Abstract
Introduction: We aimed to identify causes of false-positives in ultrasound scanning of synovial/tenosynovial/bursal inflammation and provide corresponding imaging examples.
Methods: We first performed systematic literature review to identify previously reported causes of false-positives. We next determined causes of false-positives and corresponding example images for educational material through Delphi exercises and discussion by 15 experts who were an instructor and/or a lecturer in the 2013 advanced course for musculoskeletal ultrasound organized by Japan College of Rheumatology Committee for the Standardization of Musculoskeletal Ultrasonography.
Results: Systematic literature review identified 11 articles relevant to sonographic false-positives of synovial/tenosynovial inflammation. Based on these studies, 21 candidate causes of false-positives were identified in the consensus meeting. Of these items, 11 achieved a predefined consensus (≥ 80%) in Delphi exercise and were classified as follows: (I) Gray-scale assessment [(A) non-specific synovial findings and (B) normal anatomical structures which can mimic synovial lesions due to either their low echogenicity or anisotropy]; (II) Doppler assessment [(A) Intra-articular normal vessels and (B) reverberation)]. Twenty-four corresponding examples with 49 still and 23 video images also achieved consensus.
Conclusions: Our study provides a set of representative images that can help sonographers to understand false-positives in ultrasound scanning of synovitis and tenosynovitis.
Introduction
Musculoskeletal ultrasonography visualizes inflammation in the synovial tissues as synovial hypertrophy with or without Doppler signals inside or as synovial fluid [Citation1]. A number of studies have shown that ultrasound detects synovial inflammation more sensitively than does clinical joint examination [Citation2–5] and ultrasound-detected synovitis correlates well with magnetic resonance imaging (MRI) [Citation4,Citation6–8] and histopathological findings [Citation9,Citation10]. As synovial inflammation is the most characteristic pathophysiology of rheumatoid arthritis (RA), sonographic assessment of synovial inflammation has been shown to improve the accuracy of diagnosis [Citation11–16], optimize the assessment of disease activity [Citation15,Citation17–22], predict relapse after discontinuation of biological treatment [Citation23], and also improve the physicians’ joint examination skill [Citation24] in the management of RA. Therefore, a rapidly increasing number of rheumatologists now use ultrasound for the assessment of synovial inflammation in daily practice, training, and education [Citation25].
One of the advantages of ultrasound over other imaging modalities is its flexibility. In order to visualize highly polymorphic synovial lesions in various joints in patients under various conditions, the transducer of ultrasound can be placed at any joints, from various angles, using a variety of dynamic procedures (e.g. joint movement and applying pressure). The downside, however, is that this flexibility allows for variable imaging planes under variable conditions, which can result in variable interpretation of the same joint lesion, necessitating the standardization of the image acquisition and interpretation.
Japan College of Rheumatology Committee for the Standardization of Musculoskeletal Ultrasonography (JCR-CoSMUS) has previously shown that the sonographic assessment of milder synovial inflammation is less reproducible than that of more severe synovial inflammation even among experts with training and experience [Citation26]. These data indicate that the assessment of mild or equivocal images is frequently affected by artifacts and within-normal/non-specific findings and may explain the limited specificity of low-grade sonographic findings for synovitis reported in recent studies [Citation27–29]. Because joints with mild or equivocal inflammation are the ones where imaging plays a significant role in the management of rheumatic conditions, these sonographic false-positives need to be characterized for sonographers to discriminate between normal and arthritic joints and maximize the utility of ultrasound. However, no studies have systematically identified the causes of false-positives in imaging synovial inflammation with ultrasound.
The present study was undertaken by JCR-CoSMUS, aiming to identify causes of false-positives in ultrasound scanning of synovial/tenosynovial/bursal inflammation and provide a set of example images which represent the false-positives. For this purpose, we performed systematic literature review and built consensus through Delphi exercises and discussion.
