBS for further analysis.
Evaluation of scavenging enzymes
Tissue homogenates of liver, kidney and spleen were assessed for levels of SOD, CAT and GPx. SOD was estimated using a modification of the method described by Marklund and Marklund (Citation1974). Briefly, the reaction mixture contained 100 μg tissue homogenate in 2 mL of 50 mM Tris EDTA buffer (pH 8.2) and 1 mL of 4 mM pyrogallol. Absorbance was measured every minute for 3 min at 420 nm.
CAT was estimated using a modification of the method described by Chance and Herbert (Citation1950). Briefly, the reaction mixture contained 200 μg of tissue homogenate and 19.6 mM H2O2 in 2 mL of 10 mM potassium phosphate buffer (pH 7.4). Absorbance was measured every minute for 2 min at 240 nm.
GPx was estimated using a modification of the method described by Awasthi et al. (Citation1979). Briefly, the reaction mixture contained, 100 μg tissue homogenate, 10 mM sodium azide, 1 mM reduced glutathione and 2 mM H2O2 in 0.2 mL of 0.2 M phosphate buffer (pH 7.6). Following incubation at 37 °C for 10 min, 0.4 mL of 5% TCA was added and centrifuged at 3200g for 20 min. One micromolar Ellman’s reagent (1 mL) was added to 0.2 mL supernatant and incubated for 5 min. The absorbance was measured at 412 nm.
Evaluation of inflammatory markers
The serum samples were assessed for levels of CRP and PGE2. The CRP was estimated using CRP latex kit (Aspen-Rapid Diagnostic Co. Kit, Delhi, India). The PGE2 was estimated using Prostaglandin E2 assay kit as per manufacturer’s instructions (Catalog No- KGE004B, R&D Systems, McKinley Place NE, Minneapolis, MN). The absorbance was measured at 450 and 570 nm on an ELISA plate reader (BIO-RAD, CA).
Histological analysis of paw
Histological studies of the left hind paw of the animals were performed. The tissues were fixed in formalin, decalcified and embedded in paraffin blocks. The tissue sections were stained with haematoxylin and eosin and examined under microscope.
Statistical analysis
The values obtained are expressed as mean ± SEM. The data were analysed by a one-way ANOVA, followed by Dunnett’s post-test using Prism 5.0 (GraphPad Software, Inc., CA). p-Values less than 0.05 (p < 0.05) were considered as significant.
Results
Extraction of plant material
The yield of the extract from powdered fruit peel was 28.14%. The gas chromatography analysis of the dried extract showed the presence of approximately 7.63% w/w of methanol content. For all the assays conducted in the study, the extract suspended in distilled water at concentrations of 50, 100, 250 and 500 mg/kg b.w. was administered orally. The methanol content received by the different groups of animals was estimated to be 3.815, 7.63, 19.08, 38.15 mg/kg b.w., respectively. The minimum lethal dose of methanol via oral administration has been reported to be approximately 5628 mg/kg b.w. (Sax & Lewis Citation1989), indicating that the extract was not toxic to the animals. This was also confirmed by histopathological analysis where no difference was observed between control and test groups (data not shown).
Phytochemical component analysis of MEFP
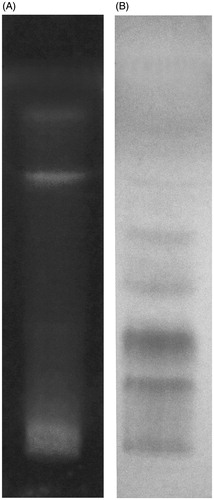
Qualitative phytochemical analysis of MEFP of A. comosus showed the presence of phytoconstituents, namely flavonoids, tannins, triterpenoids and phytosterols. The TLC analysis also showed presence of flavonoids () and tannins () in MEFP when derivatized with NP reagent and vanillin sulphuric acid reagent, respectively. The contents of flavonoids and tannins in MEFP were estimated to be 1.76 ± 0.067 mg/g and 4.52 ± 0.45 mg/g, respectively.
Acute toxicity studies
No deaths were observed in the group of animals administered with 2000 mg/kg b.w. of MEFP. No effect on behavioural or any other toxicological parameters was observed during the 14-day observation period. Hence, MEFP was considered to be safe.
