Abstract
Objectives. Asymptomatic atrial fibrillation (AF) presents the same risk for cardioembolic events as symptomatic AF. Intermittent ECG recordings may be helpful in detecting asymptomatic paroxysmal arrhythmias. The objective of this study was to compare short intermittent heart rhythm recordings with or without symptoms with continuous ECG recordings. Design. Twenty-two patients with diagnosed symptomatic paroxysmal AF participated. Patients used a handheld transtelephonic ECG device for a 30-day period performing two registrations of 10 seconds per day. Additionally patients were asked to make registrations if arrhythmia symptoms occurred. Patients also performed a 24-hour ambulatory continuous ECG recording during a single twenty-four hours period of the 30 day period. AF was defined as an irregular ventricular rhythm without visible p-waves for at least 10 seconds.Results. 1425 intermittent ECG recordings were performed. AF episodes were diagnosed in 18 (82%) patients compared with 7 (32%) patients using continuous ECG, (p = 0.001). All patients with AF detected by continuous monitoring were also detected by intermittent recordings. Of the episodes, 16% were judged as symptomatic. Conclusion. Short-term ECG registrations over extended time periods seem to be a more sensitive tool, compared with short continuous ECG recordings, for detection of AF episodes.
Introduction
Atrial fibrillation (AF) is a major risk factor for morbidity and mortality and may be symptomatic or asymptomatic (Citation1–2). Patients with AF frequently present with paroxysmal AF and asymptomatic episodes are estimated to be 12-times more frequent than symptomatic episodes (Citation3). In recent years, awareness has grown concerning asymptomatic AF and its implications. Several studies have found that asymptomatic paroxysmal AF presents with as high a risk of ischemic stroke as permanent AF (Citation4–7). It is therefore of importance to identify these subjects, and if necessary, provide adequate anticoagulant treatment (Citation8).
Suspected arrhythmias can be screened for by various methods such as 24-hour continuous ECG recorders (Holter) and event recorders (Citation9,Citation10). These methods allow for heart rhythm monitoring over extended periods of time. Longer monitoring periods might provide higher diagnostic certainty but may also lower patient compliance (Citation11). Thus, the different methods have various advantages and disadvantages. It is possible that intermittent rhythm recordings over a longer time period is an alternative to continuous heart rhythm monitoring for a relatively short time period (24–48 hours), to screen for asymptomatic AF. The aim of this study was to examine whether short, episodes of intermittent monitoring is superior compared to 24-hour continuous ECG recordings in detecting AF episodes. This work, to our knowledge, is the first that has tried to compare the efficiency between intermittent and continuous ECG monitoring in detection of AF.
Methods
Patients
Patients were recruited from two cardiology departments in the Stockholm area using existing patient registers based on the diagnosis using the ICD 10 system. Screening was performed in patients who had been in contact with the cardiology departments within the last 12 months either in an emergency setting or as a planned follow-up visit with the diagnosis of paroxysmal AF. Patients were contacted consecutively by telephone and interviewed regarding arrhythmia symptoms. Enrolled patients medical records were scrutinized and medical information was examined and validated according to a predefined protocol, including co-morbidity and risk factors according to the CHA2DS2-VASc scoring system (Citation12). Furthermore, medication and echocardiography examinations were documented.
Participating patients should have a verified diagnosis of paroxysmal AF and symptomatic episodes of palpitations at least once every 3 months. The study was performed in accordance with the Declaration of Helsinki in regards to research protocol approval from the local ethics committee and informed consent from study participants.
Registrations
The subjects were asked to use a handheld patient triggered ECG recorder (Zenicor EKG ®) that allows for 10 seconds of ECG recordings from both thumbs (i.e. lead I), twice daily; once in the morning and once in the evening for a 30 day period. In addition, patients were asked to make recordings in case of arrhythmia symptoms. Thus, recorded registrations, apart from the predetermined morning and evening hours, were considered as symptomatic. Recordings were sent by telephone and stored at a secure SSL encrypted Internet database. An example of recordings of sinus rhythm and AF is presented in .
