Abstract
Objectives. The long-term prognostic value (>5 years) of elevated cardiac biomarkers after elective coronary angioplasty is yet not clear. Most previous studies have included high risk, unstable patients. The aim of this study was to determine the prognostic value of CK-MB mass ≥ three times the reference after elective angioplasty in low-risk patients with stable angina. Methods. A total of 278 consecutive patients were included in the final analysis. Patients with elevated CK-MB values at baseline, and those suffering an acute coronary syndrome <1 month before the time of inclusion, were excluded. Blood samples were drawn just before and 1-3 hours, 4-8 hours after the procedure and the next morning. Nineteen patients (6.8%) had peak CK-MB mass values ≥15 μg/L (three times the reference), and 259 patients had values <15 μg/L (defined as controls). No patient developed new Q-waves on ECG. The median follow-up time was 80.5 months. Results. None of the patients died during the procedure or within the first 30 days after angioplasty, confirming a low risk cohort. All cause mortality, readmission for acute coronary syndromes and target lesion revascularisation (TLR) were more frequent in patients with high CK-MB, 42.1% vs. 22.8%, p=0.034 (log rank). In a multivariate logistic regression analysis, high CK-MB and former angioplasty were the only variables independently related to a reduced event-free survival. Conclusions. CK-MB mass values ≥ three times the reference after elective angioplasty predicts reduced long-term event-free survival.
Periprocedural myocardial infarction (PMI) is a harmful event that implies worsened short- and long-term prognosis (Citation1–9). Electrocardiographic (ECG) or echocardiographic changes indicative of PMI are frequently absent. The precise cut-off level of the cardiac biomarkers identifying acute MI has also been difficult to determine due to marker release caused by the percutaneous procedure itself. Furthermore, the impact of very small release of cardiac biomarkers has been disputed (Citation8) in otherwise uncomplicated procedures.
The cardiospecific troponins are increasingly dominating the field of cardiac biomarkers but CK-MB mass is still an important biochemical cardiac marker in the setting of PMI. Most previous studies having assessed the prognostic value of CK-MB in PCI patients (Citation1,Citation3,Citation4,Citation6) included unstable and more high risk patients. Several of these studies were based on large registries (Citation1, Citation3, Citation8). Thus, use of stents, medication and analytical methods varied over time and makes it more difficult to generalize the results.
Although large-scale studies (Citation5, Citation7) have evaluated the prognostic value of CK-MB after percutaneous coronary intervention (PCI), this issue has not yet been well characterized in low-risk patients. The aim of this study was therefore to assess the long-term prognostic value of CK-MB mass (three times the reference) in patients with stable angina.
Methods
Study patients
In this prospective study, 285 consecutive patients with stable angina for at least 1 month prior to the procedure were screened for inclusion during the period from 1998 to 1999. Seven patients were excluded. Exclusion criteria were elevated or missing CK-MB values just before angioplasty, unavailability for follow-up, recent acute coronary syndrome (ACS) <1 month, malignant disease, or creatinine >200 μmol/L. In line with previous studies, a CK-MB value three times the reference was used as the cutoff (Citation10). Patients were compared according to CK-MB values, i.e. patients with CK-MB <15 μg/L were defined as controls, and patients with CK-MB ≥ 15 μg/L were defined as the high CK-MB group. The study was approved by the regional ethical committee, and informed written consent was obtained from all patients.
Study end points
Predefined major adverse clinical events were the composite end point of all cause mortality (>24 hr after procedure), readmission for acute coronary syndromes, and target lesion revascularisation (TLR). ACS (unstable angina, ST-elevation and non ST-elevation myocardial infarction) were diagnosed according to AMI definition (Citation11).
PCI technique
The patients underwent angioplasty according to the hospitals standard techniques. All patients received aspirin and a thienopyridine agent at least 48 hrs before procedure and heparin was administered intravenously during procedure to achieve an ACT around 300. Stented patients were treated with a thienopyridine agent for 1 month post-PCI. All patients were recommended life-long aspirin treatment.