Methods
Systematic literature review
We searched for original articles in English concerning humans, published between January 1985 and December 2013, and relevant to the false-positives in the sonographic evaluation of synovial inflammation using PUBMED and EMBASE databases. We first used the term, (synovitis OR tenosynovitis OR bursitis) AND (sonography OR ultrasound) AND (pitfall OR false positive), which identified a limited number of articles. Therefore, we next used the broader term, (synovitis OR tenosynovitis OR bursitis) AND (sonography OR ultrasound). Titles, abstracts, and full reports of articles identified were systematically screened for inclusion by two authors (Ikeda and Nakamura). We only included articles with data which identify the factor related to false-positives. Data were extracted from the selected articles using a standardized spreadsheet with particular attention paid to the following: (1) Who were the study subjects (e.g. healthy volunteers)? (2) Which joints were assessed? (3) What was the gold standard or comparator?
Consensus meeting and Delphi process
The consensus meeting was held during the JCR advanced course on musculoskeletal ultrasound in Tokyo in September 2013. Fifteen experts in musculoskeletal ultrasound (Ikeda, Narita, Ogasawara, Ohno, Kawahito, Kawakami, Ito, Matsushita, Suzuki, Misaki, Ogura, Kamishima, Seto, Nakahara, Kaneko), who were an instructor and/or a lecturer in the JCR advanced course, participated the consensus meeting and the Delphi processes. In the meeting, candidate factors, which possibly cause false-positives in the sonographic evaluation of synovial inflammation, were identified based on the preliminary results of systematic literature review and also on their knowledge and experience. The first Delphi process was undertaken to determine the final items from these candidates. The participants were asked in questionnaire whether these factors should be presented as pitfalls in educational materials and asked to rate their level of agreement or disagreement with each candidate. The second Delphi process asked the participants whether the sample ultrasound images were appropriate. Each Delphi process took one round, in which participants were asked to answer according to a 1–5 Likert scale (1 = strongly disagree; 5 = strongly agree) and were also asked for the reason when the answer was not 5. Space for additional free comments was also provided. We predefined that group consensus was achieved when 80% or larger proportion of participants scored an item as 4 or 5.
Ultrasound images
Representative ultrasound images, which illustrate the identified causes of false-positives, were collected. All images were obtained from asymptomatic joints in healthy subjects using a HI VISION Avius with a linear array multi-frequency transducer (5–18 MHz for GS) (Hitachi Medical Corporation, Tokyo, Japan). Power Doppler images were obtained with a pulse repetition frequency of 800 Hz and a color gain of 40 dB.
Results
Systematic literature review
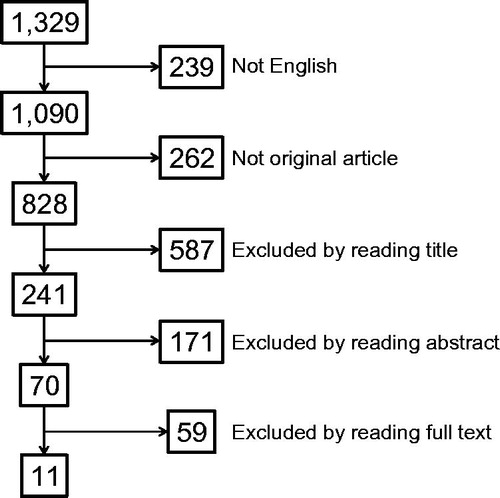
The first search identified a total of 17 articles, of which 11 were original articles in English. Six of these 11 articles were relevant to the topic. The second search identified a total of 1329 articles, of which 828 were original articles in English (). Screening process, however, identified only 11 articles that are relevant to sonographic false-positives of synovial/tenosynovial inflammation and none for bursitis [Citation30–40].
Figure 1. Flow chart of article selection. Shown in the box is the number of articles. The chart starts with the search results in the PUBMED and EMBASE databases from January 1985 to December 2013 using the terms (synovitis OR tenosynovitis OR bursitis) AND (sonography OR ultrasound) filtered for human research.
