Abstract
Background We sought to evaluate left atrial (LA) and right atrial (RA) phasic function and deformation in the subclinical hyperthyroidism (SCH) using two- and three-echocardiographic (2DE and 3DE) methods. Methods We included 45 untreated women with SCH and 45 healthy women who underwent comprehensive 2DE and 3DE examination. Results Total and passive LA emptying fractions (EF) were decreased, whereas active EF was increased among the SCH participants. RA total EFs were similar between the controls and the SCH subjects; passive EF was reduced; and active EF was amplified in the SCH group. TSH correlated with 2DE LA passive and active EFs, 3DE LA total, passive and active EFs, as well as 2DE LA positive longitudinal strain. Additionally, TSH correlated with 2DE RA passive and active EFs, 3DE LA and RA passive EF, 2DE LA and RA positive longitudinal strain. However, after adjustment for the parameters of left and right ventricular diastolic function and structure, the TSH level remained associated only with LA conduit and booster pump functions, as well as RA pump function. Conclusion Biatrial phasic function evaluated by 2DE and 3DE is significantly impaired in the SCH subjects. TSH level correlates with LA and RA conduit and pump functions.
Introduction
The influence of subclinical thyroid disorders on cardiovascular morbidity and mortality still represents a topic of debate. Recently published large studies have shown that subclinical hyperthyroidism (SCH) increased all-cause mortality, risk of new atrial fibrillation onset, risk of major adverse cardiovascular events, especially due to the increased risk of heart failure.[Citation1,Citation2] On the other side, Cappola et al.[Citation3] reported no relationship between SCH and the risk of coronary heart disease, cerebrovascular disease, or cardiovascular or all-cause mortality; but the authors still demonstrated the association between SCH and atrial fibrillation occurrence.
The role of atrial remodeling in SCH has not been enlightened yet, although it would be of clinical relevance to clarify the pathophysiology of atrial fibrillation and heart failure development in these subjects. There is only one study that investigated phasic atrial function in patients with overt hyperthyroidism.[Citation4] The authors used traditional two-dimensional echocardiographic (2DE) methods for evaluation of left atrial (LA) phasic function – volumetric method and revealed left atrial dysfunction.[Citation4] Nacar et al.[Citation5] investigated the subjects with SCH and did not find any difference between the left and the right atrial volumes. Both groups of authors found inter-, right and left intra-atrial electromechanical delay in the subjects with hyperthyroidism (overt and subclinical).[Citation4,Citation5] Right atrial (RA) phasic function and mechanics have not been investigated in the SCH subjects so far.
The aim of the present study was to investigate left and right atrial remodeling in the SCH subjects using comprehensive two- and three-dimensional echocardiography, including two-dimensional speckle tracking analysis.
Methodology
The investigation involved 45 female individuals with untreated subclinical hyperthyroidism and 45 healthy female controls of similar age. The participants were consequently included in the study. The study was performed between January 2011 and May 2015. The inclusion criteria were the decreased serum TSH level (<0.4 μIU/ml) with normal levels of T4 and FT4 in at least two separate measurements taken 3-6 months apart. According to their TSH concentrations, all SCH participants were classified in two groups: those with slightly low values (>0.1 to 0.4 μIU/ml) - SCH Citation1 group; and those with low values (<0.1 μIU/ml) - SCH Citation2 group. Subjects with heart failure, coronary artery disease, previous cerebrovascular insult, atrial fibrillation, congenital heart disease, valvular heart disease, arterial hypertension, obesity, asthma, chronic obstructive lung disease, neoplastic disease, cirrhosis of the liver, kidney failure or endocrine diseases, including type 2 diabetes mellitus, were excluded from the study.