Evaluation of anti-rheumatic activity of MEFP
CFA induced arthritis in rats
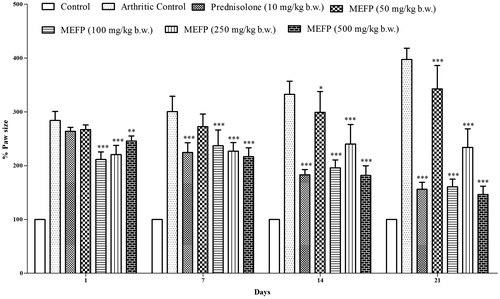
The paw size was measured on day 1 following injection with FCA in the left hind paw. The treatment was initiated on day 1 and the paw size was measured daily for 21 days. shows mean paw size for animals in each group at days 1, 7, 14 and 21. The CFA-induced arthritis control group showed continuous increase in the paw size until day 21, indicating no improvement in the arthritic lesions. The inflammation was significantly lower in groups treated with MEFP when compared to the arthritic control group. The reduction in inflammation with 500 mg/kg b.w. of MEFP was 77.01 ± 10.53% and 49.11 ± 20.69% with prednisolone.
Evaluation of scavenging enzymes
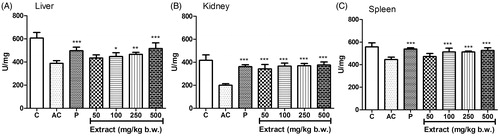
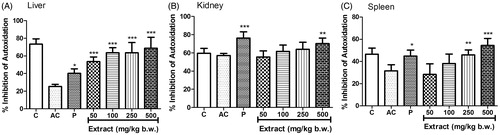
The level of SOD activity decreased significantly in tissue homogenates of liver () and spleen () in the arthritic control group compared to the normal control; however, the decrease in tissue homogenate of kidney was not significant (). The SOD activity significantly increased in liver (EC50 26.84 ± 16.37), kidney (EC50 261.75 ± 81.5) and spleen (EC50 152.14 ± 39.57) when treated with MEFP with maximum increase at 500 mg/kg b.w.
Figure 3. Effect of MEFP on SOD levels in liver, kidney and spleen. The values are expressed as mean ± SD. p < 0.05 was considered significant with respect to arthritic control group (*p < 0.05; **p < 0.01; ***p < 0.001).
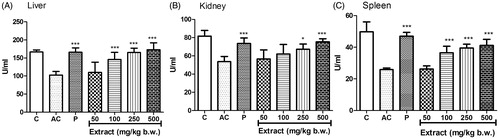
The level of CAT activity decreased significantly in the tissue homogenates of liver (), kidney () and spleen () in the arthritic control group compared to the normal control. The CAT activity increased in a dose-dependent manner in liver (EC50 68.37 ± 19.22), kidney (EC50 176.38 ± 8.08) and spleen (EC50 83.97 ± 14.6) when treated with MEFP with a significant increase at 250 and 500 mg/kg b.w. compared to the arthritic control group in all three tissue homogenates.
Figure 4. Effect of MEFP on CAT levels in liver, kidney and spleen. The values are expressed as mean ± SD. p < 0.05 was considered significant with respect to arthritic control group (*p < 0.05; ***p < 0.001).
The level of GPx activity decreased significantly in the tissue homogenates of liver (), kidney () and spleen () in the arthritic control group compared to the normal control. The GPx activity increased in a dose-dependent manner in liver (EC50 106.54 ± 34.81), kidney (EC50 14.32 ± 6.64), spleen (EC50 47.1 ± 10.45) when treated with MEFP with a significant increase at 100, 250 and 500 mg/kg b.w. compared to the arthritic control group in all three tissue homogenates.
Effect on inflammatory markers
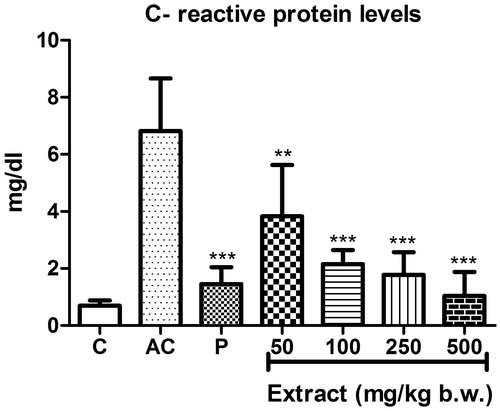
The levels of CRP increased significantly in serum samples of rats in the arthritic control group compared to that of the normal control (). When treated with different concentrations of MEFP, the levels of CRP decreased in a dose-dependent manner with all the concentrations (EC50 36.37 ± 12.4), showing significant decrease compared to the arthritic control group.
