Abstract
Objective. We investigated the myocardial protective effect of sevoflurane in patients receiving off-pump coronary artery bypass grafting (OPCABG) and the role of brain natriuretic peptide (BNP). Design. Forty-eight patients receiving elective OPCABG were randomly assigned to a control group, and to 0.75 MAC, 1.0 MAC and 1.5 MAC sevoflurane groups. Blood samples were collected and levels of BNP and cardiac troponin I (cTnI) were measured before anesthesia, and immediately, 24, 48 and 72 h after surgery. Results. Dopamine was necessary to maintain blood pressure in the sevoflurane groups, but not in the control group (p < 0.002). 1.0 MAC sevoflurane significantly decreased post-surgical cTnI levels (p < 0.001). 0.75 MAC had no significant effect, and increasing sevoflurane concentrations to 1.5 MAC caused no further decrease in cTnI concentrations. There was no significant difference in BNP level among the groups (p = 0.227) or between any two groups, although values of BNP showed a significant correlation with cTnI values in control subjects immediately after (r = 0.847) and 24 h after (r = 0.661) surgery. Conclusions. Our results demonstrated that 1.0 MAC and 1.5 MAC sevoflurane can exert a significant myocardial protective effect. BNP cannot be used to predict the myocardial protective effect of sevoflurane in OPCABG.
Introduction
The myocardial protective effect of volatile anesthetics such as sevoflurane has been extensively investigated, and several potential mechanisms have been identified including modulation of gene expression (Citation1,Citation2). In humans, myocardial protective effects of sevoflurane have been investigated when sevoflurane is used before, during, and after on-pump coronary bypass surgery (Citation3–6), and protective effects have been observed in most studies. Cardioprotection has been shown to vary with the dose of sevoflurane, with the protocol used (Citation7), and with the type of surgery (Citation8,Citation9). In addition, pre-treatment with sevoflurane at 1.0 MAC has only limited myocardial protection. Few studies, however, have examined the cardioprotective effect of sevoflurane in off-pump coronary artery bypass surgery.
One study of sevoflurane use during off-pump coronary artery bypass grafting (OPCABG) showed a myocardial protective effect, but did not examine the dose range over which the effect occurred (Citation10). Similarly, Bein et al. (Citation11) studied the effects of sevoflurane on left ventricular performance during minimally invasive direct coronary artery bypass grafting (MIDCAB) without cardiopulmonary bypass and found that sevoflurane preserved myocardial function better than propofol, though again the dose range was not studied. Whether sevoflurane at any concentration exerts a protective effect requires further study.
Cardiac troponin I (cTnI) is a sensitive indicator of myocardial damage and dysfunction (Citation12–14), and the myocardial protective effect of sevoflurane is characterized by a reduction of the cTnI level. Brain natriuretic peptide (BNP) is also an indicator of myocardial damage and dysfunction, and clinically BNP has been used in diagnosis, treatment, and determination of the prognosis of heart failure and many studies have investigated its use for the prediction of severe post-operative complications (Citation15). In coronary artery bypass surgery, however, repeated movement of the heart as well as post-operative ischemia may stimulate the secretion of BNP.
The primary goal of the present study was to investigate the concentration of sevoflurane that provides a cardioprotective effect and has a minimal cardiac inhibitory effect in off-pump CABG by comparing post-surgery cTnI and BNP levels. In addition, we sought to determine the clinical usefulness of BNP in assessing myocardial damage after off-pump CABG.
Patients and methods
Patients
The present study was approved by the institutional review board of our hospital, and written informed consent was obtained from each subject before the study. This trial was registered at www.chictr.org (registration number, ChiCTR-TTRCC-12002691). A total of 48 patients undergoing elective OPCABG were recruited into this prospective, randomized and controlled study. Inclusion criteria were: (Citation1) lesions in 2 or more coronary artery branches, or in the left coronary artery trunk; (Citation2) recent myocardial infarction or unstable angina; (Citation3) obvious regional left ventricular wall motion abnormality (Citation4) ejection fraction (EF) > 40% in pre-operative echocardiography. Left ventricular wall motion abnormality was determined by cardiac ultrasonography, and the wall motion score index (WMSI) was defined as follows: score 1, normal kinesis; score 2, hyperkinesis; score 3, akinesis; score 4, dyskinesis; and score 5, ventricular aneurysm. Exclusion criteria were as follows: severe arrhythmia, valvular lesions, concomitant congenital heart disease, severe peripheral vascular occlusive disease, patients receiving intra-aortic balloon pumping (IABP), intra-operative change into cardiopulmonary bypass, intra-operative administration of adrenaline, stroke patients, and patients with diabetes mellitus.