Anthropometric measures (height, weight), smoking habits and laboratory analyses (the level of thyroid hormones, total cholesterol and triglycerides) were taken from all the subjects included in the study. Fasting venous blood samples were drawn between 08:00 and 09:00 O’clock in the morning. FT3 level was determined by IMMULITE 1000, a competitive analog based immunoassay; FT4 level was assessed by IMMULITE 2000 enzyme-labeled chemiluminescent competitive immunoassay; and TSH level was determined by using IMMULITE 2000, third generation TSH, two-site chemiluminescent immunometric assay. None of the participants used any medications before the inclusion in the study. Normal ranges for T3, T4, FT4, TSH, were 1.3-2.6 nmol/l, 58-161 nmol/l, 11.5-22.7 pmol/l, 0.4–4 μIU/ml, respectively.
Body mass index and body surface area were computed for each included subject. The local Ethics Committee approved the protocol, and informed consent was obtained from all the participants.
Echocardiography
A 2.5 MHz transducer was used for the echocardiographic investigation. 3D data set acquisition was obtained by the 3V matrix probe and a Vivid 7 ultrasound machine (GE Healthcare, Horten, Norway). Two investigators performed all echocardiographic acquisition and analysis.
Standard two-dimensional echocardiographic examination
All 2DE parameters were assessed as the average value of three consecutive cardiac cycles. The left ventricular end-systolic and end-diastolic diameters (LVEDD), LV posterior wall (PWT) and interventricular septum thickness were evaluated according to the current guidelines.[Citation6] Relative wall thickness was calculated using the formula: (2 × PWT)/LVEDD. LV ejection fraction was obtained by using the biplane method. Left ventricular mass was calculated by using the Penn formula,[Citation7] and indexed for height.[Citation2,Citation7]
Transmitral velocities were obtained in the apical 4-chamber view. Pulsed Doppler measurements involved the transmitral early and late diastolic peak flow velocity (E and A), their ratio (E/A) and deceleration time (DTm).[Citation8] The average of the peak early diastolic velocity (é) of the septal and lateral mitral annulus obtained by the tissue Doppler was computed, and the E/é ratio was calculated.
The parasternal long-axis view was used for the assessment of the RV internal diameter.[Citation9] The RV thickness was measured in the subcostal view.[Citation9] Tricuspid flow velocities were evaluated by the pulsed wave Doppler in the apical four-chamber view. The following parameters were determined: early and late diastolic peak flow velocity (Et and At), their ratio (E/A)t, and deceleration time (DTt). The RV myocardial velocities in diastole (ét) were obtained by tissue Doppler imaging,[Citation9] and tricuspid (E/é)t ratio was calculated.
The tricuspid annular plane systolic excursion (TAPSE) was used for the assessment of RV global systolic function.[Citation9] RV systolic blood pressure (SPAP) was evaluated in the subjects with tricuspid regurgitation according to the formula: SPAP = (tricuspid regurgitation velocity)2 + RA pressure.[Citation9] RA pressure was determined according to vena cava inferior (IVC) diameter. Thus, IVC diameter <2.1 cm that collapses >50% suggests normal RA pressure of 3 mmHg (range, 0-5 mmHg), whereas IVC diameter >2.1 cm that collapses <50% suggests high RA pressure of 15 mmHg (range, 10-20 mmHg).[Citation9]
