Abstract
Objectives. To optimize the efficacy of treatment with tocilizumab for rheumatoid arthritis (RA), we comparatively analyzed the outcome of tocilizumab treatment in patients with normal background changes associated closely with IL-6.
Patients and Methods. The study involved 87 patients with RA satisfying the diagnostic criteria of the American College of Rheumatology (ACR) and receiving continuous tocilizumab treatment for 24 weeks or longer. The outcome of tocilizumab treatment in these patients was comparatively analyzed in relation to the baseline platelet count (the high platelet count group and the normal group), pretreatment hemoglobin levels (the low group and the normal platelet count group), and speed of bone destruction (the rapid progression group and slow progression group).
Results. Treatment with tocilizumab significantly improved the 28-joint disease activity score using the erythrocyte sedimentation rate (DAS28-ESR) and Clinical Disease Activity Index (CDAI), regardless of baseline platelet count, hemoglobin level, or annual speed of bone destruction (ΔTSS). The margins of improvement in DAS28-ESR and CDAI did not differ depending on baseline hemoglobin level or ΔTSS, but the improvement was significantly greater in the high platelet count group than in the normal platelet count group.
Conclusions. These results suggest that in patients with high platelet count, IL-6 is a more important factor involved in RA pathogenesis and that tocilizumab is suitable as a first-line biologic for the treatment of RA patients with high platelet count.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease of unknown etiology and is characterized by arthritis. Inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), and interleukin-1 (IL-1) are involved closely with the onset of RA. Blood cell components such as macrophages, T lymphocytes, and B lymphocytes also serve as important regulatory factors [Citation1].
After the 2000s, the use of biologics that directly suppress these cytokines and inflammatory cells was expanded to achieve higher clinical efficacy. The TNF inhibitors available for clinical use include infliximab, etanercept, adalimumab, golimumab, and certolizumab. In addition, tocilizumab (an IL-6 inhibitor), abatacept (a T lymphocyte antagonist), and rituximab (a B lymphocyte inhibitor) are now used clinically.
According to the National Institute for Health and Care Excellence (NICE) Guidance, abatacept and rituximab (non-TNF inhibitory biopharmaceuticals) are unsuitable as the first-line biologics (1st Bio), and it was recommended that these drugs be used as the second-line biologics instead, specifically in cases with a failed response to the 1st Bio [Citation2].
According to the NICE Guidance, a TNF inhibitor or IL-6 inhibitor is to be used as the 1st Bio. The Guidance, however, does not provide specific criteria for selecting a TNF inhibitor or IL-6 inhibitor as the 1st Bio in different cases.
TNF is a cytokine located upstream of IL-6 [Citation3]. Considering the pathological mechanism of RA, it is unlikely that patients who fail to respond to TNF inhibitors will respond to IL-6 inhibitors. However, a clinical study has reported that non-responders to TNF inhibitors show response to tocilizumab (an IL-6 inhibitor) [Citation4], suggesting that in some cases of RA, IL-6 rather than TNF is primarily involved in the pathogenesis.
Both TNF and IL-6 have many common actions in patients with RA, such as synovial cell proliferation, inflammatory cell induction, persistence of inflammation, osteoclast activation, and cartilage destruction [Citation1].
However, some actions are confined to IL-6 and are absent in TNF. One such action is the activity on bone marrow megakaryocytes to increase peripheral blood platelets [Citation5]. Furthermore, IL-6 acts on hepatocytes, inducing hepcidin and inflammatory anemia [Citation6]. It has also been estimated that TNF differs from IL-6 in terms of involvement in bone destruction. A study using transgenic mice revealed articular destruction akin to RA in TNF transgenic mice [Citation7]. In contrast, IL-6 transgenic mice were free of articular destruction and presented with hepatosplenomegaly alone, resembling human Cattleman's disease [Citation8]. As described above, it seems likely that TNF is closely involved with the articular destruction found in RA patients and that IL-6 is closely involved with the increase in platelet number and anemia. Therefore, distinction between these phenotypes may enable identification of IL-6-dominant RA cases. If IL-6 dominancy can be judged before treatment, tocilizumab can be selected as the 1st Bio for treatment, thus enabling establishment of a more effective treatment method. We retrospectively analyzed the outcome of tocilizumab treatment in patients with abnormal background associated with IL-6.