Figure 6. Effect of MEFP on CRP levels. The values are expressed as mean ± SD. p < 0.05 was considered significant with respect to arthritic control group. (**p < 0.01; ***p < 0.001).
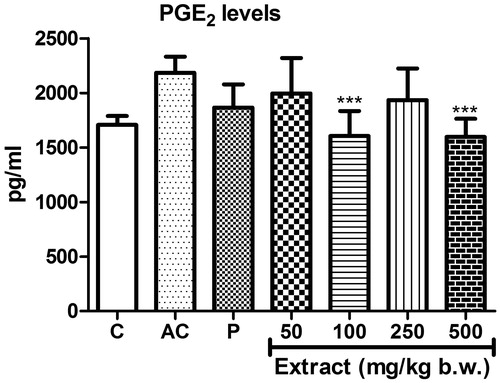
Treatment with MEFP showed decrease in levels of PGE2 in the serum samples of rats (EC50 191.06 ± 71.54) compared to the arthritic control when treated with significant decrease at concentrations of 100 and 500 mg/kg b.w. ().
Histopathological analysis of paw
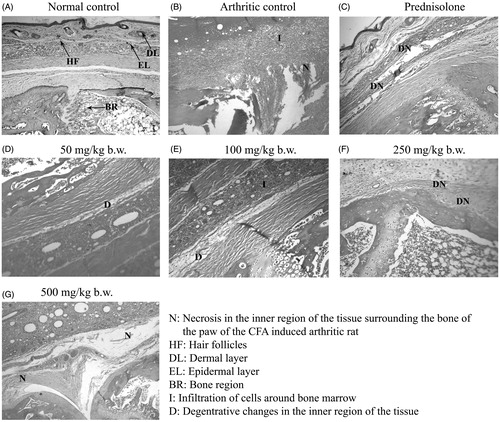
The histopathological analysis of hind paw of the arthritic control group rats injected with FCA showed infiltration of mononuclear cells, inflammatory reaction around the bone and under the subcutaneous tissue; necrosis and degeneration in the inner regions of the tissue surrounding the bone (). Treatment with MEFP showed reduction in necrosis at all the concentrations tested.
Discussion
CFA-induced arthritis in rats show clinical and pathological similarity to human RA and is thus the most widely used chronic test model (Pearson & Wood Citation1959). Most researchers have reported that inhibition of adjuvant-induced arthritis in rats is one of the most suitable methods to screen anti-arthritic agents. In this study, we investigated the effect of MEFP of A. comosus on paw inflammation following injection of FCA in hind paw. The extract showed a significant reduction in inflammation measured in terms of paw-size at 100 and 500 mg/kg b.w., which was comparable to the effect shown by the standard drug prednisolone at a concentration of 10 mg/kg b.w. The histopathological analysis also showed reduction in necrosis when treated with MEFP.
The body possesses antioxidant defences, repair mechanisms, physical defences and preventive mechanisms against free radical oxidative stress. Enzymes such as SOD, CAT and GPx are necessary for protective mechanisms including quenching of singlet oxygen, potential complexing of pro-oxidant metals and free radical scavenging and reducing activity.
SOD acts as the first line of defence against oxygen derived free radicals and protects cells by converting superoxide anion to hydrogen peroxide (Afonso et al. Citation2007). In the present study, although there was significant decrease in level of SOD in liver and spleen, there was no significant decrease in level of SOD in kidney. This is because the body tries to overcome the oxidative stress by maintaining the levels of SOD in kidney unlike in liver and spleen (Chandankhede & Gupta Citation2013). The increase in SOD activity observed in liver, kidney and spleen when treated with MEFP exhibit the protective effect of the extract against the extracellular oxygen-derived free radicals.