Grouping
All patients were numbered, and randomly assigned into the following 4 groups using a random number table: control group, 0.75 MAC group, 1 MAC group, and 1.5 MAC group (n = 12/group). Each random number was sealed in an individual envelope which was opened before anesthesia. Patients, examiners, and statisticians were blind to the assignment. Anesthesia and surgery were performed by the same team, and patients were followed-up for 3 days.
Anesthesia
All cardiovascular drugs except for angiotensin-converting enzyme inhibitors were administered until the day of surgery. All patients received a standard pre-operative treatment: 0.1 mg/kg morphine and 0.3 mg of scopolamine, given by intramuscular injection, 30 min before surgery. Upon entry into the operating room, electrocardiogram, entropy index, and oxygen saturation (SpO2) were monitored, and oxygen inhalation by mask was administered. A peripheral pathway was established through the left upper extremity vein, and blood pressure was measured through left radial artery catheterization.
Anesthesia induction was identical in the 4 groups and consisted of 0.05–0.1 mg/kg midazolam, 15 μg/kg fentanyl, and 0.1 mg/kg vecuronium, and was followed by endotracheal intubation and mechanical ventilation. In the control group, anesthesia maintenance consisted of midazolam (0.1–0.15 mg/kg/h), fentanyl (≤ 30 μg/kg), and vecuronium (0.1 mg/kg/h). The dosage of propofol in the control group was 20–50 mg. Anesthesia maintenance in the experimental groups was midazolam (0.05 mg/kg/h), fentanyl (≤ 30 μg/kg), and vecuronium (0.05 mg/kg/h). Midazolam and vercuronium are used to decrease entropy during anesthesia. Because the use of sevoflurane alone can decrease the entropy index, when sevoflurane is used, smaller amounts of midazolam and vecuronium are required to maintain the entropy index (Citation16). Although the methods for anesthesia were different, the depth of anesthesia was controlled to the same level. Entropy and oxygen saturation were determined using the Datex-Ohmeda S/5 ADU (GE, USA). Ventilatory conditions were tidal volume, 8–10 ml/kg; respiratory rate, 10–12 breaths/min, and end-tidal carbon dioxide (PETCO2), 35–40 mm Hg. During anesthesia maintenance, the concentration of anesthetic gas was monitored. In the control group, no sevoflurane was used. In the 3 remaining groups, 0.75 MAC, 1 MAC, and 1.5 MAC sevoflurane were administered and the oxygen flow rate was 5 L/min. The target concentration was reached within about 2 min, and after that the oxygen flow rate was adjusted to 2 L/min. Oxygen inhalation was continued until completion of surgery.
During the peri-operative period, midazolam (0.05–0.15 mg/kg/h), vecuronium (0.05–0.1 mg/kg/h), and fentanyl were administered intermittently, the total dose was lower than 20 μg/kg. The entropy index was maintained at 40–50 and train-of-four (TOF) was maintained at < 10%. When the entropy index was ≥ 50, midazolam was administered at 0.1 mg/kg.
Sevoflurane can exert a direct suppressive effect on the myocardium. When the concentration of sevoflurane was at a low level, we administered dopamine to increase myocardial contractility. When the sevoflurane concentration was at a high level, the suppressive effect of sevoflurane was more potent and the requirement of dopamine was also increased. When the dose of dopamine was elevated to a certain extent, additional adrenaline was required or extracorporeal circulation was necessary for safety of the surgery. During surgery, nitroglycerin (0.5–5 μg/kg/min) was given when blood pressure was ≥ 90 mm Hg. If blood pressure was < 90 mm Hg, dopamine was given at 2–8 μg/kg/min. When the dose of dopamine was > 8 μg/kg/min, adrenaline was given or the study was discontinued. Lactated Ringers solution and Voluven (6% hydroxyethyl starch 130/0.4, lot #: WF7316-08, Fresenius Kabi, Germany) were used for fluid replacement. When the hemoglobin (Hb) was ≤ 90 mg/ml, red blood cells were infused.
