Abstract
Context: Skin microbiota takes part in the control of cutaneous inflammation. In skin diseases such as atopic dermatitis (AD) cutaneous dysbiosis and the emergence of Staphylococcus aureus contribute to the pathophysiology of the disease. New therapeutic approaches consist in topical application of natural products able to counteract S. aureus effects through activation of resident immune cells producing anti-inflammatory cytokines such as IL-10.
Objective: This study investigates the potential immunosuppressive properties of Aquaphilus dolomiae (Neisseriaceae), a flagellated bacterium contained in Avène Thermal Spring Water used in hydrotherapy treatments of AD patients.
Materials and methods: An aqueous protein extract of Aquaphilus dolomiae (ADE, 60 μg/mL) was added to human monocyte-derived dendritic cells (moDC) for 24 h. Expression of HLA-DR, CD86 and CD83 was evaluated by flow cytometry and released cytokines (IL-10, IL-12) by cytometry bead array assay. The proliferation of allogeneic CFSE-labelled CD4+ T cells stimulated with ADE-conditioned moDC and S. aureus secretome was analysed by flow cytometry.
Results: MoDC exposed to ADE expressed lower levels of HLA-DR and CD86 than untreated cells, no CD83 and secreted barely detectable IL-12 but high amounts of IL-10 (N = 12, p < 0.0002). The proliferative effect of S. aureus secretome on CD4+ T cells was reduced (p < 0.001) in the presence of ADE-moDC.
Conclusion: ADE counteracted the mitogenic effect of a S. aureus secretome on CD4+T cells. Owing to the role of S. aureus colonization in driving inflammation in AD the immunosuppressive property of the ADE might be useful to reduce disease severity.
Introduction
The pathophysiology of atopic dermatitis (AD), an inflammatory skin disease that primarily affects young children, is based on genetic and acquired defects characterized by barrier dysfunction and impaired anti-inflammatory skin-associated immunity (Bieber Citation2008; Biedermann et al. Citation2015). In healthy skin, interactions between cutaneous microbiota and resident cells including keratinocytes, dendritic cells (DC) and CD4+T cells contribute to maintain skin homeostasis. Commensally, bacteria play a critical role in this process by suppressing inflammatory responses and by inducing tolerance through multiple mechanisms including secretion of IL-10 by DC and regulatory T cells (Treg). Accordingly, cutaneous dysbiosis and the emergence of Staphylococcus aureus in skin lesions at the expense of commensal bacteria such as Staphylococcus epidermidis, contribute to the pathophysiology of AD (Naik et al. Citation2015). Indeed, S. aureus colonization could promote the expansion of Th2 cells with inhibitory effect on the production of antimicrobial peptides by keratinocytes, and the inhibition of the suppressive activity of Treg (Cardona et al. Citation2006; Gittler et al. Citation2012). We previously demonstrated that the secretome of S. aureus isolated from skin of atopic children promoted CD4+T cell proliferation and Treg silencing and we suggested that both processes could be counteracted and counterbalanced by IL-10 secreted by monocyte-derived dendritic cells (moDC) exposed to the S. epidermidis secretome (Laborel-Préneron et al. Citation2015). Therefore, induction of tolerance to allergen in AD should be based on both anergy of Th2 cells and activation/induction of Treg cells. To achieve this goal both IL-10-producing DC and IL-10-treated DC with tolerogenic and regulatory properties could play an important role and therapeutic approaches against AD could consist in stimulating and/or inducing such DC populations. This could be done topically by using preparations with tolerogenic properties and/or formulations able to break dysbiosis to promote anti-inflammatory capacities of skin commensals such as S. epidermidis. Such a strategy consisting in exposure of skin from AD patients to Vitreoscilla filiformis bacterial lysates proved to be effective (Gueniche et al. Citation2008; Volz et al. Citation2014). In this study, we investigated the potential immunosuppressive activity of an Aquaphilus dolomiae (Neisseriaceae) extract (ADE). We demonstrated that exposure of moDC to ADE induced the secretion of substantial amounts of IL-10 providing conditions to reduce activation of CD4 + T cells induced by S. aureus secretome.