CAT, localized in peroxisomes or microperoxisomes (Devi et al. Citation2007) cleaves hydrogen peroxide generated in the body to water and oxygen, and thus, protects the cell from oxidative damage. Increase in CAT activity in liver, kidney and spleen when administered with MEFP again exhibits its protective effect against oxygen-derived free radicals.
GPx, a detoxifying enzyme, forms the primary mitochondrial defence against peroxides (Devi et al. Citation2007). Selenium is related closely with GPx activities in tissues (Rister & Bancher Citation1976). In RA, the free radicals decrease levels of selenium thereby decreasing the activity of GPx. Similar to as observed with SOD and CAT, the increase in GPx activity in liver, kidney and spleen when administered with MEFP again exhibits its protective effect against oxygen-derived free radicals through restoration of activity of radical scavenging enzymes. The results were similar to as observed with the standard drug, prednisolone.
CRP is a member of the class of acute phase reactants that is produced only in liver (Bashir et al. Citation2014). CRP acts as a marker of systemic inflammation since during inflammatory processes, its levels rise dramatically in the body as was observed in case of arthritic control rats in the present study (). Significant reduction in CRP levels in rats when administered with MEFP compared to arthritic control rats indicates the potential of MEFP as an anti-inflammatory agent.
PGE2, the main products of COX pathways (Fattahi & Mirshafiey Citation2012), are important mediators of inflammation in RA (Ricciotti & Fitzgerald Citation2011). Production of PGE2 by rheumatoid synovial tissue causes erosion of cartilage and juxta atricular bone. The decrease in the levels of PGE2 when administered with MEFP indicates the anti-inflammatory potential of A. comosus. It also suggests the possible use of MEFP for protection of cartilage from damage caused during RA.
The qualitative phytochemical analysis of MEFP showed the presence of flavonoids, tannins, triterpenoids and phytosterols. Of these, flavonoids and tannins can be attributed with the anti-rheumatic activity observed in the present study. Various studies have reported the anti-inflammatory properties of flavonoids and tannins from plants (Jiang et al. Citation2014). Reports have shown that triterpenoids and phytosterols do not possess anti-inflammatory properties (Saxena et al. Citation2013). There are no reports on the phytochemical composition of A. comosus fruit peel. Studies related to identification of active constituents through bioassay-guided fractionation is presently ongoing.
Conclusion
The MEFP of A. comosus exhibited anti-rheumatic activity by increasing the levels of SOD, CAT and GPx in liver, kidney and spleen, and by decreasing the levels of CRP and PGE2 prostaglandin in serum of arthritic rats. The histopathology of paw also exhibited reduction in necrosis when treated with the extract. We can conclude that flavonoids and tannins present in the crude extract may be responsible for the anti-arthritic activity as observed in this study.
Acknowledgements
The authors acknowledge Dr. Tannaz Birdi, Deputy Director, The Foundation for Medical Research, Mumbai and Dr. Kirti S. Laddha, Dean ICD and Professor of Pharmacognocy, Institute of Chemical Technology, Mumbai, for their critical inputs throughout the study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. 2007. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 74:324–329.
- Awasthi YC, Dao DD, Lal AK, Srivastava SK. 1979. Purification and properties of glutathione peroxidase from human placenta. Biochem J. 177:471–476.
- Bashir MU, Qureshi HJ, Mumtaz U. 2014. Effect of Nigella sativa seeds extract on serum C-reactive protein in albino rats. J Coll Physicians Surg Pak. 20:35–39.
- Bhattacharyya BK. 2008. Bromelain: an overview. Nat Prod Radiance. 7:359–363.
- Chance B, Herbert D. 1950. The enzyme substrate compounds of bacterial catalase and peroxides. Biochem J. 46:402–414.
- Chandankhede MS, Gupta MM. 2013. Oxidative stress and antioxidant status in patients with rheumatoid arthritis. Int J Biol Med Res. 4:3088–3090.
- Chandana R, Prabhukaran V. 2014. A study on antiepileptic activity of eugenol excluded aqueous extract of Eugenia caryophyllus. Int J Exp Pharmacol. 4:153–158.
- Chang C, Yang M, Wen H, Chern J. 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 10:178–182.
- Chao M, Sheng-Yuan X, Zhen-Guo L, Wang W, Li Jun D. 2007. Characterization of active phenolic components in the ethanolic extract of Ananas comosus L. leaves using high performance liquid chromatography with diode array detection and tandem mass spectrometry. J Chromatogr. 1165:39–44.