Patients and methods
Patients
The study involved 87 patients with RA satisfying the American College of Rheumatology (ACR) 1987 or 2010 revised criteria for the classification of RA [Citation9,Citation10] and receiving continued tocilizumab treatment for 24 weeks or longer. There were 18 men and 69 women. The average age was 61.3 ± 12.4 years. All patients received methotrexate (MTX) concomitantly. The mean MTX dose level was 9.2 ± 2.9 mg/week. Mean duration of RA was 9.8 ± 10.8 years. The mean tocilizumab treatment period was 92.0 ± 76.8 weeks.
Responses to treatment analyzed using baseline platelet count
Responses to tocilizumab treatment were analyzed in relation to the baseline peripheral blood platelet count: the normal platelet count group (less than 400 000 platelets/μL) versus the high platelet count group (400 000 platelets/μL or more). Sixty-four patients were allocated to the normal platelet count group, and 23 patients were allocated to the high platelet count group (). Of the baseline background variables, C-reactive protein (CRP) levels, hemoglobin (Hb) levels, platelet count, matrix metalloproteinase-3 (MMP-3) levels, DAS28-ESR, swollen joint count (SJC), and erythrocyte sedimentation rate (ESR) differed significantly between the two groups.
Table 1. Baseline characteristics of patients which are divided according to platelet count, hemoglobin level or ΔTSS.
Responses to treatment analyzed using baseline hemoglobin levels
Responses to tocilizumab treatment were analyzed in relation to the baseline Hb levels (g/dL): the low Hb group (less than 13 g/dL in men and less than 12 g/dL in women) versus the normal Hb group. Forty patients were allocated to the normal Hb group and 47 patients to the low Hb group (). Of the baseline background variables, CRP levels, Hb levels, platelet count, MMP-3 levels, DAS28-ESR, and ESR differed significantly between the two groups.
Table 2. CDAI at baseline and after treatment of patients which are divided according to platelet count, hemoglobin level or ΔTSS.
Responses to treatment analyzed using baseline radiographic stages of bone destruction
In each patient, the baseline-modified sharp Heidji score [Citation11] was evaluated and divided by the duration of RA to obtain ΔTSS. Responses to tocilizumab treatment were analyzed in relation to ΔTSS: the group showing slow progression of bone destruction (ΔTSS < 50) vs the group showing rapid progression of bone destruction (ΔTSS ≥ 50). Sixty patients were allocated to the slow progression group and 27 patients to the rapid progression group (). Of the baseline background variables, ΔTSS and duration of RA differed significantly between the two groups.
Table 3. Changes in DAS28-ESR and CDAI from baseline of patients which were divided according to platelet count, hemoglobin level or ΔTSS.
Evaluation of disease activity
Disease activity was evaluated using two indicators: DAS28-ESR [Citation12] and Clinical Disease Activity Index (CDAI) [Citation13].
Statistical analysis
Background variables were statistically analyzed using the unpaired t-test. CDAI was analyzed using the paired t-test between pre- and posttreatment measurements. ΔDAS28-ESR and ΔCDAI were analyzed using unpaired t-test between groups defined by platelet count, Hb level or delta TSS. The Wilcoxon signed rank sum test was used for analysis of a difference between a distribution ratio of patients with each state of disease activity before and after treatment, with p < 0.05 considered statistically significant.