2DE assessment of left atrial volumes and function
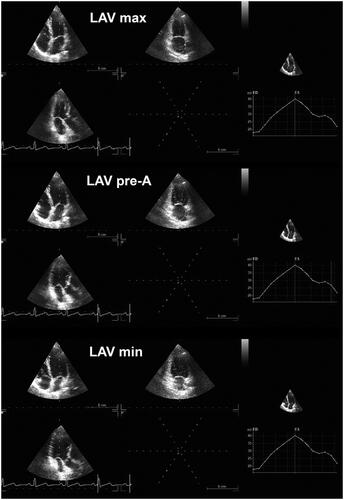
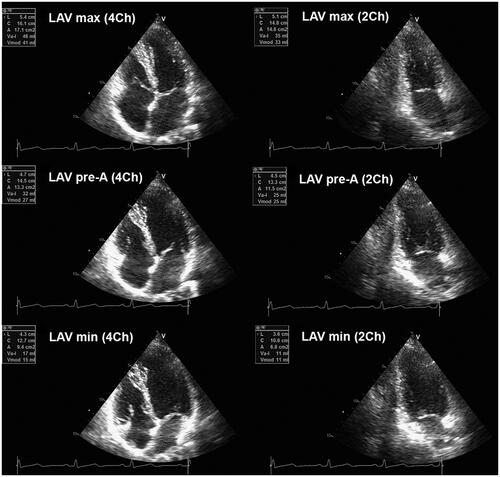
Left atrial (LA) volumes were evaluated in three different periods of the cardiac cycle in 4Ch- and 2Ch-views, as we have previously reported.[Citation10]: (i) maximal LA volume was assessed just before the mitral valve opening; (ii) pre-A (pre atrial contraction) LA volume was calculated at the beginning of atrial systole (peak of P wave in ECG); and (iii) minimal LA volume was evaluated at the mitral valve closure (). The biplane method was used for calculation of all LA volumes, and all values were indexed for BSA. The total emptying volume, indicator of the LA reservoir function, was estimated as the difference between maximum and minimum LA volume. The LA passive emptying volume, parameter of LA conduit function, was assessed as the difference between maximum and pre-A LA volume. The LA active emptying volume, which corresponds to LA booster function, was calculated as the difference between pre-A and minimum LA volume. Therefore, total emptying fraction was estimated as the ratio between total emptying volume and maximum LA volumes; passive emptying fraction was calculated as the ratio between passive and maximum LA volumes; whereas active emptying fraction was computed as the proportion between active and pre-A LA volumes.
Figure 1. Maximum, minimum, and pre-A left atrial volume (LAV) assessed by two-dimensional echocardiography.
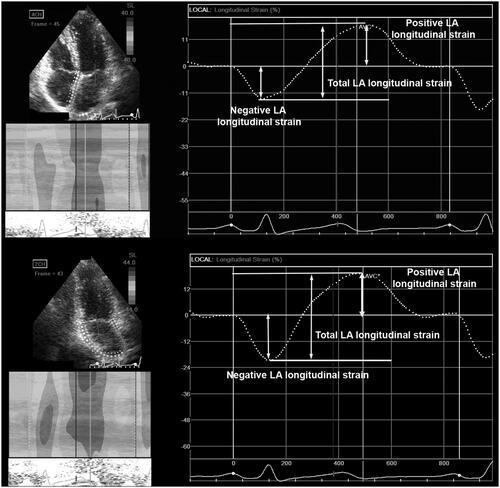
2DE strain imaging was done in the apical 4- and 2-chamber views,[Citation11] and EchoPAC 112 software (GE-Healthcare, Horten, Norway) was used for the 2DE strain analysis. LA strain was evaluated from the peak of P wave to the peak of the P wave in the next cardiac cycle. The software automatically generated an average longitudinal strain curve that included the negative and positive deflections. LA negative longitudinal strain expresses LA contraction, whereas LA positive strain shows LA filling. Their summation represents the total LA longitudinal strain. LA positive, negative and total strains were calculated as the average of the values assessed in 4- and 2-chamber apical views ().
2DE assessment of right atrial volumes and function
RA volumes were measured in the same way as LA volumes[Citation10]: (i) maximal RA volume was evaluated just before the tricuspid valve opening; (ii) pre-A RA volume was measured at the onset of atrial systole (peak of P wave in ECG); and (iii) minimal RA volume was determined at the tricuspid valve closure. Only 4-chamber view was used for determination of all RA volumes, and all parameters were indexed for body surface area. Active, passive and total RA volumes, together with the matching emptying fractions were computed using the same formula as for the LA.
2DE strain imaging was performed only in the apical 4-chamber view,[Citation9] and the same software for the strain analysis. Total, positive and negative RA longitudinal strain values were evaluated likewise as it was performed for LA strain evaluation.