Thoracotomy was performed at the midline, and the internal mammary artery and saphenous vein of appropriate length were separated. Intravenous heparin (1.0 mg/kg) was administered and the activated clotting time (ACT) was controlled at > 300 s. A heart stabilizer was placed and vascular anastomosis was performed after placement of a split bolt at the blood vessels undergoing anastomosis. The lateral wall of blood vessels was clamped to reduce the diameter by 2/3 followed by distal anastomosis. Heparin was administered intravenously at 1.0 mg/kg and nasopharyngeal temperature was maintained at 35–37° by using a heating pad.
The Cardiac Index (CI) was measured by cannulating the left radial artery with a standard arterial catheter, and the first measurement was carried out under partial anesthesia. A FloTracTM-sensor was connected directly to the arterial line and a Vigileo monitor (Edwards, Irvine, CA, USA) was used to measure CI at five time points: pre-operation (baseline), after anesthesia, after 15 min sevoflurane, after the operation, and 12 h after the operation. Measurements at these times included mean arterial pressure (MAP), heart rate (HR), cardiac output (CO), stroke volume (SV), CI, and stroke volume index (SVI).
Sample collection
Before anesthesia, and immediately postoperatively, 24, 48, and 72 h after surgery arterial blood samples were collected. The samples were immediately centrifuged and the supernatant was collected and stored at − 80°C until use. Assays were performed within 2 months of blood collection. A double antibody sandwich ELISA method was employed to detect the levels of NT-BNP (lot #: 02828M500; Abbott Laboratories, USA) and cTnI (lot#: 021471; Beckman Coulter, Inc., USA) according to the manufacturer's instructions. The sensitivity of the BNP assay was 1.0 fmol/ml, and the sensitivity of the cTnI assay was 0.1 ng/ml. For both assays, the calculated overall intra-assay coefficient of variation was < 5%, and the calculated overall inter-assay coefficient of variation was < 10%.
Sample size calculation
The first 6 subjects were included in the pilot study. Based on the cTnI levels in the pilot study, the following formula was used for calculation of sample size in comparisons of multiple samples: 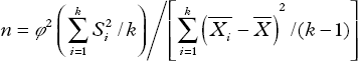 (where n is sample size, k is number of groups, and
(where n is sample size, k is number of groups, and ![]() and Si are the primarily estimated mean and standard deviation of sample I). The value for ϕ was obtained from the ϕ distribution table on the basis of α, β, ν1 = κ − 1, ν2 = ∞ followed by calculation of n1. The value for n2 was calculated on the basis of ν1 = κ − 1, ν2 = κ[n1 − 1] according to ϕ distribution table. The calculation was continued until the two n's were similar, and this n represents the number of samples sufficient for analysis (sample size).
and Si are the primarily estimated mean and standard deviation of sample I). The value for ϕ was obtained from the ϕ distribution table on the basis of α, β, ν1 = κ − 1, ν2 = ∞ followed by calculation of n1. The value for n2 was calculated on the basis of ν1 = κ − 1, ν2 = κ[n1 − 1] according to ϕ distribution table. The calculation was continued until the two n's were similar, and this n represents the number of samples sufficient for analysis (sample size).
According to the pilot study, the mean cTnI in the control group, 0.75 MAC group, 1.0 MAC group, and 1.5 MAC group was 0.610 nmol/L, 0.464 nmol/L, 0.300 nmol/L, and 0.193 nmol/L, respectively, and the standard deviation was 0.361 nmol/L, 0.199 nmol/L, 0.190 nmol/L, and 0.151 nmol/L, respectively. On the basis that ϕ1 = 2.17, ϕ2 = 2.33, and ϕ3 = 2.31, n1, n2, and n3 were calculated (n1 = 8, n2 = 9, and n3 = 9). Nine was present in both calculations, and thus the sample size was determined as 9. Considering that some patients might withdraw from the study, the sample size was increased by 20%. Finally, the sample size was determined as 12, and was identical among 4 groups.
