Abstract
Objectives. Platelet-to-lymphocyte ratio (PLR) provides a simple method for assessment of inflammatory status. The aim of the present study was to investigate the predictive value of preprocedural PLR on development of in-stent restenosis in patients undergoing bare-metal stent (BMS) implantation. Design. Six hundred and seventy-five consecutive patients (mean age: 60.6 ± 8.3, 66% men) who had undergone successful BMS implantation and additional coronary angiography for stable or unstable angina pectoris were analyzed. Mean period between 2 coronary angiographies was 14.3 ± 3.4 months. Results. Patients were divided into tertiles based on preprocedural PLR. Restenosis occurred in 58 patients (26%) in the lowest tertile, in 82 (36%) in the middle tertile, and in 115 (51%) in the highest tertile (p < 0.001). Serum C-reactive protein levels were also significantly higher in patients in tertile 3 than in those in tertiles 1 and 2 (p < 0.001). Smoking, diabetes mellitus, high-density lipoprotein, stent length, preprocedural PLR, and C-reactive protein levels emerged as independent predictors of in-stent restenosis. In receiver-operating characteristics curve analysis, PLR >122 had 81% sensitivity and 72% specificity in predicting in-stent restenosis. Conclusions. High preprocedural PLR is a powerful and independent predictor of BMS restenosis in patients with stable and unstable angina pectoris.
Introduction
Inflammatory processes play an important role not only in initiation and progression of atherosclerosis, but also in development of stent restenosis (Citation1,Citation2). Despite major advances in interventional techniques and drug therapies, in-stent restenosis remains a major problem in interventional cardiology (Citation3). Vascular responses after percutaneous coronary intervention (PCI)-induced endothelial injury are characterized by the sequence of inflammation, granulation, extracellular matrix remodeling, and smooth muscle cell (SMC) proliferation and migration, which leads to neointimal hyperplasia and restenosis (Citation4,Citation5). Besides technical and mechanical conditions associated with PCI, inflammatory status before and after stent implantation is a significant risk factor for in-stent restenosis (Citation6).
Platelets secrete proinflammatory substances such as chemokines and cytokines that mediate vascular inflammation after being activated by substances released from cells of the vascular wall. Platelet– lymphocyte ratio (PLR) is a novel biomarker showing inflammation in cardiac and non-cardiac patients (Citation7). Previous studies demonstrated an association between major adverse cardiovascular outcomes with higher platelet and lower lymphocyte counts (Citation8,Citation9). The aim of the present study was to evaluate the usefulness of PLR before successful bare-metal stent (BMS) implantation in predicting in-stent restenosis in patients with stable and unstable angina pectoris.
Methods
We retrospectively analyzed clinical, laboratory, and angiographic data from consecutive patients who underwent successful BMS implantation from January 2010 to December 2012 at Türkiye Yüksek Ihtisas Educational and Research Hospital (Ankara, Turkey). Patients who underwent coronary angiography because of stable or unstable angina pectoris and succesful BMS implantation were included in this study. For patients’ data, we retrospectively gained access to the records at the time of interest, when the patients underwent BMS implantation during coronary angiographybecause of clinical indications, including symptoms of angina and abnormal non-invasive test results (either treadmill exercise tests or myocardial perfusion scintigraphy), thus recalling clinical, angiographic, and laboratory characteristics at that time. As part of our preprocedural protocol, complete hemogram analysis and peripheral differential counts had already been available before coronary angiography for all patients. Patients were excluded from study if they had clinical evidence of cancer, chronic inflammatory diseases, hepatic or hemolytic disorders, rheumatologic diseases, or any active infectious diseases. Finally, 675 patients were enrolled in this study.
Patients’ clinical and demographic characteristics, encompassing age, gender, history of arterial hypertension, diabetes mellitus, tobacco use, and the left ventricular ejection fraction, were noted. In addition, serum levels of fasting blood glucose, hemoglobin, C-reactive protein (CRP), and a lipid panel including low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were also recorded. The local ethics committee approved the study protocol.
All laboratory data were obtained from venous blood samples up to 6 h before stent implantation. Total platelet, lymphocyte, and monocyte counts were calculated using an automated blood cell counter (ADVIA 2120i Hematology System, Siemens Healthcare Diagnostics, Deerfield, IL). PLR was calculated as the preprocedural ratio of platelets to lymphocytes obtained from the same blood samples. CRP was measured by an immunonephelometric method (Roche Diagnostics GmbH, Marburg, Germany).