3DE assessment of left and right atrial volumes and function
A full-volume acquisition of the entire heart required for further analyses was obtained. Six electrocardiogram-gated consecutive beats were acquired during end-expiratory breath-hold to generate full volume. Three-dimensional echocardiography LA and RA analyses were performed using LVQ software (EchoPAC 112, GE-Healthcare, Horten, Norway). The software provided maximum and minimum volumes, whereas pre-A atrial volume was acquired from the time-volume curve. Maximal LA and RA volumes were measured just before the mitral/tricuspid valve opening; pre-A LA and RA volumes were assessed at the onset of atrial systole (peak of P wave in ECG); whereas minimal LA and RA volumes were measured at the mitral/tricuspid valve closure ().
Statistical analysis
Continuous variables were presented as mean ± standard deviation and compared by the analysis of equal variance (ANOVA), as they showed normal distribution. The Bonferroni post hoc analysis was used for the comparison between different groups. The Kruskal-Wallis test was used in the case when the distribution was not normal, and the Mann–Whitney U-test was performed for the post hoc analysis. The differences in proportions were compared by using the χ2 test. The correlations were determined by Pearson’s correlation test, after TSH values were transformed by natural logarithm logarithmic in order to obtain normal distribution. The stepwise multiple regression analyses included age, BMI and parameters of LV and RV structure and diastolic function. Inter- and intraobserver variability was examined by using bivariate two-tailed correlations. The two-tailed p value <0.05 was considered statistically significant.
Results
Age, BMI, BSA, heart rate, prevalence of smoking and blood pressure were similar between the observed groups. T3 level was similar between the groups, while T4 and fT4 levels were higher in the SCH subjects, but still in normal range. TSH gradually decreased from the controls to the patients with low TSH level (). Triglycerides and total cholesterol levels were similar between the controls and the SCH participants.
Table 1. Demographic and clinical parameters of study population.
2DE left ventricular and atrial parameters
LV diameters were similar between the observed groups; interventricular septum thickness was increased in the patients with low TSH level in comparison with the controls; while relative wall thickness, LV mass and mass indexes gradually increased from the healthy subjects to the participants with low TSH level (). There is no difference between the SCH subjects with low and borderline TSH level. LV ejection fraction was similar between the observed groups (). Mitral E/A ratio gradually decreased, while mitral E/e' increased, from the healthy controls to the SCH subjects with low TSH. Mitral deceleration time was significantly increased in the SCH groups comparing with the controls ().
Table 2. Echocardiographic parameters of left and right ventricular structure and function in the study population.
RV diameter and wall thickness were similar among the observed groups. TAPSE, parameter of RV systolic function, was similar between the three observed groups (). Tricuspid E/A and E/e' ratios, parameters of RV diastolic function, were impaired in both SCH groups. However, there was no difference between the subjects with slightly low and low TSH (). Tricuspid deceleration time was significantly increased in the SCH groups compared with the controls (). RV systolic pressure was higher in the SCH subjects with low TSH compared with the control participants ().
2DE and 3DE left atrial phasic function and deformation
Maximal and minimal LA volumes indexed for BSA were higher in the SCH patients with low TSH level in comparison with the controls (). Pre-A LA volume indexed for BSA gradually and significantly increased from the control group to the subjects with low TSH level. Total LA volume was similar among the groups; passive LA volume progressively decreased; whereas active LA volume gradually increased from the healthy individuals to the patients with low TSH level. Interestingly, passive and active LA volumes were also different between the subjects with low and slightly low TSH level ().
Table 3. Echocardiographic parameters of left atrial function and mechanics in the study population.
The results obtained by 3DE were similar to the 2DE (). 3DE LA total and passive EFs were lower in the SCH subjects, whereas 3DE LA active EF was higher among the SCH participants (). Interestingly, only 3DE LA passive EF was different between the SCH subjects with borderline and low TSH level.
The two-dimensional speckle tracking analysis showed that total LA longitudinal strain was lower in the low TSH group in comparison with the controls, while positive LA longitudinal strain gradually decreased from the healthy controls to the subjects with low TSH (). Negative LA longitudinal strain was higher in both SCH groups than in the controls (). This demonstrates that LA conduit function is reduced in both SCH groups, though LA booster pump function was compensatorily increased in the SCH subjects.