Statistical analysis
Continuous and categorical variables were compared by one way analysis of variance (ANOVA) and Fisher's exact test, respectively. Data were described with means and standard deviations or number (percentage). If the continuous variable was non-normally distributed (skewed) data, non-parametric analysis (Kruskal–Wallis test) was used instead, and data were displayed as median (interquartile range). Ordinal data, cardiac function, and number of bypass grafts were compared using the Kruskal–Wallis test and data were displayed as number and percentage. When a significant difference between groups was apparent, multiple comparisons of means were performed using the Bonferroni procedure with type-I error adjustment. Myocardial wall motion data before and after surgery were examined using a paired t test. Because of the significantly different intra-operative dopamine usage among the 4 groups, repeated measurements controlling for intra-operative dopamine usage and including Bonferroni adjustment were used to determine the difference over time, and whether this difference over time was different between groups. The correlations between cTnI and BNP in each group at each time point were determined using the Pearson correlation coefficient. All statistical assessments were two-sided and evaluated at the 0.05 level of significant difference. Statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc., Chicago, IL, USA).
Results
The demographic and clinical characteristics of patients in the four groups are presented in . There were no significant differences in age, gender, height or weight, cardiac function, nitroglycerin and fentanyl usage, post-operative drainage, anesthesia or surgical periods, and intravenous fluid administration (all, p > 0.05). Dopamine usage increased as sevoflurane dosage increased (p = 0.006). However, no patients require adrenaline intra-operatively. There were no deaths.
Table I. Demographic and clinical characteristics of the four groups.
Vital signs were similar among groups at all times except for slight, although statistically significant, differences in nasopharyngeal temperature and diastolic blood pressure after anesthesia (p < 0.05) (data not shown). There were no significant differences among the four groups in the hemodynamic indicators measured (SV, SVI, CO, CI) at any time point ().
Table II. Hemodynamic status of the four groups at each time point.
Administration of sevoflurane decreased cTnI levels after surgery (), and this decrease was still apparent 72 h later. After controlling for intra-operative dopamine usage, there was a significant time effect in cTnI levels (p < 0.001). In addition, a significant difference over time in cTnI levels among the four groups was found (p < 0.001). The cTnI level in the control group was significantly higher than that in the 1 MAC and 1.5 MAC groups (both, p < 0.001). However, there was no significant difference in cTnI levels between the control and 0.75 MAC group, or between the 1 MAC and 1.5 MAC group (both, p > 0.05).
Table III. cTnI levels at each time point among the four groups.
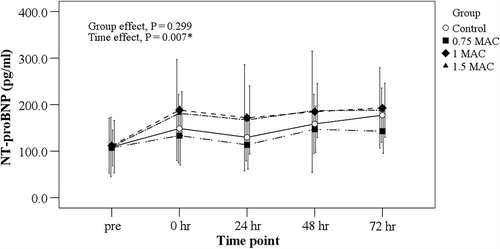
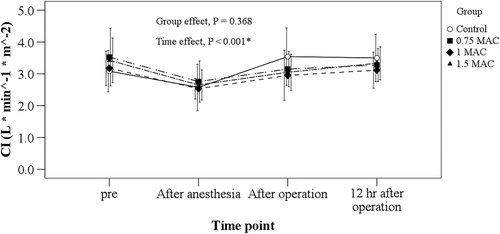
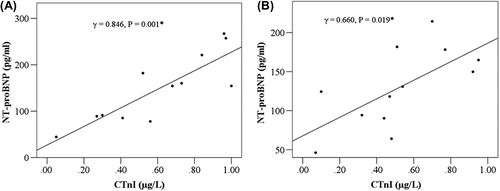
BNP levels in the control group were correlated with cTnI levels both immediately after and 24 h after surgery (r = 0.846, p = 0.001; r = 0.660, p = 0.019, respectively, ). BNP levels rose slightly with time after surgery in all four groups after controlling for intra-operative dopamine usage (p < 0.001), but sevoflurane administration did not cause a significant difference from control levels (p = 0.299, ). After controlling for intra- operative dopamine usage, the cardiac index (CI) levels rose slightly after surgery over time in all four groups (p < 0.001), but no significant difference was found among the four groups (p = 0.368, ).