Results
Responses to tocilizumab evaluated on the basis of the DAS28 remission rate
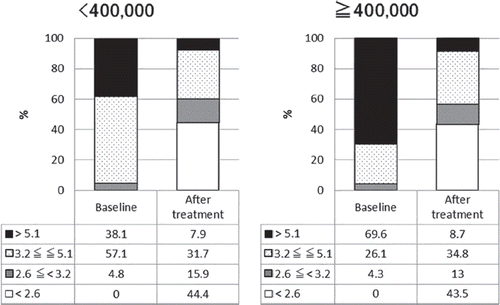
shows the responses to tocilizumab treatment in the two groups divided according to the platelet count. The DAS28-ESR remission rates increased significantly after tocilizumab treatment in both the normal platelet count group and the high platelet count group ( and ). The percentage of patients with high baseline disease activity rated using DAS28-ESR was higher in the high platelet count group than in the normal platelet count group (69.6% vs. 38.1%), reflecting the differences in background variables.
Figure 1. Proportion of patients in each disease activity (patients divided according to platelet count). Figure in left represents the result of patients with high platelet counts in baseline (< 400 000). Figure in right represents the result of patients with normal platelet counts in baseline (≦ 400 000). Numbers in the table below each bar charts represent percentage of patients in each disease activity. Patients are graded into four group according to DAS28-ESR, DAS28-ESR > 5.1, 3.2 ≦ DAS28-ESR ≦ 5.1, 2.6 ≦ DAS28-ESR < 3.2, DAS28-ESR < 2.6. Percentages of patients with each state of disease activity after the treatment is significantly changed form before the treatment (P < 0.0001).
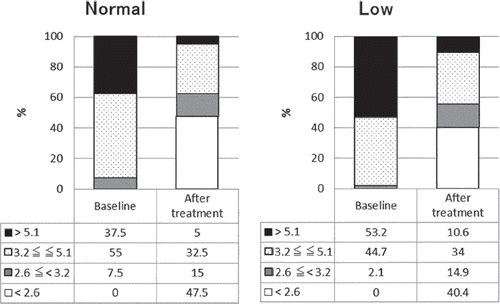
shows the responses to tocilizumab treatment in the two groups divided according to Hb level. The DAS28-ESR remission rates increased significantly after tocilizumab treatment in both the normal Hb group and the low Hb group ( and ). The percentage of patients with high baseline disease activity rated using DAS28-ESR was higher in the low Hb group than in the normal Hb group (53.2% vs. 37.5%), reflecting the differences in background variables.
Figure 2. Proportion of patients in each disease activity (patients divided according to hemoglobin level). Figure in left represents the result of patients with normal hemoglobin level in baseline. Figure in right represents the result of patients with low hemoglobin level in baseline. Numbers in the table below each bar charts represent percentage of patients in each disease activity. Patients are graded into four group according to DAS28-ESR, DAS28-ESR > 5.1, 3.2 ≦ DAS28-ESR ≦ 5.1, 2.6 ≦ DAS28-ESR < 3.2, DAS28-ESR < 2.6. Percentages of patients with each state of disease activity after the treatment is significantly changed form before the treatment (P < 0.0001).
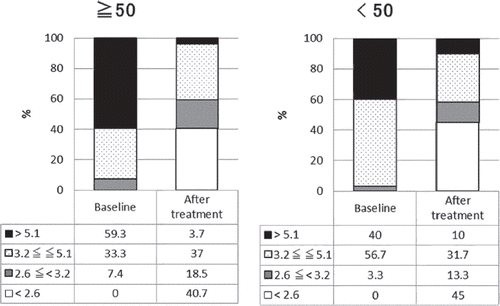
shows the responses to tocilizumab treatment in the two groups divided according to the radiographic stage of bone destruction. The DAS28-ESR remission rate increased significantly after tocilizumab treatment in both the slow progression group and the rapid progression group. The DAS28-ESR remission rate at baseline did not differ between these two groups ( and ).