As shown in , three articles studied only patients with pain or arthritic conditions, three only healthy volunteers, four both patients and healthy volunteers, and one healthy volunteer and a cadaveric specimen. Patients studied were mostly those with RA. The earliest four studies performed ultrasound on large joints in patients and compared the findings with other imaging techniques such as computed tomography (CT), MRI, and arthroscopy. More recent studies focused on smaller joints in healthy subjects and reported non-specific findings and technical pitfalls. Most of the studies reported false-positives in the gray-scale evaluation of intra-articular synovitis, whereas one study reported that in the gray-scale evaluation of tenosynovitis and two studies reported those in the Doppler assessment of intra-articular synovitis. These results indicate that both non-specific findings and technical/interpretational pitfalls can cause false-positives in gray-scale and Doppler assessment of intra- and extra-articular synovial inflammation.
Table 1. Causes of false positives identified in the systematic literature review.
Consensus-based identification of causes of false-positives
Given the preliminary results of systematic literature review, a wide variety of candidate causes of false-positives were proposed and discussed in the consensus meeting. Twenty-one candidates were identified, which included 15 items for gray-scale assessment and six for Doppler assessment ().
Table 2. Rating and agreement in expert panel on candidate causes of false-positives which should be presented as pitfalls in educational materials.
In the Delphi process, predefined consensus (≥80%) was achieved in 11 of 21 items (). For all of the 10 items which did not achieve consensus, the major reason for disagreement was that the item is too rudimentary and hardly causes false-positives in practice.
The 11 items, which achieved consensus, are listed in . These items are classified as factors related to false-positives in gray-scale assessment and those in Doppler assessment. Gray-scale items are further classified as non-specific synovial findings and normal anatomical structures, which can mimic synovial lesions due to either their low echogenicity or anisotropy. “Reverberation/mirror image artefact” was rephrased as “Reverberation” because a couple of participants pointed out that the major explanation for this artifact which appears below strong Doppler signal is reverberation instead of mirror image and all participants agreed with this correction.
Table 3. Systematic classification of factors which cause false-positives in sonographic evaluation of synovial inflammation.
Representative ultrasound images
We next collected ultrasound images which represent 11 identified factors related to false-positives. Twenty-seven examples with a total of 55 still images and 26 video clips were obtained. In the second Delphi process, two examples did not reach predefined consensus agreement and three examples were excluded (). Representative example images are shown in and . All examples and video images are available as the Supplementary material.
Figure 2. Representative images of non-specific thickening of synovial membrane. Dorsal aspect of metatarsophalangeal joint in the right first toe, longitudinal view. (A) gray-scale image and (B) power Doppler image. Asterisks indicate non-specific thickening of synovial membrane.
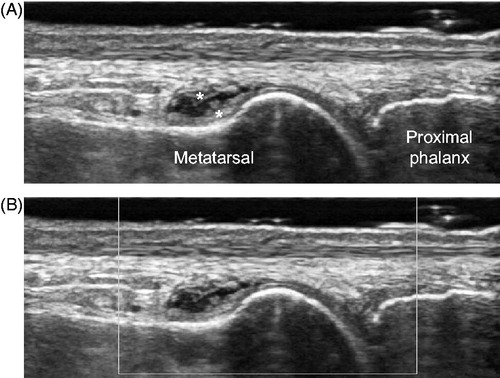
Figure 3. Representative images of reverberation mimicking synovial Doppler signals. Dorsal aspect of the left first metacarpophalangeal joint, longitudinal view. (A) gray-scale image and (B) power Doppler image. Arrows indicate reverberation on the hyaline cartilage due to superficial artery (see also Video 22).