Figure 1. Correlation between cTnI and BNP in control subjects. (A) Immediately after surgery in the control group. (B) 24 h after surgery in the control group.
Intra-operative data and adverse events of the four groups are presented in . There was a significant difference in the extubation time among the four groups (p = 0.023). In addition, patients in the 1.0 MAC group had significantly shorter extubation time than those in the control group (13.4 ± 2.7 h vs 19.9 ± 8.1 h, p = 0.017). No significant differences in adverse events (malignant arrhythmia, myocardial ischemia, myocardial infarction, death, and redo surgery) were found. The results of WMSI measurement are shown in and . There was a significant difference between pre- and post-operative WMSI in the 1.5 MAC group (1.6 ± 0.8 vs 1.1 ± 0.3, p = 0.026), but not in the other three groups. In addition, no significant difference in the change in WMSI before and after surgery among the four groups was found (p = 0.170, ).
Table IV. Intra-operative data and adverse events among the four groups.
Table V. The wall motion score index data before and after surgery.
Table VI. Change in wall motion score index before and after surgery among the four groups.
Discussion
Our results showed a 1 MAC dose of sevoflurane to be the optimal dose to provide myocardial protection during OPCABG; a lower dose (0.75 MAC) provided no significant protection and a higher dose (1.5 MAC) provided no additional protection. The data also showed BNP to be ineffective as a biomarker of ischemia/reperfusion injury in OPCABG, although it correlated well with cTnI levels in control subjects.
The 1 MAC dose, the optimal cardioprotective dose in our study, corresponds to the 2% sevoflurane dose most commonly used in other clinical studies of sevoflurane cardioprotection. However, clinical studies do not always use a strictly defined dose, and no dose-response studies have been reported. One in vivo animal study of sevoflurane protection against reperfusion injury showed a similarly narrow dose range in that 1 MAC, but not higher or lower doses, provided significant cardioprotection (Citation17). The narrow range of effectiveness of sevoflurane seen in our study is somewhat, but not completely, surprising. In our study, the use of 1.5 MAC sevoflurane necessitated an infusion of dopamine to maintain cardiac function. However, the presence of dopamine has been linked to an increase of cTnI (Citation18). It is possible that any decrease in cTnI that may have been the result of 1.5 MAC sevoflurane may have been offset by an increase in cTnI secondary to the dopamine infusion.
Sevoflurane has number of actions known to decrease ischemia/reperfusion injury, as well as actions that might increase this injury. Actions that decrease injury are (Citation1) a negative inotropic and chronotropic action that decreases oxygen consumption and thus lessens the effect of ischemia; (Citation2) an inhibition of neutrophil adhesion to vascular endothelium that decreases the inflammatory mediator-induced increase in reactive oxygen species; and (Citation3) an increase in reperfusion injury salvage kinases (RISKs) that decreases ischemia-caused apoptosis (Citation1). These protective mechanisms are counterbalanced by the fact that higher doses of sevoflurane depress cardiac function to a degree that has a negative effect on cardioprotection. Our results demonstrated the myocardial depressant effect of sevoflurane, as well as the cardioprotective effect. When sevoflurane was used, dopamine was required to maintain the target blood pressure, and the dopamine dose was increased with increase in sevoflurane concentration, although this increase did not reach statistical significance. The lack of statistical significance may be attributed to the small sample size. Sevoflurane not only exerts a suppressive effect on the myocardium requiring dopamine infusion, but also a vaso-dilative effect that could be treated with the same drug (Citation19,Citation20). Gravel et al. (Citation21) compared the hemodynamic effects of sevoflurane induction and maintenance supplemented with sufentanil with total intravenous anesthesia using midazolam and propofol in patients undergoing CABG and found that the hemodynamic responses in the two groups were comparable, but that intraoperative control of systemic blood pressure was achieved with fewer interventions in the sevoflurane group.