Materials and methods
Characterization of the ADE
A Gram negative bacteria strain I-4290, Aquaphilus dolomiae has been isolated from Avene Spring water (southwest France), fully characterized by genotyping and phenotyping and deposited at CNCM (Collection nationale de Cultures de Microorganismes, Institut Pasteur Paris) (Bourrain et al. Citation2012). The strain forms a distinct lineage within the family Neisseriaceae of the β-Proteobacteria class. Upstream and downstream processing was performed at the Centre d’Immunologie Pierre Fabre (St-Julien-en-Genevois, France) according to Good Manufacturing Practice, as following: bacteria from working cell bank were cultivated in complex defined medium in aerobic conditions at 27 °C and pH 7.0 in controlled bioreactor. The culture was carried out in three successive increasing volume steps from 1 L shaking flask to 25 and 350 L volume-stirred bioreactors. The high cell density fermentation was carried out in fed-batch mode with sterile glucose solution. The cell growth was monitored by optical density measurement at OD 620 nm and dry cell weight determination. At the end of the fed-batch fermentation, bacterial cells were processed to specifically release periplasmic and membrane proteins, peptides and lipopolysaccharides in an aqueous basic buffer (pH 11) at 4 °C for 30 min in a stirred reactor. The bacterial cells were removed by centrifugation on disk separator followed by depth filtration. The supernatant extract was filtered through a 0.2-μm polyethersulphone filter. The clear and homogeneous aqueous solution was stored at −20 °C. The solution namely “ADE” was tittered in protein content (⋍2 mg/mL) according to DC Protein Assay Kit II (Bio-Rad®, Marnes-la-Coquette, France) protocol.
Cell toxicity assay
Toxicity of the bacterial extract on moDC was determined by using the CellTox™ Green Cytotoxicity Assay (Proméga, G8741, Charbonni res-les-Bains, France) that measures changes in membrane integrity occurring as a result of cell death. MoDC suspension either treated or not with ADE was supplemented with cyanine dye and incubated for 24 h before recording fluorescence intensity. Fluorescence was normalized to untreated and lysed cells (lysis solution) according to the manufacturer’s instructions. ADE was considered as toxic when the percentage of cell mortality was more than 5%. The assay was performed in duplicate with N = 3. A maximum final concentration of 60 μg/mL was determined and used in all the experiments.
Preparation and characterization of skin bacterial secretomes
Secretomes were prepared with S. aureus and S. epidermidis clones isolated from the skin of AD children by overnight culture in RPMI1640 medium supplemented with 10% FCS until stationary phase was reached as described by Laborel-Préneron et al. (Citation2015). In this study, secretomes from patient P04 were used at 5% (v/v) concentration.
Monocyte-derived dendritic cells
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats (blood donors from EFS, Toulouse) by Ficoll separation (PAA). Monocytes were isolated from PBMC with the use of CD14 microbeads and a magnetic bead separation system (Miltenyi, Paris, France). Monocytes were maintained for 5 days in RPMI medium supplemented with 10% FCS, 1% penicillin–streptomycin, 20 ng/mL recombinant GM-CSF (Miltenyi, Paris, France) and 50 ng/mL IL-13 (donated by Sanofi-Aventis). On day 5, non-adherent moDC were recovered and stimulated for 24 h with either S. aureus or S. epidermidis secretome at 5% (v/v), or LPS at 100 ng/mL or ADE (μg/mL) as indicated. Cells were stained and submitted to flow cytometry analysis on a FACSCalibur cytometer (BD Biosciences, Le Pont de Claix, France) as detailed in the flow cytometry analysis section.
CD4+T cells
Naive CD4 + CD45RA+ T cells were isolated from PBMC with the use of naive CD4+ T cell isolation kit II and CD4 T cell isolation kit II (Miltenyi, Paris, France). For proliferation assays, CD4+T cells were stained with CFSE (Invitrogen, Waltham, MA) and co-cultured with allogeneic moDC as indicated for 5 days in 96-well plates at a ratio of 1 stimulator to 10 T cells. Proliferation (%) was assessed by flow cytometry.
Flow cytometry analysis
Cells were stained with monoclonal antibodies directed against: CD1a-PE, HLA-DR-FITC, CD86-APC, (Biolegend, San Diego, CA), CD83-PE (eBioscience, San Diego, CA). Analyses were performed with LSRII flow cytometer (BD Biosciences, Le Pont de Claix, France). All the cells used in this study expressed CD1a (#100%) at their surface.
Cytokines quantification
The production of secreted cytokines (IL-6, IL-12, IL-10) was quantified with cytometric bead array kits (BD Biosciences, Le Pont de Claix, France).
Statistical Analysis
Data were presented as mean ± standard error. Statistical analyses were performed using the paired t-test; p values less than 0.05 were considered as significant.