2DE and 3DE right atrial phasic function and deformation
Two-dimensional maximal and minimal volumes indexed for BSA were higher in the SCH group with low TSH, while there was no difference between the subjects with low and slightly low TSH level (). Pre-contraction RA volume was significantly higher in the patients with low TSH in comparison with other two groups (). There was no important difference in total RA EF between the three groups. Passive RA EF gradually decreased, while active RA EF increased, from the healthy individuals to the patients with low TSH. Interestingly, there was no statistically significant difference between the healthy controls and the SCH subjects with borderline TSH level in most of 2DE and 3DE parameters.
Table 4. Echocardiographic parameters of right atrial function and mechanics in the study population.
Analogous results were obtained by 3DE. Maximal 3DE RA volume was similar among the three groups, minimal 3DE RA volume increased, while pre-A RA volume decreased from the control group to the patients with the lowest TSH level (). RA reservoir function assessed by 3DE was similar between the groups; conduit function was the lowest in the SCH subjects with low TSH; whereas RA pump function was the highest in these subjects.
The two-dimensional strain analysis demonstrated that RA reservoir function was reduced in the patients with low TSH level; while RA booster pump function was increased in these subjects (). RA conduit function, estimated by 2DE strain, gradually and significantly decreased from the controls to the subjects with low TSH level.
Correlation analyses
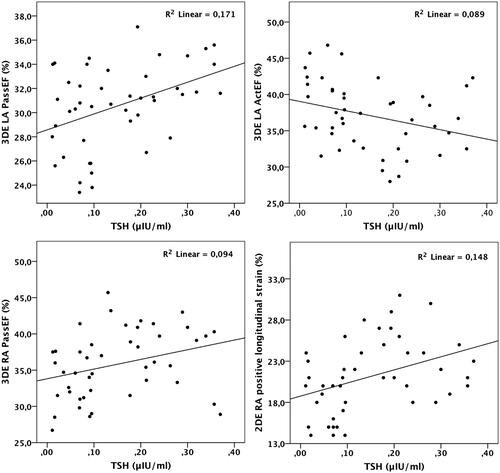
Among 2DE and 3DE LA parameters, the analyses showed that TSH correlated with 2DE LA passive emptying fraction (r=0.35, p=0.015), 2DE LA active emptying fraction (r=−0.31, p=0.038), 3DE LA passive emptying fraction (r=0.41, p<0.01), 3DE LA active emptying fraction (r=−0.30, p=0.047), 2DE LA positive longitudinal strain (r=0.39, p<0.01) (). After the adjustment for the left ventricular mass index and transmitral E/e', 2DE LA passive emptying fraction (β=0.3, p<0.01) and 2DE LA positive longitudinal strain (β=0.29, p<0.01) were associated with the TSH level in all the SCH subjects.
The analysis that included 2DE and 3DE parameters of the right atrium revealed that TSH correlated with 2DE RA passive emptying fraction (r=0.28, p=0.049), 2DE RA active emptying fraction (r=-0.30, p<0.01), 3DE RA passive emptying fraction (r=0.31, p=0.04), 2DE RA positive longitudinal strain (r=0.38, p<0.01) (). After the adjustment for the right ventricular thickness and tricuspid E/e', only 2DE RA positive longitudinal strain (β=0.33, p<0.01) was associated with the TSH level in all the SCH subjects.
Inter-observer variability
Pearson’s correlations: 3DE LA maximal volume: r=0.83, p<0.001; 3DE LA minimal volume: r=0.80, p<0.001; 3DE pre-A LA volume: r=0.70, p<0.001; 2DE LA longitudinal strain: r=0.85, p<0.001; 3DE RA maximal volume: r=0.79, p<0.001; 3DE RA minimal volume: r=0.72, p<0.001; 3DE pre-A RA volume: r=0.70, p<0.001; 2DE RA longitudinal strain: r=0.78, p<0.001.