A number of studies have examined the effects of volatile anesthetics on cardiac complications in patients undergoing CABG. Yao et al. (Citation22) conducted a systematic review of randomized controlled trials comparing the myocardial protection profiles of sevoflurane and propofol in patients undergoing CABG and the analysis included 13 studies with 402 patients in the sevoflurane group and 294 in the propofol group. Patients in the sevoflurane group were found to have significantly higher post-bypass CI, lower troponin I level, lower incidence of myocardial ischemia, and shorter ICU and hospital stays. In another metaanalysis including 2,841 patients that received CABG, Yu et al. (Citation23) reported that sevoflurane and desflurane were associated with a reduced postoperative rise in cTnI and in one study the sevoflurane-mediated reduction in troponin was associated with improved long-term outcomes. However, the meta-analysis did not show that the positive effects of volatile anesthetic on troponin were associated with improved long-term clinical outcomes.
Our result showing a shorter time to extubation in the 1 MAC group than in the control group is similar to the work of others. Venkatesh et al. (Citation24) compared sevoflurane and isoflurane in OPCAB surgery and reported that time of awakening (48 ± 13 vs 114 ± 21 min; p < 0.001) and subsequent extubation (124 ± 25 vs 177 ± 36 min, p < 0.001) was earlier in sevoflurane group than in isoflurane group.
cTnI and BNP are two sensitive and specific biomarkers of myocardial injury and dysfunction. cTnI, a protein that regulates myocardiocyte contraction, is released by myocardial cells as a result of cardiac injury and can then be detected in peripheral blood. It has a high sensitivity and specificity for myocardial injury (Citation25), and is the “gold standard” for biochemical detection of myocardial damage. There have been a number of studies examining the relevance of cTnI levels in patients undergoing CABG. van Geene et al. (Citation26) reported that a cTnI level of > 4.25 μg/L measured within the first hour after cardiac surgery was 70% sensitive and 89% specific for predicting hospital morality. Similarly, Hashemzadeh et al. (Citation27) reported that cTnI measured 20 h after surgery was an independent predictor of in-hospital death after CABG. Amin et al. (Citation28) studied 160 consecutive patients with acute coronary syndrome who underwent CABG, and found that cTnI level measured shortly after admission to the emergency room was predictive of early and long-term adverse events after CABG. Tzimas et al. (Citation29) measured serial pre- and postoperative cTnI and CK-MB levels in patients who received CABG and found that in patients without postoperative cardiac events cTnI levels peaked at 24 h after surgery, whereas in patients with postoperative cardiac events cTnI levels peaked at 36 h after surgery, and that cTnI was superior to CK-MB in detecting postoperative cardiac events. In a study examining cTnI levels after different types of cardiac surgery (CABG, valve surgery, combined cardiac surgery), Fellahi et al. (Citation30) reported that cTnI levels were significantly different between the groups and that an elevated cTnI above a defined threshold in each group was significantly associated with a severe cardiac event and/or death.
BNP is released into peripheral blood in response to cardiomyocyte stretch. Changes in ventricular load and ventricular wall stress are the main factors stimulating the secretion of BNP (Citation31–33). In the presence of such stimulation, BNP synthesis is markedly increased resulting in an increase in serum BNP level (Citation34–36). BNP levels reached a peak immediately after surgery, then decreased within 24 h and subsequently gradually increased. The stimulation of intra-operative manipulation was stronger than any effect induced by sevoflurane. Therefore, BNP cannot be used as an indicator of the myocardial protection of sevoflurane in this type of surgery.
There are some limitations to this study, of which the first is the small sample size. In addition, the Vigileo instrument is not considered the “gold-standard” of invasive measurement devices. It is not reliable in hypothermia, and there is debate regarding its reliability for the measurement of cardiac output in cardiac surgery.
Conclusions
In conclusion, 1.0 MAC sevoflurane gives optimal myocardial protection when used for maintenance in OPCABG. In addition, BNP alone cannot be used to predict the myocardial protective effect of sevoflurane in OPCABG.
Acknowledgments
None.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Frabdorf J, De Hert S, Schlack W. Anesthesia and myocardial ischemia/reperfusion injury. Br J Anaeth. 2009;103:89–98.