Figure 1. Example of sinus rhythm (bottom) and AF (top) registration using the intermittent ECG device.
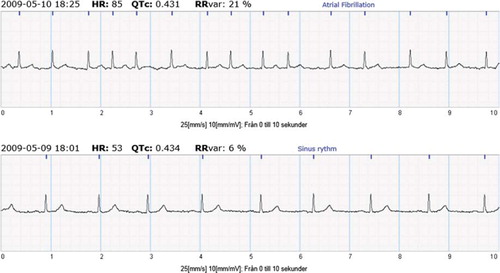
Patients were provided with an ambulatory continuous ECG device to be used for a single 24-hour period during the 30 days of patient triggered recordings. Analysis of the 24-hour continuous ECG recordings and the hand-held ECG were performed by two separate investigators in a blinded fashion. AF was defined as irregular ventricular rhythm without visible p-waves. For diagnostic consistency, the same AF definition was used for the continuous ECG recordings. The primary endpoint was detection of AF episodes as either present or absent. The diagnostic capacity for AF and feasibility of the handheld-ECG recorder has been validated previously (Citation13).
Statistics and sample size
To test the null hypothesis that there is no difference between the methods (24-hour continuous ECG vs. intermittent ECG) used to classify the patients (Negative vs. Positive AF episodes), we used McNemar's test for paired proportions. The criterion for significance (alpha) was 0.05 (two-tailed).
The study was dimensioned to detect large discrepancies between the methods. We needed 20 patients to yield a power of 80% to yield a statistically significant result based on the following population effect size: In 25% of the patients, both methods will classify a patient as negative AF, and in another 25%, both methods will classify a patient as positive. A discrepancy between the two methods was assumed in the population as follows: In 5% of all patients only one test will show an outcome of positive, while 45% of all patients will show an outcome of positive AF for the other method. The power calculation was performed in Sample Power 2.0. Patients acted as their own controls. Twenty-two patients were calculated to be needed.
The statistical analysis was performed in SPSS version 17.
Results
Twenty-five patients were initially recruited for participation in the study. Three patients were excluded due to progression into persistent AF, leaving 22 patients eligible for participation out of which 73% (n = 16) were male and 27% (n = 6) women with a median age of 63 years (range 46–77). (Further background data are presented in .)
Table I. Clinical characteristics of 22 patients with documented paroxysmal atrial fibrillation.
Continuous 24-hour ECG recordings
Using the 24-hour continuous ECG recording 13 AF episodes were detected in 7 of the 22 patients (32%). Three of these recordings revealed constant AF during the recording, whereas the rest revealed shorter bursts ranging from minutes to hours. The median number of AF episodes per patient was 1 (Citation1–6). The average recorded time was 22 hours and 24 minutes per patient, (16:29–24:00).
Intermittent ECG recordings
In total, the twenty-two patients transmitted 1425 registrations (mean: 65 per patient), out of which 1399, that is, 98.2% were interpretable. Recordings were, based on findings, divided into: sinus rhythm, AF, erroneous registrations, and missing registrations. AF episodes (n = 250) were detected in 18 patients (82%).
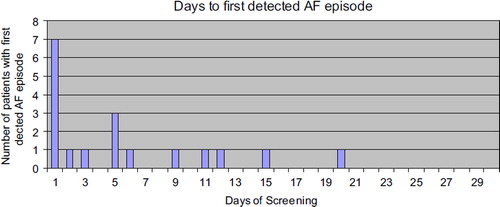
AF episodes were distributed evenly during the day with 124 registrations in the evening and 117 during the morning hours. In patients with AF episodes, 17 out of 18 patients were identified within 15 days of monitoring (). Of all AF episodes, 16% were additional symptom triggered recordings.
In twelve patients with a negative 24-hour continuous ECG recording, intermittent ECG recordings revealed an average of 7 (range 1–17) AF episodes per patient. Sixteen patients transmitted an additional 104 registrations due to arrhythmia symptoms. The number of transmissions spanned from 1–19 per patient. Out of all AF episodes 16% were symptomatic.
Figure 2. Patients with first detected AF episode;17 out of 18 first AF episodes were detected within 15 days.