Biochemistry, ECG and angiography
Venous blood samples were prospectively collected just prior to angioplasty in all patients. Blood samples were also sampled 1-3 and 4-8 hr after the procedure, and next morning thereafter. CK-MB mass (reference limit <5 μg/L) was assayed with an established immunoassay, the Technicon Immuno 1 System (Bayer Business Group Diagnostics, Tarrytown, NY, USA) according to the manufacturer.
ECG was taken before and at least once after the procedure and changes were assessed according to the Minnesota Code for defining myocardial infarction based on new Q-waves (Citation12). During follow-up, we were able to investigate the medical records of all the patients as well as the Central Population Register and local patient administrative systems for predefined end points and other adverse clinical events. The vast majority (97.8%) of the patients was living near the two largest regional hospitals.
All patients underwent angiography before PCI according to established routines in our institution (Citation13) and further treatment strategy was decided by a joint meeting of experienced invasive cardiologists and thoracic surgeons.
Statistical analysis
Data are presented as mean and 95% confidence interval (CI) unless otherwise stated. Student t-test was used when comparing continuous variables and χ2 test (or Fisher exact test) for analysis of discrete variables (SPSS, version 14.0). Analysis of variance for repeated measurements was applied to determine the p-value for trend. The Kaplan-Meier method and log-rank test were used to evaluate differences in event-free survival between patients with CK-MB ≥ three times the reference vs. controls. Univariate and multivariate linear analyses were performed to assess which variables influenced peak CK-MB values. The independent ability of CK-MB to predict worse outcome was assessed by a univariate and multivariate logistic regression analysis. All clinical relevant baseline and procedural variables presented in and were tested in the univariate analysis. Variables with a univariate p-value <0.10 were adjusted for both in the linear and logistic regression multivariate analysis. Differences were considered significant with p < 0.05 if not otherwise stated.
Table I. Patient characteristics.
Table II. Procedural data.
Results
Baseline and procedural characteristics
and show baseline characteristics and procedural data. The mean age of the 278 patients included was 65 years, range 45-85. A trend of more women in patients with CK-MB values ≥ 15 μg/L as compared with controls was demonstrated. The mean stent length was significantly longer in the high CK-MB group as well as the number of stents implanted per procedure. Complications were also more frequent in the high CK-MB group (52.6% vs. 17.3% among controls). Two hundred and fourteen patients (77.0%), mean age 66 years, range 41-85, were stented (bare metal stents). Creatinine was above 150 μg/L in two patients (values of 154 μg/L and 172 μg/L) and the number of patients with EF less than 30% and 40% was one (0.4%) and nine (3.4%).
CK-MB values
The mean peak CK-MB value was 4.8 μg/L at 20-24 hrs after PCI in the total study cohort (), P-value for trend <0.001. Sixteen point five percent of all patients had values above 5 μg/L and 19 patients (6.8%) had CK-MB values above ≥ three times the reference post procedure. In a multivariate linear analysis, long lesions, the number of stents per procedure, gender and complications were predicting high CK-MB values post-PCI.
Table III. Temporal pattern of CK-MB mass before and after angioplasty.
Clinical end-points
None of the patients died during the procedure or within the first 30 days after angioplasty, and only 11 patients (4%) died during a median long-term follow-up time of 80.5 months (mean of 79.9 months), confirming a low-risk cohort. Seventy seven point two percent of controls compared to 57.9% in the high CK-MB group were free of predefined events (p = 0.030, , ). Freedom from all-cause mortality was 100.0% and 95.8% in the controls and high CK-MB group, respectively (p = 0.452, ). Thirteen point five percent of controls underwent TLR vs. 26.3% of patients with CK-MB ≥ 15 μg/L, p = 0.125. These reinterventions were all due to restenosis.
Figure 1. Kaplan Meier plot of event-free survival (all patients, n = 278). P-value = 0.034 (log-rank test).