- Lucchinetti E, Hofer C, Bestmann L, Hersberger M, Feng J, Zhu M, et al. Gene regulatory control of myocardial energy metabolism predicts postoperative cardiac function in patients undergoing off-pump coronary artery bypass graft surgery. Anesthesiology. 2007;106:444–57.
- Ceyhan D, Tanriverdi B, Bilir A. Comparison of the effects of sevoflurane and isoflurane on myocardial protection in coronary bypass surgery. Anadolu Kardiyol Derg. 2011; 11:257–62.
- De Hert SG, Cromheecke S, ten Broecke PW, Mertens E, De Blier IG, Stockman BA, et al. Effects of sevoflurane, desflurane, and propofol on recover of myocardial function after coronary surgery in elderl high-risk patients. Anesthesiology. 2003;99:314–23.
- De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, ten Broecke PW, De Blier IG, et al. Choice of primary anesthetic regimen can influence intensive care unit stay after coronary surgery with cardiac bypass. Anesthesiology. 2004; 101:9–20.
- Piriou V, Mantz J, Goldfarb G, Kitakaze M, Chiari P, Paquin S, et al. Sevoflurane preconditioning at 1 MAC only provides limited protection in patients undergoing coronary artery bypass surgery: a randomized bi-centre trial. Brit J Anaesth. 2007;99:624–31.
- Zitta K, Meybohm P, Bein B, Ohnesorge H, Steinfath M, Scholz J, et al. Cytoprotective effects of the volatile anesthetic sevoflurane are highly dependent on timing and duration of sevoflurane conditioning: findings from a human, in-vitro hypoxia model. Eur J Pharmacol. 2010;645:39–46.
- Bignami E, Landoni G, Gerli C, Testa V, Mizzi A, Fano G, et al. Sevoflurane vs. propofol in patients with coronary disease undergoing mitral surgery: a randomised study. Acta Anaesthesiol Scand. 2011;56:482–90.
- Cromheecke S, Pepermans V, Hendrickx E, Lorsomradee S, Ten Broecke PW, Stockman BA, et al. Cardioprotective properties of sevoflurane in patients undergoing aortic valve replacement with cardiopulmonary bypass. Anesth Analg. 2006;103:289–96.
- Conzen PF, Fischer S, Deter C, Peter K. Sevoflurane provides greater protection of the myocardium than propofol in patients undergoing off-pump coronary artery bypass surgery. Anesthesiology. 2003;99:826–33.
- Bein B, Renner J, Caliebe D, Scholz J, Paris A, Fraund S, et al. Sevoflurane but not propofol preserves myocardial function during minimally invasive direct coronary artery bypass surgery. Anesth Analg. 2005;100:610–6.
- Nordenskjöld AM, Ahlström H, Eggers KM, Fröbert O, Jaffe AS, Venge P, et al. Short- and long-term individual variation in cardiac troponin in patients with stable coronary artery disease. Clin Chem. 2012 Nov 26. [Epub aheCad of print].
- Røsjø H, Kravdal G, Høiseth AD, Jørgensen M, Badr P, Røysland R, et al. Troponin I measured by a high-sensitivity assay in patients with suspected reversible myocardial ischemia: data from the Akershus Cardiac Examination (ACE) 1 Study. Clin Chem. 2012;58:1565–73.
- McQueen MJ, Kavsak PA, Xu L, Shestakovska O, Yusuf S. Predicting myocardial infarction and other serious cardiac outcomes using high-sensitivity cardiac troponin T in a high-risk stable population. Clin Biochem. 2012 Oct 9. [Epub ahead of print].
- Berendes E, Schmidt C, Van Aken H, Hartlage MG, Rothenburger M, Wirtz S, et al. A-type and B-type natriuretic peptides in cardiac surgical procedures. Anesth Analg. 2004;98:11–9.
- Prabhakar H, Ali Z, Bithal PK, Rath GP, Singh D, Dash HH. Isoflurane and sevoflurane decrease entropy indices more than halothane at equal mac values. J Anesth. 2009;23:154–7.
- Obal D, Preckel B, Scharbatke H, Müllenheim J, Höterkes F, Thämer V, et al. One MAC of sevoflurane provides protection against reperfusion injury in the rat heart in vivo. Br J Anaesth. 2001;87:905–11.