There was a difference (p = 0,001, McNemar) in the ability to classify AF patients between the methods in favor of intermittent recordings, which more often detected AF episodes ().
Table II. Comparison of the ability to detect atrial fibrillation between continuous 2-hour ECG recordings and intermittent hand-held ECG recordings.
Intermittent ECG recording were observed to cause little or no disturbance in the patient's everyday life although the study period stretched over 30 days [13]. Of the scheduled planned recordings at fixed time intervals, 5.9% were missing.
Intermittent recordings made during the 30-day period showed an even distribution between preset hours and extra registrations, which imply the possibility to diagnose both arrhythmia like symptoms and asymptomatic AF at random controls.
Discussion
Extensive heart rhythm monitoring reveals more arrhythmias (Citation9,Citation10). However, the optimal method how this should be performed non-invasively has not been fully evaluated. Several studies for screening of new AF in ischemic stroke patients have yielded various results irrespective of method and monitoring period (Citation14). However, Piorkowski et al. described comparable results when comparing repetitive transtelephonic ECGs and 7-day Holters for AF detection in post ablation patients due to symptomatic AF (Citation15).
Our findings suggest that short episodes of monitoring over longer time periods detect more episodes of AF than continuous recordings over a limited time sequence. Similar findings have been reported in AF patients post ablation (Citation16). This might reflect the fact that both symptomatic and asymptomatic attacks of AF episodes have a more unpredictable pattern than anticipated, including a strategy of spot checks at certain time intervals being more effective. In our study, the time to first AF episode detection spanned from 1–20 days, which gives an indication of the screening period necessary to find AF episodes. One might argue that the screening period should be prolonged in patients where no previous arrhythmia symptoms have been documented, to increase diagnostic certainty, in comparison to recently ablated AF patients with an increased risk of postablation AF episodes.
Further, since it has been shown that there often is no correlation between the subjective sensation of arrhythmia episodes and the actual rhythm, it seems important to perform intermittent recordings not only in case of symptoms but rather with regular time intervals for improved detection of asymptomatic AF episodes (Citation3,Citation17,Citation18,Citation19). This was also seen in our study where only 16% of the recorded AF episodes were symptomatic.
This was also the reason why we abstained from using symptom based assessment for quality of life, especially since this paper is focused on silent AF episodes.
An alternative method to detect episodes of asymptomatic silent AF could be the use of implantable loop recorders, which provide extensive arrhythmia monitoring over long time periods. Ziegler et al. showed continuous monitoring with an implanted device to be significantly reliable in assessing AF burden compared with intermittent monitoring (Citation18).
However, such a strategy is invasive and implies a high up-front cost.
Can our data be generalized to other populations?
Whether our findings, from patients with known symptomatic paroxysmal atrial fibrillation, can be applied as a screening tool in patients without a history of AF, but with a high risk for stroke is an important question.
In a recently presented study, we used the same two techniques in 236 patients with ischemic stroke with no obvious cause, including no history of AF (Citation20). All patients underwent 24-hour ECG and 30-day handheld ECG. A total of 17 (7.2%) patients were detected to have silent AF.
In 12 out of the 17 patients, the hand held ECG exclusively detected AF. Only in 2 out of the 17 patients, 24-hour ECG monitoring added information. In the remaining patients, both methods detected AF. In our view, this strongly supports that intermittent ECG recording is a more sensitive method to detect silent AF.
The results from this study are derived from a small group of patients with a high density of symptomatic attacks of AF. Thus, one should be cautious to generalize the findings to larger patient groups with less risk for silent AF undergoing screening. One might, however, argue as such: It is not known today what AF burden is required to pose a significant risk of stroke. A higher AF burden seems to increase the risk of stroke. Apart from devices able to constantly monitor the heart rhythm, screening methods only present a fraction of the heart rhythm. An arrhythmia diagnosis is then based upon either one single recorded event or added results from several recordings when using methods such as the intermittent ECG recorder. In patient without previously known AF found to have short AF episodes during screening, it is for the treating physician to make a judgment based on the overall patient risk profile to initiate OAC medication or not.