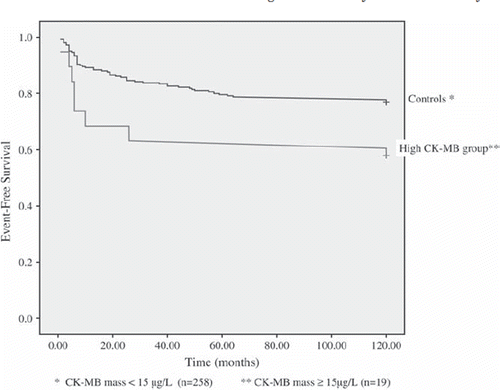
Table V. Logistic regression analysis in all patients (n = 278) with event-free survival as dependant variable.
Independent predictors of event-free survival
In a univariate logistic analysis, all variables in and were tested for. Only ostial lesion (p = 0.014), former PCI (p = 0.002), smoking (never, current, or ever smoker, p = 0.095) and CK-MB with a cut-point of 15 μg/L (p = 0.064) had a p-value <0.10. When the variables mentioned above were put in the same multivariate analysis, the p-value of CK-MB was 0.049. Non-fatal complications was a significant predictor of elevated CK-MB () but did not predict event-free survival (univariate p-value was 0.708). Stenting was not associated with higher number of predefined events during follow-up (the univariate p-value was 0.600).
Table IV. Univariate and multivariate linear regression analysis with peak CK-MB mass after PCI as dependent variable (all patients, n = 278).
Discussion
The study showed that in these stable patients at a clinical and procedural low risk, the CK-MB was elevated above the reference level of 5 μg/L in about 16.5%. Peak CK-MB values were ≥ three times the reference (the recommended cut-off value of CK-MB post PCI) in 6.8% of the patients. None had ECG changes or chest pain post-PCI suggestive of new MI and thus, the traditional signs of acute MI were not present. Importantly, TLR was twice as frequent in patients with CK-MB mass ≥ 15 μg/L compared to the controls. There was no significant difference in all cause mortality during follow-up. Nonfatal complications (treated with an additional stent in cases with focal dissection or abrupt closure and in some patients glycoprotein IIb/IIIa inhibitor was added) were more frequent in the high CK-MB group and seemed to be the major cause of CK-MB elevation in more than one half of the patients.
Previous studies
Several studies including two large metaanalysis (Citation1–9) have assessed the prognostic value of CK-MB. Many of the large scale studies, however, have been based upon PCI registries (Citation13) or clinical trial cohorts, raising important methodological issues (89). One of the first large-scale study by Abdelmeguid et al. (Citation1) included 4 484 patients. However, 68% of all patients had unstable angina and around 16% had EF <40% compared to 0.4% in our study. The mortality was about twice as high as compared to the present study. Thus, the study included patients at a much higher risk than the present study. CK-MB was measured as catalytic activity and not as mass and mean follow-up time was much shorter than in our study. Similarly, in the large study by Ellis et al. (8 409 patients), 63% of all included patients had unstable angina and mean follow-up time was 38 months. Mortality in the subgroup with the lowest CK-MB values was around 15% after 4 years, much higher than in the present study.
The study by Brener et al. is probably the largest previous study having assessed the prognostic value of CK-MB after PCI in stented patients only. However, the study included 64% unstable and high risk patients (Citation6). Consequently the mortality rates were much higher compared to the stented patients in the present study (11% vs. 4.7% in our study). This is well explained by the fact that the Brener study did include more patients with renal failure, 4.0% compared to 0.4% in our study, as well as more patients with heart failure and low ejection fraction (about 22% with EF < 40% vs. 8.0% in the present study). Furthermore, the mean follow-up time was much shorter than in our study (15 vs. about 80 months).
In the metaanalysis by Roe et al. (Citation5), acute coronary syndrome was the main inclusion criterium and thus, the results can not be automatically extrapolated to patients at lower clinical risk. Another drawback of this metaanalysis was the selection bias in that CK-MB levels may have been measured in patients at highest risk. In the metaanalysis by Ionnadis et al., the authors included studies with unstable patients as well as studies where large fractions of patients underwent directional or rotational artherectomy (Citation7). Different types of analytical methods in the same study and uncertain adherence to guidelines for CK-MB sampling were also a methodological problem in several of the large-scale studies (Citation8).