Coronary interventions were performed according to current practice guidelines and recorded in digital storage for further analysis (Citation10). The degree of coronary stenosis was visually estimated by experienced interventional cardiologists. Luminal narrowing > 50% in a major subepicardial vessel (left anterior descending, left circumflex, or right coronary artery) was defined as significant stenosis. Each patient received aspirin plus clopidogrel (loading dose 300 or 600 mg) before or during coronary intervention. Unfractionated heparin (100 U/kg) was administered at the beginning of the procedure to keep the activated clotting time > 200 s. The access site for intervention was at the physician's preference (femoral or radial). Use of glycoprotein IIb/IIIa inhibitors and predilatation or postdilatation after stent implantation of the lesion was at the operator's discretion. A successful PCI was previously defined as the achievement of a minimum stenosis diameter reduction to < 50% in the presence of grade-3 Thrombolysis In Myocardial Infarction (TIMI) flow (assessed by angiography) without side branch loss, flow-limiting dissection, or angiographic thrombus (Citation11). We also defined successful PCI as a reduction of stenosis diameter to lower than 50% narrowing at final angiography.
During routine clinical follow-up, coronary angiography was performed on clinical indications in patients with stable or unstable angina pectoris. Control coronary angiograms were recorded using the Judkins technique and interpreted by two independent cardiologists who were blinded to patients’ data. The evaluation of stenosis was carried out using the conventional visual assessment technique. Stent restenosis was accepted as narrowing > 50% in a vessel of otherwise normal diameter, including 5 mm proximal and distal to the stent edge. Intra- and interobserver variability of stent restenosis analysis was minimal in a representative subset of 80 patients. The interpretations of the two investigators on the presence or absence of in-stent restenosis agreed in 95% (76 of 80) and 96% (77 of 80), respectively. Intraobserver variability was assessed by one investigator. The two readings were concordant for the presence or absence of in-stent restenosis in 96% (77 of 80) and 97.5% (78 of 80), respectively.
Analyses were performed using SPSS 20.0 (SPSS, Inc., Chicago, IL). Continuous data were presented as median and interquartile range or mean ± SD. To test the distribution pattern, the Kolmogorov–Smirnov test was used. The study population was assigned into tertiles based on PLRs at admission. Comparisons of multiple mean values were carried out by Kruskal–Wallis tests or analysis of variance, as appropriate. Categorical variables were summarized as percentages and compared with chi-square test. Spearman correlation coefficient was computed to examine the association between two continuous variables. Effects of different variables on in-stent restenosis were calculated in univariate analysis for each. Variables for which the unadjusted p value was < 0.10 in logistic regression analysis were identified as potential risk markers and included in the full model. We reduced the model using stepwise multivariate logistic regression analyses and eliminated potential risk markers using likelihood ratio tests. A p value < 0.05 was considered statistically significant and the confidence interval was 95%. An exploratory evaluation of additional cut points was performed using receiver-operating characteristics curve analysis. A two-sided p value < 0.05 was considered statistically significant.
Results
The baseline clinical and procedural characteristics of the study population are summarized in . A total of 675 patients (mean age: 60.6 ± 8.3 years, 66% men) were divided into tertiles according to preprocedural PLR: 94 ± 41 in tertile 1, 115 ± 54 in tertile 2, and 147 ± 58 in tertile 3. Each group was composed of 225 patients. The mean period between the two coronary angiographic studies for all study population was 14.3 ± 3.4 months.
Table I. Baseline characteristics of patients according to preprocedural PLR tertiles.
Patients in tertile 3 had significantly higher rates of in-stent restenosis compared with those in tertiles 1 and 2 (p < 0.001, ). A positive correlation was observed between PLR and CRP levels (r = 0.35, p < 0.001). Higher PLRs before stent deployment were associated with an increased in-stent restenosis by logistic regression analysis. Diabetes mellitus, smoking, high-density lipoprotein cholesterol, stent length, and preprocedural PLR and CRP levels were found to be independent predictors of in-stent restenosis (). Receiver-operating characteristic curve analysis was used to explore the relation between preprocedural PLR and in-stent restenosis. The area under the curve was 0.82 (95% confidence interval: 0.77–0.91, p < 0.001). Preprocedural PLR with a cut-off level of > 122 predicted in-stent restenosis with sensitivity of 81% and specificity of 72% ().