Results
moDC exposed to the ADE differentiate into IL-10 producing semi-mature dendritic cells
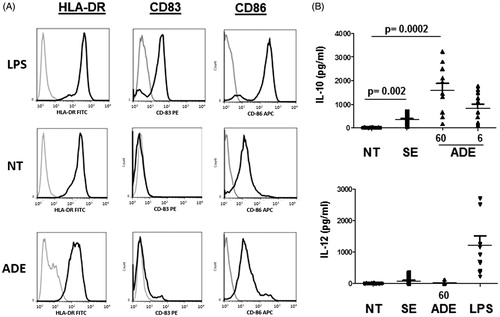
Cutaneous inflammatory DC that derive from the differentiation of monocytes recruited in inflamed skin (moDC) can induce activation of resident T cells and then contribute to immune response and tolerance. Skin DC receive various stimuli through their constant exposure to bacterial products coming from the commensal flora or from opportunistic pathogens. We previously showed that components contained in bacterial secretomes prepared from S. aureus and S. epidermidis isolated from the skin of atopic children exerted pro- and anti-inflammatory effects, respectively (Laborel-Préneron et al. Citation2015). Indeed, the S. epidermidis secretome induced secretion of IL-10 by moDC with immunosuppressive activity on CD4+ T cell activation that was induced by S. aureus secretome. In this study, we addressed the effect of an ADE on the phenotype of moDC. Cells were exposed for 24 h to medium (NT), S. epidermidis secretome (SE) and ADE as indicated (μg/mL) and analysed for the expression of the surface markers HLA-DR, CD83, CD86 by flow cytometry. Under treatment with LPS, cells exhibited a fully mature phenotype characterized by the induction of CD83, a strong up-regulation of HLA-DR and CD86 expression () and secretion of IL-6 (not shown) and IL-12 (mean ± SEM = 1227 pg/mL). In contrast, exposure of cells to ADE (60 μg/mL) induced moDC that did not express CD83, and HLA-DR and CD86 at a lower level than untreated cells (). Cells secreted IL-10 (mean ± SEM = 1579 pg/mL) at levels higher than those of cells exposed to SE (mean ± SEM = 339 pg/mL) but very low levels of IL-12 (mean ± SEM = 15.9 pg/mL) (). When using 0.6 μg/mL concentration of ADE, moDC phenotype was identical to untreated cells and IL-10 was barely undetectable (not shown). These data show that the ADE induced semi-mature DC devoid of maturation/activation markers with potential immunosuppressive capacities due to IL-10 secretion. We then addressed how ADE-conditioned moDC could influence CD4 + T cells proliferation.
Figure 1. Phenotype of moDC exposed to the ADE. (A) Activation profile (CD86, CD83 and HLA-DR surface expression) of moDC exposed for 24 h to either LPS, medium alone (NT), or the ADE at 60 μg/mL. Similar results were obtained in three independent experiments. (B) Levels of secreted IL-10 (pg/mL) and IL-12 (pg/mL) by moDC exposed to medium alone (NT), S. epidermidis secretome (SE), ADE as indicated (μg/mL) and LPS. Mean values with SEM are shown, p values less than 0.05 were considered as significant, N = 12.
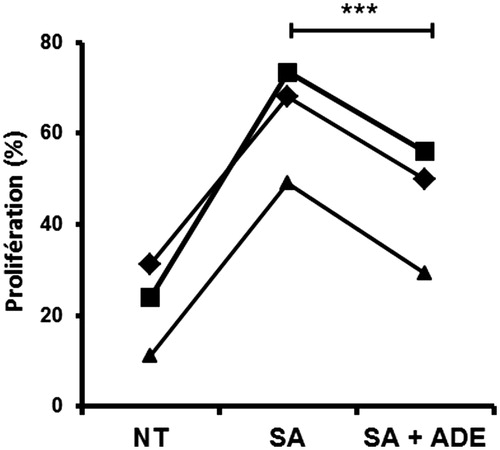
ADE counteracted S. aureus effect on CD4+T cells proliferation
According to the contribution of S. aureus secretome as a major activator of T cell proliferation in AD due to the presence of superantigens (SAg), we addressed the effect of ADE on the capacity of moDC exposed to S. aureus secretome (SA) to modulate the proliferation of allogeneic naive CD4+ T cells. shows that exposure of moDC to the S. aureus secretome (SA) induced a strong increase in T cell proliferation which was significantly decreased (p < 0.001, N = 3) by the addition of ADE to SA. Owing to a substantial number of T cells that are activated by powerful mitogens represented by SAg contained in the S. aureus secretome, these data point out the outstanding anti-inflammatory potential of the ADE.