Specificity of CK-MB
Former studies (28) have shown increased mortality among patients who sustained periprocedural MI during PCI. The diagnostic criteria of myocardial infarction, however, were usually based on new Q-waves and/or raise in conventional cardiac bio-markers (Citation8). As shown also by us in previous studies (Citation14), CK does not discriminate between extra-cardiac and cardiac injury with certainty. This might have led to overestimation of perioperative myocardial infarction in previous studies. Furthermore, the use of new Q-waves as a diagnostic tool has been debated (Citation15).
CK-MB is present in the skeletal muscle in around 1-3% and therefore not completely heart specific (Citation16). The insertion of the sheath might possibly influence the CK-MB release. However, in a previous study by us mean peak CK-MB was 5 μg/L after lung surgery and only one patient had a peak value above 10 μg/L (Citation14). Thus, we suggest that the contribution of extra-cardiac release of CK-MB release would be very small, if any, in the present cohort of patients with less trauma on the extracar-dial tissue. It seems clear that the elevation of CK-MB is caused primarily by cardiac injury, possibly from ischemic driven myocardial injury. The true cut-off value of CK-MB for diagnosing periprocedural MI as well as the clinical relevance has been debated and the present guidelines is still based on expert consensus (Citation9,Citation10). Whether small release of CK-MB without symptoms or ECG changes represents myocyte necrosis has also been debated (Citation17–23). However, whatever the cause of elevation, CK-MB values ≥ three times the upper normal limit clearly predicted worse long-term outcome in the present study.
Causes of CK-MB elevation after PCI
This study was not aimed at clarifying causes for CK-MB elevation. Several patient, procedural and lesion related factors have been associated with periprocedural MI (Citation8). Rotablational or directional atherectomy, dissections and abrupt closure, bifurcation stenting and side-branch occlusion as well as distal embolization have, among others, been mentioned as possible causes of elevated CK-MB after PCI (Citation8) but the exact mechanisms of marker release remain unclear in many cases.
Rotablation was performed in only 0.3% of the patients in the present study. There was implanted significantly more stents in the high CK-MB group compared to the controls and bifurcation lesions as well as the use of glycoprotein IIb/IIIa receptor inhibitor tended to be more frequent in these patients. More patients with high CK-MB had non-fatal complications. Thus, challenging anatomical lesions were more frequent in the high CK-MB group, probably resulting in more dissections and transient chest pain and more stents were needed to successfully finish the procedure.
Strength/limitations
In this study we were able to assess the temporal pattern of CK-MB with certainty up to 20–24 hrs. According to previous studies (Citation10, Citation14), CK-MB peaks around this time interval. In all patients included in the study, normal baseline values were identified and blood samples were also collected immediately after the procedure to make sure that no other factors than the procedure itself influenced the CK-MB levels. The results of this study were also strengthened by the very long follow-up time. To our knowledge, this is the only report on stable patients at low risk with a median follow-up time close to 7 years (approximately 2 000 patient-years of follow-up time). The study was limited by the fact that it was a single-center study with a relatively small cohort of patients, although the number of patient years during follow-up was quite high. We were not able to differentiate between all-cause mortality and cardiac mortality in this study but the overall number of deaths was very low during follow-up and not different between controls and patients with high CK-MB mass. On the other hand, all cause death is a robust and objective end-point and uncertainty regarding the accuracy of death certificates was avoided (Citation24).
In conclusion, this study suggests that even in stable, low-risk patients undergoing PCI, CK-MB values three times the reference imply worse outcome during long-term follow-up whatever cause of the elevation. The study was not aimed at defining the diagnostic cut-off for periprocedural infarction. However, we believe the present results add important information to the newly published definitions for diagnosing acute MI and support the clinical value of the chosen cut-off value of three times the reference in both high- and low-risk patients. In these patients, more aggressive medical treatment as well as out-patient follow-up should be considered. Furthermore, since high CK-MB values seem related to procedural complications, it is important to prevent these events by good preparations and ensure proper indication for the procedure.
Declaration of interest: All authors declare no conflict of interest.
References
- Abdelmeguid AE, Topol EJ, Whitlow PL, Sapp SK, Ellis SG. Significance of mild transient release of creatine kinase-MB fraction after percutaneous coronary interventions. Circulation. 1996;94:1528–36.