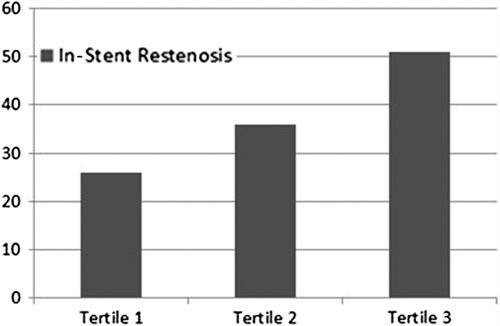
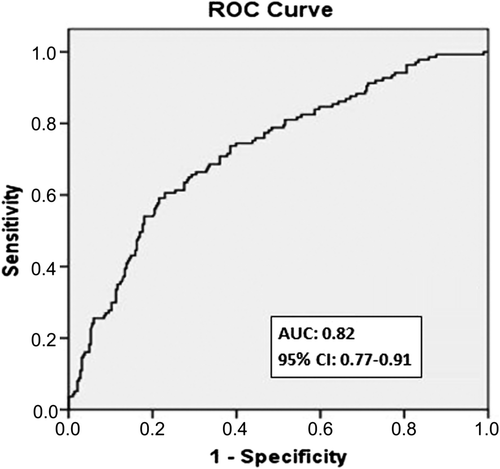
Table II. Odds ratio (OR) for in-stent restenosis in univariate and multivariate logistic regression analysis.
Discussion
In the present study, we investigated the relationship between preprocedural PLR and development of in-stent restenosis. We demonstrated that PLR is a powerful and independent predictor of in-stent restenosis in patients undergoing successful BMS implantation. Patients in the highest tertile of PLR were at greater risk, and PLR > 122 measured before stent placement had an 81% sensitivity and a 72% specificity in predicting in-stent restenosis.
Adverse reactions to BMS implantation include stent thrombosis and restenosis. Although thrombosis in BMSs mainly seems to be affected by procedural factors, restenosis is a more complex procedure involving mechanisms of tissue repair after vessel injury occurring during implantation (Citation12). The cellular response to mechanical vascular damage initiated immediately after PCI includes an early phase consisting of platelet activation and inflammation, followed by an intermediate phase of granulation tissue corresponding to SMC migration and proliferation, a late phase of tissue remodeling (neointima), and finally progression to in-stent restenosis (Citation2). Although the exact causative mechanisms remain to be completely elucidated, pre- and postprocedural inflammatory status seem to be the most important risk factors for in-stent restenosis (Citation13). Several inflammatory biomarkers have been investigated in the setting of coronary stenting to stratify the risk of both angiographic and clinical outcomes. CRP is the most widely studied biomarker in patients undergoing PCI, and represents a sensitive marker of systemic inflammation. A meta-analysis of nine studies showed that preprocedural CRP levels were a significantly predictor of in-stent restenosis after BMS placement (Citation14). Many other proinflammatory biomarkers, including fibrinogen (Citation15), interleukin-1 (Citation15), neutrophil–lymphocyte ratio (Citation16), lipoprotein a (Citation17), interleukin-6 (Citation17), and soluble CD40 ligand (Citation18) have also been found to be related to preprocedural inflammatory status and prediction of in-stent restenosis.
Previous studies have demonstrated that higher platelet and lower lymphocyte counts are associated with poor clinical outcomes in various cardiovascular diseases (Citation8,Citation19–21). The PLR, as an independent predictor, merges the predictive risk of platelet and lymphocyte counts into a single risk factor. It has been found that a higher PLR is associated with severity and complexity of coronary artery disease (Citation22), poor coronary collateral devolepment (Citation23), no re-flow after primary PCI (Citation24), non-dipper hypertension (Citation25), and occlusive peripheral arterial disease (Citation26). A recent study by Temiz et al. showed that in patients with ST-elevated myocardial infarction, in-hospital mortality was increased in the high PLR group when compared with that in the low PLR group (12.7% vs. 5.9%, p = 0.004) (Citation27). In addition, Azab et al. showed that higher PLR is a significant independent marker of long-term mortality in patients with non-ST elavated myocardial infarction (Citation28). To the best of our knowledge, we have for the first time demonstrated an association of PLR with in-stent restenosis in patients presenting with stable or unstable angina pectoris. According to our findings, higher PLR values on admission were significantly associated with in-stent restenosis during follow-up.