Figure 2. ADE counteracts the effect of S. aureus secretome on CD4 + T cells proliferation. MoDC were exposed for 24 h to either medium alone (NT), or S. aureus secretome (SA) alone, or a mixture of (SA) with ADE at 60 μg/mL. Then, moDC were co-cultured with CFSE-labelled allogeneic CD4+ T cells. Proliferation of T cells was quantified by flow cytometry (%). Paired t-test, ***p < 0.001, N = 3.
Discussion
In inflammatory skin disorders such as AD, cutaneous dysbiosis contributes to pathophysiology of the disease (Kobayashi et al. Citation2015). In addition to the direct effects due to the emergence of S. aureus in skin lesions, harmful concomitant effects could be due to a defect in commensals that protect the skin through induction of anti-inflammatory cytokines like IL-10 by immune cells, including resident DC (Laborel-Préneron et al. Citation2015). Restoring suitable conditions to favour the anti-inflammatory properties of skin commensals and/or application on the skin of formulation with anti-inflammatory properties appear as new therapeutic approaches. In line with our previous work on the effect of the secretome of S. aureus prepared from atopic children microbiota on CD4+ T cells activation, the anti-inflammatory properties of the ADE was investigated. Here, we show that the extract induced secretion of IL-10 by moDC and that these cells could counteract the effect of the S. aureus secretome containing SAg on allogeneic CD4+T cell proliferation. This effect was more likely due to a decreased expression of MHC-II and co-stimulatory markers on moDC as we observed, leading to a decrease in the number of MHC-II-SAg complexes and T cell stimulatory capacity. These data provide similar results to those obtained when moDC were exposed to a mixture of S. aureus and S. epidermidis secretomes, suggesting that ADE could have a therapeutic benefit by complementing the defect of commensals in AD skin. We previously demonstrated that S. aureus secretome could impair the suppressive activity of Treg, a defect that could worsen the disease. Therefore, counterbalancing this effect could be beneficial to the patients. As a flagellated bacterium, Aquaphilus dolomiae could be a good candidate to enhance Treg activity since binding of flagellin to TLR5 was shown to enhance the suppressive capacity and expression of Foxp3 in CD4 + CD25+ Treg cells (Crellin et al. Citation2005). This requires further exploration. Overall, our observations point out the anti-inflammatory properties of Aquaphilus dolomiae extract for topical therapeutic approach in AD.
Funding information
This work was supported by Pierre Fabre Dermo-cosmétique (Toulouse). The study was sponsored by Pierre Fabre Dermo-cosmétique.
Disclosure statement
T.N., A-M.S. and D.R. are employees of Pierre Fabre Dermo-cosmétique. There is no conflict of interest for H.M., E.L-P., F.F. and C.D.
References
- Bieber T. 2008. Atopic dermatitis. N Engl J Med. 358:1483–1494.
- Biedermann T, Skabytska Y, Kaesler S, Volz T. 2015. Regulation of T cell immunity in atopic dermatitis by microbes: the yin and yang of cutaneous inflammation. Front Immunol. 6:353–361.
- Bourrain M, Villette C, Nguyen T, Lebaron P. 2012. Aquaphilus dolomiae Gen. Nov., Sp. Nov., isolated from a deep aquifer. Life Environ. 62:191–195.
- Cardona ID, Goleva E, Ou LS, Leung DY. 2006. Staphylococcal enterotoxin B inhibits regulatory T cells by inducing glucocorticoid-induced TNF receptor-related protein ligand on monocytes. J Allergy Clin Immunol. 117:688–695.
- Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. 2005. Human CD4+T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4 + CD25+ T regulatory cells. J Immunol. 175:8051–8059.
- Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, Mitsui H, Cardinale I, de Guzman Strong C, Krueger JG. 2012. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 130:1344–1354.
- Gueniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, Röcken M, Breton L, Biedermann T. 2008. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled clinical study. Br J Dermatol. 159:1357–1363.
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, Kong HH, Amagai M, Nagao K. 2015. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 42:756–766.
- Laborel-Préneron E, Bianchi P, Boralevi F, Lehours P, Fraysse F, Morice-Picard F, Sugai M, Sato’o Y, Badiou C, Lina G, et al. 2015. Correction: effects of the Staphylococcus aureus and Staphylococcus epidermidis secretomes isolated from the skin microbiota of atopic children on CD4+ T cell activation. PLoS One. 10:e0144323.
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 520:104–108.
- Volz T, Skabytska Y, Guenova E, Chen KM, Frick JS, Kirschning CJ, Kaesler S, Röcken M, Biedermann T. 2014. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol. 134:96–104.