Figure 3. Proportion of patients in each disease activity (patients divided according to ΔTSS). Figure in left represents the result of patients with rapid progression in bone destruction in baseline (ΔTSS ≧ 50). Figure in right represents the result of patients with slow progression in bone destruction in baseline (ΔTSS < 50). Numbers in the table below each bar charts represent percentage of patients in each disease activity. Patients are graded into four groups according to DAS28-ESR, DAS28-ESR > 5.1, 3.2 ≦ DAS28-ESR ≦ 5.1, 2.6 ≦ DAS28-ESR < 3.2, DAS28-ESR < 2.6. Percentages of patients with each state of disease activity after the treatment is significantly changed form before the treatment (P < 0.0001).
Responses to tocilizumab treatment rated with ΔDAS28-ESR
shows the responses to tocilizumab treatment rated with ΔDAS28-ESR (posttreatment DAS28-ESR—baseline DAS28-ESR) in the two groups of patients divided according to platelet count, Hb level, or ΔTSS. The ΔDAS28-ESR improved significantly in the high platelet count group as compared to the normal platelet count group (p = 0.029), whereas there was no statistically significant difference in ΔDAS28-ESR between the normal Hb group and the low Hb group or between the group with slow progression of bone destruction and the group with rapid progression of bone destruction.
Responses to tocilizumab treatment rated with CDAI
shows the results related to improvement in CDAI after treatment in the two groups of patients divided according to platelet count, Hb level, or ΔTSS. As shown in this table, significant improvement was seen in each group after tocilizumab treatment.
Responses to tocilizumab treatment rated with ΔCDAI
shows the results related to ΔCDAI (posttreatment CDAI—baseline CDAI) in the two groups of patients divided according to platelet count, Hb level, or ΔTSS. The ΔCDAI improved significantly in the high platelet count group as compared to the normal platelet count group, whereas there was no statistically significant difference in the ΔCDAI between the normal Hb group and the low Hb group or between the group with slow progression of bone destruction and the group with rapid progression of bone destruction. On the other hand, from the investigation for correlation of ΔDAS28-ESR or ΔCDAI with joint space narrowing score (JSN) or erosion score (ERO), there was no correlation between ΔDAS28-ESR or ΔCDAI and JSN or ERO (ΔDAS28-ESR vs. JSN; r = 0.2403, ΔDAS28-ESR vs. ERO; r = 0.0932, ΔCDAI vs. JSN; r = 0.2516, ΔCDAI vs. ERO; r = 0.1028).
Cases requiring discontinuation of tocilizumab treatment
No discontinuation of tocilizumab treatment for adverse reactions was required in any of the patients studied. For two patients, tocilizumab treatment was discontinued in less than 8 weeks after the start of treatment because of poor response to the treatment. These two cases were excluded from analysis.
Discussion
Treatment using biologics is an effective means of treating RA clinically. However, selection of the most effective drug in individual cases remains unclear. Biologics have excellent efficacy, but their high cost is a concern [Citation14]. Therefore, there is a need to minimize the risk of prolonged administration of poorly effective treatment by replacing drugs of unknown efficacy with other more efficacious drugs. Attention has been focused on the physiological actions of cytokines and the considerations on how to achieve the best use of TNF and IL-6 inhibitors that were identified as 1st Bio in the NICE Guidance [Citation15]. The present study was designed to examine whether platelet count and Hb levels (which are largely affected by the physiological activity of IL-6) and radiographic changes (seemingly affected less by IL-6) serve as indicators in identifying patients who are highly sensitive to IL-6 inhibitory treatment.
In this study, background variables (including disease activity) at the start of treatment differed significantly between the two groups of patients divided according to platelet count or Hb level. This probably reflects the large influence of IL-6 in the high platelet count group and the low Hb group and also appears to be closely associated with elevation in the indicators of inflammation such as CRP and ESR ().