Intra-observer variability
Pearson’s correlations: 3DE LA maximal volume: r=0.90, p<0.001; 3DE LA minimal volume: r=0.86, p<0.001; 3DE pre-A LA volume: r=0.77, p<0.001; 3DE LA longitudinal strain: r=0.92, p<0.001; 3DE RA maximal volume: r=0.85, p<0.001; 3DE RA minimal volume: r=0.80, p<0.001; 3DE pre-A RA volume: r=0.77, p<0.001; 2DE RA longitudinal strain: r=0.83, p<0.001.
Discussion
Our investigation has revealed several new findings: (i) LA and RA volumes estimated by 2DE and 3DE are increased in the SCH subjects; (ii) biatrial reservoir and conduit functions assessed by echocardiographic volumetric and speckle tracking methods are reduced, whereas booster pump function is amplified among the SCH participants; (iii) deterioration of LA and RA phasic function occurs gradually – from the control group, throughout individuals with slightly decreased TSH level, to the subjects with low TSH level. However, the progressive function decline is more pronounced for the LA.
It is very important to investigate the possible relationship between SCH and biatrial functional and mechanical changes. Sawin et al.[Citation12] reported that even patients with slightly low TSH level have higher risk of atrial fibrillation occurrence, which is why we decided to compare the patients with borderline and low TSH level.
Our findings revealed higher LA volumes in the patients with SCH, evaluated both with 2DE and 3DE. There is borderline difference in maximal LA volume, but minimal and pre-contraction LA volumes are significantly higher among the SCH participants. Ayhan et al.[Citation4] found no significant difference in maximal and minimal LA volumes between the healthy controls and the patients with overt hyperthyroidism, whereas pre-A LA volume was significantly increased in these patients. A possible reason for this difference could be a small sample size that precludes reaching statistical significance. Our results showed that conduit and reservoir LA functions were reduced, while LA pump function was increased in the SCH subjects. The same results were obtained by Ayhan et al.[Citation4] Other several studies that included only LA maximum volume showed no difference between the controls and the SCH subjects.[Citation5,Citation13] Interestingly, our study also revealed that subjects with borderline TSH level also have reduced LA conduit and reservoir functions in comparison with the healthy controls, but still better than the patients with low TSH level.
The advantage of our investigation is the concomitant usage of 2DE and 3DE examination that has enabled a detailed insight in atrial phasic function. The main advantage of 3DE atrial assessment is the ability to investigate atria from different planes and angles, which is especially important for the RA that is difficult for evaluation because of its unique position and geometry. Furthermore, 3DE enables assessment of all LA and RA volumes from only one data set, from the same cardiac cycle, which is not possible with 2DE. Another important benefit of 3DE usage over 2DE imaging is significantly better accuracy and reproducibility of volumetric estimation, comparable with computed tomography and magnetic resonance.[Citation14–16] Our findings revealed that 3DE LA and RA volumes were higher than 2DE counterparts, which was previously shown.[Citation17,Citation18] However, we showed that biatrial phasic functions assessed by total, passive and active emptying fractions were comparable between 2DE and 3DE technique.
The two-dimensional strain analysis in our study confirmed 2DE and 3DE LA volumetric analysis regarding reduced LA conduit and reservoir functions, and compensatory amplified LA pump function in the SCH subjects. To our knowledge, there is no similar investigation that used atrial speckle tracking analysis in this population of patients.
Right atrial volumes assessed by 2DE were increased among the SCH subjects in our research. 3DE analysis showed that RA reservoir function was similar between the groups; conduit function was reduced in the SCH groups; whereas pump function was increased in these patients. The RA phasic function (especially RA conduit function) gradually deteriorated from the controls, throughout the subjects with borderline TSH, to the individuals with low TSH. To our knowledge, Nacar et al.[Citation5] were the only authors who determined RA maximal volume in the SCH patients. They did not find any important difference between the SCH group and the controls, which agrees with our results. However, the researchers did not investigate other aspects of atrial phasic function.