- Cohen A, Goldman J. 1964. Bromelains therapy in rheumatoid arthritis. PA Med J. 67:27–30.
- Coppens d’Eeckenbrugge G, Leal F. 2003. Morphology, anatomy, and taxonomy. In: Bartholomew DP, Paull RE, Rohrbach KG, editors. The pineapple: botany, production, and uses. Wallingford (CT): UCP. p. 93–96.
- Devi PR, Kumari SK, Kokilavani C. 2007. Effect of Vitex negundo leaf extract on the free radicals scavengers in complete Freund's adjuvant induced arthritic rats. Indian J Clin Biochem. 22:143–147.
- Dougnon TJ, Kpodekon TM, Laleye A. 2009. Protective effects of pineapple (Ananas comosus) on liver and kidney of Wistar rats intoxicated with doliprane. Int J Biol Chem Sci. 3:532–537.
- Engwerda CR, Andrew D, Ladhams A, Mynott TL. 2001. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell Immunol. 210:66–75.
- Eric R, Secor Jr, William F, Carson IV., Cloutier MM, Guernsey LA, Schramm CM, Wu CA, Thrall RS. 2005. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cellular Immunol. 237:68–75.
- Fattahi MJ, Mirshafiey A. 2012. Prostaglandins and rheumatoid arthritis. Arthritis. 2012:239–310.
- Feldman JM, Lee EM. 1985. Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid. Am J Clin Nutr. 42:639–643.
- Gaspani L, Limiroli E, Ferrario P, Bianchi M. 2002. In vivo and in vitro effects of bromelain on PGE(2) and SP concentrations in the inflammatory exudate in rats. Pharmacology. 65:83–86.
- Gillian S, Jerzy MB, David JB, Ian RD. 2004. Natural plant cysteine proteinases as anthelmintics. Trends Parasitol. 20:322–327.
- Graham HD. 1992. Stabilization of the Prussian blue color in the determination of polyphenols. J Agric Food Chem. 40:801–805.
- Jiang CP, He X, Yang XL, Zhang SL, Li H, Song ZJ, Zhang CF, Yang ZL, Li P, Wang CZ, et al. 2014. Anti-rheumatoid arthritic activity of flavonoids from Daphne genkwa. Phytomedicine. 21:830–837.
- Kalra N, Bhui KB, Roy P, Srivastava S, George J, Prasad S, Shukla Y. 2008. Regulation of p53, nuclear factor κB and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skin. Toxicol Appl Pharm. 226:30–37.
- Makoto S, Keisuke K, Takanori M, Santoshi M, Meiko F. 2007. Hyperthermia-related testicular toxicity of reserpine in mice. Exp Toxicol Pathol. 59:187–195.
- Marklund S, Marklund G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474.
- Odjakova M, Hadjiivanova C. 1997. Animal neurotransmitter substances in plants. J Plant Physiol. 23:94–102.
- Pearson CM, Wood FD. 1959. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. General clinical and pathological characteristics and some modifying factors. Arthritis Rheum. 2:440–459.
- Ricciotti E, Fitzgerald G. 2011. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 31:986–1000.
- Rister M, Bancher RL. 1976. The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest. 58:1174–1184.
- Sax NI, Lewis RJ Jr. 1989. Dangerous properties of industrial materials. New York (NY): Van Nostrand Reinhold.
- Saxena M, Saxena J, Nema R, Singh D, Gupta A. 2013. Phytochemistry of medicinal plants. J Pharmacog Phytochem. 1:168–182.
- Secor ER Jr, William F, Carson IV, Cloutier MM, Guernsey LA, Schramm CM, Wu CA, Thrall RS. 2005. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell Immunol. 237:68–75.
- Wei Z, Yang Y, Xie C, Li C, Wang G, Ma L, Chen L. 2014. Synthesis and biological evaluation of pyranoisoflavone derivatives as anti-inflammatory agents. Fitoterapia. 97:172–183.
- Weidong X, Wang W, Su H, Xing D, Cai G, Du L. 2005. Hypolipidemic mechanisms of Ananas comosus L. leaves in mice: different from fibrates but similar to statins. J Pharmacol Sci. 103:267–274.