In the analysis of ΔDAS28-ESR and ΔCDAI, improvement after tocilizumab treatment was more marked in the high platelet count group than in the normal platelet count group. However, Hb levels and radiographic changes did not demonstrate the ability to be indicators for identifying patients that respond better to IL-6 inhibitory treatment. As a reference, the association between baseline platelet count and treatment efficacy with anti-TNF agents (infliximab 36, etanercept 85, adalimumab 6, and golimumab 5) was examined using aggregated private data from 132 patient records in my clinic with a background equivalent to patients which were analyzed in the study (25 men and 107 women, 64.3 ± 12.4 years old,. Mean duration of RA; 10.2 ± 12.4 years). The data showed that number of platelet count of RA patient did not affect the efficacy of TNF inhibitors (ΔDAS28ESR; − 1.54 in < 400 000, − 1.03 in ≧ 400,00, P = 0.1358, ΔCDAI; − 8.66 in < 400 000, − 6.12 in ≧ 400 000, P = 0.4039).
In the present study, the difference in radiographic changes was not a definite indicator for selecting tocilizumab treatment. In previous studies, it has been reported that there is a time gap between the first appearance of clinical symptoms and the onset of changes that can be visualized on radiography. This may be one of the reasons for radiographic changes not being an indicator of the influence of IL-6 in the present study. Furthermore, the difference in Hb levels was not a definite indicator for selecting tocilizumab treatment. This is probably because anemia in RA patients was affected not only by hepcidin associated with IL-6 but also by various other factors, and some factors other than IL-6 may have affected Hb levels [Citation16].
When evaluated using both ΔDAS28-ESR and ΔCDAI, the margin of improvement in disease activity after treatment was different between the two groups of patients divided according to platelet count.
Platelet count before the start of treatment appears to be useful as an indicator for selecting tocilizumab (an IL-6 inhibitor) as the 1st Bio in routine clinical practice.
Conflict of interest
None.
References
- Matsuno H. Treating of rheumatoid arthritis with biological agents. In: Matsuno H, ed. Innovative Rheumatology. Intech Co. 2013. p. 95–107.
- Chiu Y, Ostor A, Hammond A, Sokoll K, Anderson M, Buch M, et al. Access to the wave of biological therapies for the treatment of arthritis in England and Wales. Clin Rheumatol. 2012;31(6):1005–12.
- Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T, et al.The role of TNF-α in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis: a study using a human RA/SCID mouse chimera. Rheumatology. 2002;41(3):329–37.
- Emery P, Keystone E, Tony HP, Cantagrel A, Vollenhoven RV, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refactory to anti-tumor necrosis factor biologicals: results from a 24-weel multicenter randomized placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–23.
- Ishibashi T, Kimura H, Shikama Y, Uchida T, Kariyone S, Hirano T, et al.Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989;74(4):1241–44.
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of imflamation. Blood. 2003;102(3):783–8.
- Katsume A, Saito H, Yamada Y, Yorozu K, Ueda O, Akamatsu K, et al.Anti-Interleukin6 (IL-6) receptor antibody suppresses Castleman's disease like symptoms emerged in IL-6 transgenic mice. Cytokine. 2002;20(6):304–11.
- Zwerinca J, Redlich K, Polzer K, Joosten L, Krӧnke G, Distler J, et al.TNF-induced structural joint damage is mediated by IL-1. PNAS. 2007;104(28):11742–47.
- Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthrithis. Arthritis Rheumatism. 1988;31(3):315–24.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham III CO, et al.Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8.
- van der Heijde DM, van Riel PL, Nuver-Zwart IH, Gribnau FW, van de Putte LB. Effect of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989;1(8646):1036–8.
- Wells G, Becker JC, Tang J, Dougado's M, Schiff M, Smolen H, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954–60.
- Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(39):S100–8.
- Matsuno H. Small molecule DMARD therapy and its position in RA treatment. In: Matsuno H, ed. Innovative Rheumatology. Intech Co. 2013. p. 165–87.
- National Institute for Health and Clinical Excellence: NICE. http://www.nice.org.uk/nicemedia/live/13669/58202/58202.pdf.
- Campbell K. Pathophysiology of anaemia. Nursing Times. 2004; 100(47):40–3.