- Califf RM, Abdelmeguid AE, Kuntz RE, Popma JJ, Davidson CJ, Cohen EA, . Myonecrosis after revascularization procedures. J Am Coll Cardiol. 1998;31:241–51.
- Ellis SG, Chew D, Chan A, Whitlow PL, Schneider JP, Topol EJ. Death following creatine kinase-MB elevation after coronary intervention:Identification of an early risk period:Importance of creatine kinase-MB level, completeness of revascularization, ventricular function, and probable benefit of statin therapy. Circulation. 2002;106:1205–10.
- Akkerhuis KM, Alexander JH, Tardiff BE, Boersma E, Harrington RA, Lincoff AM, . Minor myocardial damage and prognosis:Are spontaneous and percutaneous coronary intervention-related events different? Circulation. 2002;105:554–6.
- Roe MT, Mahaffey KW, Kilaru R, Alexander JH, Akkerhuis KM, Simoons ML, . Creatine kinase-MB elevation after percutaneous coronary intervention predicts adverse outcomes in patients with acute coronary syndromes. Eur Heart J. 2004;25:313–21.
- Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ. Association between CK-MB elevation after percutanous or surgical revascularisation and three-year mortality. J Am Coll Cardiol. 2002;40:1961–7.
- Ionnidis JPA, Karvouni E, Katritsis D. Mortality risk conferred by small elevations of creatine kinase-MB isoenzyme after percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:1406–11.
- Herrmann J. Peri-procedural myocardial injury:update. Eur Heart J. 2005;26:2493–519.
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, . Clinical end points in coronary stent trials:A case for standardized definitions. Circulation. 2007;115:2344–51.
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–38.
- The joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined–A consensus document of the joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000; 36: 959–69. doi:10.1016/S0735-10970000804-4.
- Prineas RJ, Crowe RS, Blackburn H. The Minnesota Code manual of electrocardiographic findings. Bristol:John Wright ;1982.
- Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. New Eng J Med. 1997;337:230–6.
- Vikenes K, Andersen KA, Farstad M, Nordrehaug JE. Temporal pattern of cardiac troponin I after thoracotomy and lung surgery. Int J Cardiol. 2004;96:403–7.
- Hodakowski GT, Craver JM, Jones EL, King SB 3rd, Guyton RA. Clinical significance of perioperative Q-wave myocardial infarction:The Emory Angioplasty versus Surgery Trial. J Thorac Cardiovasc Surg. 1996;112:1447–53.
- Adams JE, Bodor GS, Davila-Roman VG, Delmez JA, Apple FS, Ladenson JH, . Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation. 1993;88:101–6.
- Ahmed SA, Williamson JR, Roberts R, Clark RE, Sobel BE. The association of increased plasma MB CPK activity and irreversible ischemic myocardial injury in the dog. Circulation. 1976;54:187–93.
- Feng YJ, Chen C, Fallon JT, Lai T, Chen L, Knibbs DR, . Comparison of cardiac troponin I, creatine kinase-MB, and myoglobin for detection of acute ischemic myocardial injury in a swine model. Am J Clin Pathol. 1998;110:70–7.
- Piper HM, Schwartz P, Spahr R, Spieckermann PG. Early enzyme release from myocardial cells is not due to irreversible cell damage. J Mol Cell Cardiol. 1984;16:185–8.
- Sommers HM, Jennings RB. Experimental acute myocardial infarction:Histologic and histochemical studies of early myo-cardial infarcts induced by temporary or permanent occlusion of a coronary artery. Lab Invest. 1964;13:149–54.
- Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, . Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–3.
- Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, . Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury:Insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111:1027–32.
- Vikenes K, Westby J, Matre K, Kuiper KKJ, Farstad M, Nor-drehaug JE. Release of cardiac Troponin I after temporally graded acute coronary ischemia with electrocardiographic ST depression. Int J Cardiology. 2002;85:243–51.
- Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research:Time for a reassessment? J Am Coll Cardiol. 1999;34:618–20.