- Wiese AJ, Barter LS, Ilkiw JE, Kittleson MD, Pypendop BH. Cardiovascular and respiratory effects of incremental doses of dopamine and phenylephrine in the management of isoflurane-induced hypotension in cats with hypertrophic cardiomyopathy. Am J Vet Res. 2012;73:908–916.
- Iida H, Ohata H, Iida M, Watanabe Y, Dohi S. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology. 1998;89:954–60.
- Larach DR, Schuler HG. Direct vasodilation by sevoflurane, isoflurane, and halothane alters coronary flow reserve in the isolated rat heart. Anesthesiology. 1991;75:268–78.
- Gravel NR, Searle NR, Taillefer J, Carrier M, Roy M, Gagnon L. Comparison of the hemodynamic effects of sevoflurane anesthesia induction and maintenance vs TIVA in CABG surgery. Can J Anaesth. 1999;46:240–6.
- Yao YT, Li LH. Sevoflurane versus propofol for myocardial protection in patients undergoing coronary artery bypass grafting surgery: a meta-analysis of randomized controlled trials. Chin Med Sci J. 2009;24:133–41.
- Yu CH, Beattie WS. The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can J Anaesth. 2006;53:906–18.
- Venkatesh BG, Mehta Y, Kumar A, Trehan N. Comparison of sevoflurane and isoflurane in opcab surgery. Ann Card Anaesth. 2007;10:46–50.
- Gerhardt W, Katus H, Ravkilde J, Hamm C, Jørgensen PJ, Peheim E, et al. S-troponin T in suspected ischemic myocardial injury compared with mass and catalytic concentrations of S-creatine kinase isoenzyme MB. Clin Chem. 1991;37: 1405–11.
- van Geene Y, van Swieten HA, Noyez L. Cardiac troponin I levels after cardiac surgery as predictor for in-hospital mortality. Interact Cardiovasc Thorac Surg. 2010;10: 413–6.
- Hashemzadeh K, Dehdilani M. Postoperative cardiac troponin I is an independent predictor of in-hospital death after coronary artery bypass grafting. J Cardiovasc Surg (Torino). 2009;50:403–9.
- Amin AP, Mukhopadhyay E, Napan S, Mamtani M, Kelly RF, Kulkarni H. Value of early cardiac troponin I to predict long-term adverse events after coronary artery bypass graft surgery in patients presenting with acute coronary syndromes. Clin Cardiol. 2009;32:386–92.
- Tzimas PG, Milionis HJ, Arnaoutoglou HM, Kalantzi KJ, Pappas K, Karfis E, et al. Cardiac troponin I versus creatine kinase-MB in the detection of postoperative cardiac events after coronary artery bypass grafting surgery. J Cardiovasc Surg (Torino). 2008;49:95–101.
- Fellahi JL, Hedoire F, Le Manach Y, Monier E, Guillou L, Riou B. Determination of the threshold of cardiac troponin I associated with an adverse postoperative outcome after cardiac surgery: a comparative study between coronary artery bypass graft, valve surgery, and combined cardiac surgery. Crit Care. 2007;11:R106.
- Sudoh T, Maekawa K, Kojima M, Minamino N, Kangawa K, Matsuo H. Cloning and sequence analyhsis encoding a precursor for human brain natriuretic peptide. Biochem Biophys Res Commun. 1989;159:1427–34.
- LaPointe MC. Molecular regulation of the brain natriuretic peptide gene. Peptides. 2005;26:944–56.
- Seilhamer JJ, Arfsten A, Miller JA, Lundquist P, Scarborough RM, Lewicki JA, et al. Human and canine gene homologs of porcine brain natriuretic peptide. Biochem Biophys Res Commun. 1989;165:650–8.
- Onuoha GN, Nicholls DP, Patterson A, Beringer T. Neuropeptide secretion in exercise. Neuropeptides. 1998; 32:319–25.
- Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203.
- Bruneau BG, de Bold AJ. Selective changes in natriuretic peptide and early response gene expression in isolated rat atria following stimulation by stretch or endothelin-1. Cardiovasc Res. 1994;28:1519–25.