Our results demonstrated that the TSH level correlated with LA and RA conduit and booster pump functions, assessed by 2DE and 3DE emptying fractions and 2DE strain. However, after adjustment for transmitral E/e' and left ventricular mass index, TSH remained associated only with LA conduit function. Results concerning RA were very similar.
There are several mechanisms that could explain biatrial remodeling in subclinical hyperthyroidism. First, the direct influence of thyroid hormones on atrial myocardium, primarily on sarcoplasmic reticulum calcium transporters, as it was described in the animal model.[Citation19] Second, diastolic dysfunction of the left and right ventricle. Namely, when ventricular diastolic dysfunction develops, atrium could maintain a cardiac output only through the regulation of the reservoir and booster pump functions. In our study transmitral and tricuspid E/A ratios are reduced, whereas corresponding E/e' ratios are increased, in the SCH subjects. These findings, in combination with enlarged atria and prolonged deceleration time, indicate the existence of elevated filling pressures and presence of diastolic dysfunction. Third, the ventricular hypertrophy could be an additional mechanism that may explain both ventricular diastolic dysfunction and changes in atrial phasic function. The reason for ventricular hypertrophy possibly lies in increased activity of renin-angiotensin system, increased ventricular volumes and cardiac output in SCH subjects.[Citation20] The present investigation confirms this hypothesis because it demonstrated that left ventricular mass index and right ventricular wall thickness were higher among the SCH subjects, although still in normal ranges. However, our results demonstrate that TSH level is associated with LA and RA function and mechanics independently of left and right ventricular diastolic function and structure. Fourth, hyperthyroid patients have increased blood volumes due to increased renal sodium reabsorption and reduced systemic vascular resistance.[Citation21] These changes induce cardiac dilatation and hypertrophy, which could be responsible for atrial impairment in the SCH individuals. The changes of the endothelial function, inflammatory and hemostatic parameters, found in subclinical hypothyroidism and hyperthyroidism, could also affect the cardiac function.[Citation22,Citation23]
The present investigation showed that SCH significantly impacts LA and RA phasic function, which could be the reason of atrial fibrillation occurrence or development of heart failure in these subjects.[Citation24–27] Ayhan et al.[Citation4] have already reported prolonged atrial electromechanical intervals and impaired LA mechanical function in the patients with overt hyperthyroidism. The authors also found the correlation between TSH and atrial electromechanical delay. These findings with our results could reasonably explain the susceptibility for atrial fibrillation in the subjects with subclinical hyperthyroidism. Thus, the present findings indicate the need for closer monitoring of the patients with SCH, especially those individuals who already underwent atrial fibrillation conversion. Additionally, our results raise the question about the treatment of these patients even if they are asymptomatic.
Limitations
Our study has several limitations: (i) 3DE estimation of atrial volumes could be significantly affected by the quality of ultrasound images; (ii) 3DE volumetric evaluation of the left and right atrium was not performed by software dedicated to 3DE left atrial analysis. However, a number of studies showed that software packages designed for evaluation of the left ventricular volumes could be successfully used for assessment of both atria[Citation14,Citation16,Citation28]; (iii) a relatively small number of patients; (iv) our results are limited to women only; (v) we did not investigate the effect of antithyroid therapy in the SCH, which would be beneficial for the treatment of these patients.
Conclusion
Left and right atrial phasic function and mechanics are significantly changed in patients with subclinical hyperthyroidism. Biatrial phasic function deteriorates gradually from the healthy subjects, throughout the individuals with borderline TSH level, to the patients with low TSH level. However, this progressive function decline is more evident in LA. Further, prospective investigations are crucial for the evaluation of the necessity for early treatment in the SCH subjects and possible reverse influence of treatment on atrial remodeling.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Selmer C, Olesen JB, Hansen ML, et al. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab. 2014;99:2372–2382.
- Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172:799–809.
- Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041.