Implications
Intermittent short ECG recordings with or without symptoms during extended time periods might become an efficient tool for screening of silent intermittent AF episodes, for example, in patients with ischemic stroke or TIA without a history of AF. It may also possibly become an alternative in AF screening of certain groups in the general public with an increased risk of stroke and where the likelihood of finding AF is increased such as in persons over an age of 75, ischemic heart disease or congestive heart failure.
Acknowledgements
Zenicor-EKG equipment was provided to the investigators during the trial period by Zenicor Medical Systems AB.
Declaration of interest: Mårten Rosenqvist is working pro bono as a consultant for Zenicor Medical Systems AB. This work was supported by a grant from the Stockholm County Council and the Swedish Heart and Lung Foundation.
References
- Benjamin E, Wolf P, D’Agostino R, Silbershatz H, Kannel W, Levy D, . Impact of atrial fibrillation on the risk of death. Circulation. 1998;98:946–52.
- Fuster V, Rydén LE, Cannom DS, Crijns H, Curtis A, Ellenbogen K, . ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:700–52.
- Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett E. Asymptomatic Arrhythmias in patients symptomatic atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–27.
- Schuchert A, Behrens G, Meinertz T. Impact of long- term ECG recording on detection of paroxysmal atrial fibrillation in patients after an acute ischemic stroke. Pacing Clin Electrophysiol. 1999;22:1082–4.
- Scholten MF, Thornton AS, Mekel JM, Koudstaal PJ, Jordaens LJ. Anticoagulation in atrial fibrillation and flutter. Europace. 2005;7:492–99.
- Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort- Study of Atrial Fibrillation (SCAF). Eur Heart J. 2007;28:2346–53.
- Hart RG, Pearce LA, Rothbart RM, Mc Anulty JH, Asinger RW, Halperin JL, . Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke prevention in atrial fibrillation investigators. J Am Coll Cardiol. 2000;35:183–7.
- White H, Gruber M, Feyzi J, Kaatz S, Tse H, Husted S, . Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control. Results from SPORTIF III and V. Arch Intern Med. 2007;167: 239–45.
- Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35: 1647–51.
- Barthélémy JC, Féasson-Gérard S, Garnier P, Gaspoz JM, Da Costa A, Michel D, . Automatic cardiac event recorders reveal paroxysmal atrial fibrillation after unexplained strokes or transient ischemic attacks. Ann Noninvasive Electrocardiol. 2003;8:194–9.
- Scherr D, Dalal D, Henrikson CA, Spragg D, Berger R, Calkins H, . Prospective comparison of the diagnostic utility of a standard event monitor versus a “leadless” portable ECG monitor in the evaluation of patients with palpitations. J Interv Card Electrophysiol. 2008;22: 39–44.
- Gage BF, Waterman AD, Shannon W, Boehlert M, Rich MW, Radford MY, . Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70.
- Doliwa PS, Frykman V, Rosenqvist M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand Cardiovasc J. 2009;43:163–8.
- Liao J, Khalid Z, Scallan C, Morillo C, O´Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke, A systemic review. Stroke. 2007;38:2935–40.
- Piorkowski C, Kottkamp H, Tanner H, Kobza R, Nielsen J, Arya A, . Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:1286–92.
- Kottkamp H, Tanner H, Kobza R, Schirdewahn P, Dorszewski A, Gerds-Li JH, . Time courses and quatnative analysis of atrial finbrillation episode number and duration after circular plus linear left atrial lesions. Trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol. 2004;44:869–77.
- Nergårdh a. Frick M. Perceived heart rhythm in relation to ECG findings after direct current cardioversion of atrial fibrillation. Heart. 2006;92:1244–47.
- Quirno G, Gimmaria M, Corbucci G, Pistelli P, Turri E, Mazza A, . Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: Relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32:91–8.
- Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445–52.
- Doliwa PS, Rooth E, Frykman V, von Arbin M, Wallen H, Rosenqvist M, . Improving screening for silent atrial fibrillation after ischemic stroke. Circulation. 2010;122A: 10878.