The results of our study should be interpreted in light of several limitations. First, the study was designed in a retrospective manner and represents a single-center experience with only BMS restenosis. Second, the definition of in-stent restenosis was based on visual assessment rather than more quantitative and informative intravascular ultrasound or optical computed tomographic results. Third, a single blood sample obtained in the preprocedural period may not be representative for the long-term PLR.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43.
- Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc Interv 2011;4:1057–66.
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol 2010;56:1897–907.
- Liu MW, Roubin GS, King SB III. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–87.
- Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–76.
- Khouzam RN, Shaheen M, Aziz RK, Ibebuogu UN. The important role of inflammatory biomarkers pre and post bare–metal and drug–eluting stent implantation. Can J Cardiol. 2012;28:700–5.
- Dotsenko O, Chaturvedi N, Thom S, Wright A, Mayet J, Shore A, et al. Platelet and leukocyte activation, atherosclerosis and inflammation in European and South Asian men. J Thromb Haemost. 2007;5:2036–42.
- Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial). Am J Cardiol. 2007;99:1055–61.
- Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79:812–4.
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Catheter Cardiovas Interv. 2012;79:453–95.
- Smith Jr S, Dove JT, Jacobs A. Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty: ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PCI guidelines): executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty). J Am Coll Cardiol. 2001;37:2215–38.
- Niccoli G, Montone RA, Ferrante G, Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J Am Coll Cardiol. 2010;56:1783–93.
- Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9:53–62.
- Ferrante G, Niccoli G, Biasucci LM, Liuzzo G, Burzotta F, Galiuto L, et al. Association between C-reactive protein and angiographic restenosis after bare metal stents: an updated and comprehensive meta-analysis of 2747 patients. Cardiovasc Revasc Med. 2008;9:156–65.
- Pietersma A, Kofflard M, de Wit LE, Stijnen T, Koster JF, Serruys PW, et al. Late lumen loss after coronary angioplasty is associated with the activation status of circulating phagocytes before treatment. Circulation. 1995;91:1320–5.
- Turak O, Ozcan F, Isleyen A, Tok D, Sokmen E, Buyukkaya E, et al. Usefulness of the neutrophil-to-lymphocyte ratio to predict bare-metal stent restenosis. Am J Cardiol. 2012;110:1405–10.
- Rahel BM, Visseren FL, Suttorp MJ, Plokker TH, Kelder JC, de Jongh BM, et al. Preprocedural serum levels of acute-phase reactants and prognosis after percutaneous coronary intervention. Cardiovasc Res. 2003;60:136–40.
- Türker S, Güneri S, Akdeniz B, Özcan MA, Baris N, Badak Ö, et al. Usefulness of preprocedural soluble CD40 ligand for predicting restenosis after percutaneous coronary intervention in patients with stable coronary artery disease. Am J Cardiol. 2006;97:198–202.
- Iijima R, Byrne RA, Ndrepepa G, Braun S, Mehilli J, Berger PB, et al. Pre-procedural C-reactive protein levels and clinical outcomes after percutaneous coronary interventions with and without abciximab: pooled analysis of four ISAR trials. Heart. 2009;95:107–12.
- Vidwan P, Lee S, Rossi JS, Stouffer GA. Relation of platelet count to bleeding and vascular complications in patients undergoing coronary angiography. Am J Cardiol. 2010;105:1219–22.
- Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000;86:449–51.
- Kurtul A, Murat SN, Yarlioglues M, Duran M, Ergun G, Acikgoz SK, et al. Association of latelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol. 2014;114:972–8
- Açar G, Kalkan ME, Avci A, Alizade E, Tabakci MM, Toprak C, et al. The relation of platelet–lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris and chronic total occlusion. Clin Appl ThrombHemost. 2013:1076029613508599.
- Kurtul A, Yarlioglues M, Murat SN, Ergun G, Duran M, Kasapkara HA, et al. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow following primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am J Cardiol. 2014;114:342–7
- Sunbul M, Gerin F, Durmus E, Kivrak T, Sari I, Tigen K, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens. 2013;36:1–5.
- Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013;8:e67688.
- Temiz A, Gazi E, Güngör Ö, Barutçu A, Altun B, Bekler A, et al. Platelet/lymphocyte ratio and risk of in-hospital mortality in patients with ST-elevated myocardial infarction. Med Sci Monit. 2014;20:660–5.
- Azab B, Shah N, Akerman M, McGinn JT Jr. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. 2012;34:326–34.