- Ayhan S, Ozturk S, Dikbas O, et al. Detection of subclinical atrial dysfunction by two-dimensional echocardiography in patients with overt hyperthyroidism. Arch Cardiovasc Dis. 2012;105:631–638.
- Nacar AB, Acar G, Yorgun H, et al. The effect of antithyroid treatment on atrial conduction times in patients with subclinical hyperthyroidism. Echocardiography. 2012;29:950–955.
- Lang RM, Bierig M, Devereux RB, et al.; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108.
- de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260.
- Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713.
- Tadic M, Ilic S, Ivanovic B, et al. Left atrial phasic function and mechanics in women with subclinical hypothyroidism: the effects of levothyroxine therapy. Echocardiography. 2014;31:1221–1229.
- Todaro MC, Choudhuri I, Belohlavek M, et al. New echocardiographic techniques for evaluation of left atrial mechanics. Eur Heart J Cardiovasc Imaging. 2012;13:973–984.
- Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252.
- Abdulrahman RM, Delgado V, Ng AC, et al. Abnormal cardiac contractility in long-term exogenous subclinical hyperthyroid patients as demonstrated by two-dimensional echocardiography speckle tracking imaging. Eur J Endocrinol. 2010;163:435–441.
- Mor-Avi V, Yodwut C, Jenkins C, et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. 2012;5:769–777.
- Keller AM, Gopal AS, King DL. Left and right atrial volume by freehand three-dimensional echocardiography: in vivo validation using magnetic resonance imaging. Eur J Echocardiogr. 2000;1:55–65.
- Takahashi A, Funabashi N, Kataoka A, et al. Quantitative evaluation of right atrial volume and right atrial emptying fraction by 320-slice computed tomography compared with three-dimensional echocardiography. Int J Cardiol. 2011;146:96–99.
- Aune E, Baekkevar M, Roislien J, et al. Normal reference ranges for left and right atrial volume indexes and ejection fractions obtained with real-time three-dimensional echocardiography. Eur J Echocardiogr. 2009;10:738–744.
- Peluso D, Badano LP, Muraru D, et al. Right atrial size and function assessed with three-dimensional and speckle-tracking echocardiography in 200 healthy volunteers. Eur Heart J Cardiovasc Imaging. 2013;14:1106–1114.
- Shenoy R, Klein I, Ojamaa K. Differential regulation of SR calcium transporters by thyroid hormone in rat atria and ventricles. Am J Physiol Heart Circ Physiol. 2001;281:H1690–H1696.
- Biondi B, Palmieri EA, Klain M, et al. Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol. 2005;152:1–9.
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509.
- Alibaz Oner F, Yurdakul S, Oner E, et al. Evaluation of the effect of L-thyroxin therapy on endothelial functions in patients with subclinical hypothyroidism. Endocrine. 2011;40:280–284.
- Popławska-Kita A, Siewko K, Telejko B, et al. The changes in the endothelial function and haemostatic and inflammatory parameters in subclinical and overt hyperthyroidism. Int J Endocrinol. 2013;2013:981638. DOI: https://doi.org/10.1155/2013/981638.
- Yoon JH, Moon J, Chung HM, et al. Left atrial function assessed by Doppler echocardiography rather than left atrial volume predicts recurrence in patients with paroxysmal atrial fibrillation. Clin Cardiol. 2013;36:235–240.
- Mirza M, Caracciolo G, Khan U, et al. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two-dimensional speckle strain study. J Interv Card Electrophysiol. 2011;31:197–206.
- Gaynor SL, Maniar HS, Prasad SM, et al. Reservoir and conduit function of right atrium: impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol. 2005;288:H2140–H2145.
- Geng J, Lu W, Hu T, et al. Subclinical hyperthyroidism increases risk of coronary heart disease events in type 2 diabetes mellitus. Endocrine. 2015;49:557–559.
- Miyasaka Y, Tsujimoto S, Maeba H, et al. Left atrial volume by real-time three-dimensional echocardiography: validation by 64-slice multidetector computed tomography. J Am Soc Echocardiogr. 2011;24:680–686.