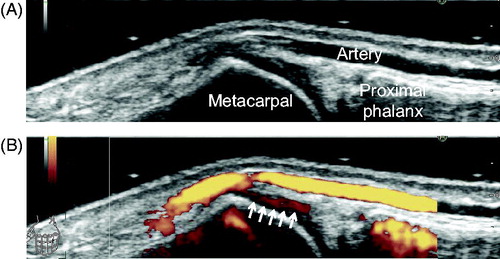
Table 4. Rating and agreement on candidate examples with still- and video-images which represent causes of false-positives in sonographic evaluation of synovial inflammation.
Discussion
In this study, we identified factors related to false-positives in ultrasound scanning of synovitis and tenosynovitis by systematic literature review, consensus exercise, and discussion. Furthermore, we provide a set of example images, which represent the identified false-positives. These images can be a unique educational material for increasing the awareness of false-positives in ultrasound scanning of synovitis and tenosynovitis.
The results of systematic literature review indicate that both non-specific findings and technical pitfalls can cause false-positives in gray-scale and Doppler assessment of intra- and extra-articular synovial inflammation (). However, JCR-CoSMUS members and experts in this field considered that these research-based false-positives identified in the literature did not cover all pitfalls we encounter in daily ultrasound examination. In fact, 5 of 11 items in the final list were ones which had not been reported in original articles in the literature but were considered important by the panel.
In the evaluation of synovitis, synovial fluid and hypertrophy are defined as “abnormal hypoechoic or anechoic-intraarticular material that is displaceable and compressible, but does not exhibit Doppler signal” and “abnormal hypoechoic intraarticular tissue that is nondisplaceable and poorly compressible and which may exhibit Doppler signal”, respectively [Citation1]. Similar definitions also apply to tenosynovitis [Citation13,Citation21,Citation23,Citation41–43]. Therefore, normal amount of synovial fluid and non-specific hypoechoic thickening of synovium/tenosynovium detected in the majority of non-arthritic joints in healthy subjects should not be considered as pathologic or clinically significant. In fact, 6 of 11 causes of false-positives identified in the literature refer to non-specific synovial fluid or thickening () [Citation33,Citation35,Citation37–40]. In addition, although not included in our systematic literature review, Hiraga et al. recently demonstrated that non-arthritic metatarsophalangeal (MTP) joints usually exhibit intraarticular lowechoic area on ultrasound in the dorsal aspect [Citation44]. Ten Cate also demonstrated that a small anechoic area is frequently observed at the distal portion of second metacarpophalangeal (MCP) joints in non-arthritic subjects [Citation45]. Whether these non-specific synovial fluid and thickening should be rated as grade 0 or grade ≥1 in semiquantitative scoring system is an important matter of debate. Grading any detectable synovial fluid and thickening allows for more sensitive and consistent assessment, while assessment which only grades pathologic ones is more specific to rheumatic conditions.
Other articles had reported a technical pitfall (i.e. anisotropy) [Citation30,Citation36] and misinterpretation of non-synovial structure [Citation31,Citation35,Citation36] that cause false-positives. These data demonstrate that any anatomical structures and any technical artifacts that exhibit hypoechogenecity in the vicinity of synovial tissues can cause false-positives in the gray-scale evaluation of synovitis/tenosynovitis. Therefore, we comprehensively included such structures, artifacts, and inappropriate machine settings as candidate pitfalls in the first Delphi process (). However, hyaline cartilage, acoustic shadow, and all inappropriate machine settings were considered too rudimentary by a significant proportion of expert panel and did not reach consensus (), while some members thought they are still important for the beginners. We all agreed that all sonographers should have good knowledge on anatomical structures of and around the joint not to confound normal low-echoic structure with gray-scale synovitis and should also find an imaging plane that demonstrates as little anisotropy as possible.
For Doppler assessment, two previous reports on false-positives were identified [Citation7,Citation32]. We excluded the blooming artifact after contrast-enhancement [Citation32] from the first Delphi process since we do not use contrast-enhancement in daily practice. In addition to the intra-articular normal vessels reported by Terslev et al. [Citation7], we included extra-articular normal vessels, three types of Doppler artifacts, and inappropriately high Doppler gain setting as candidate pitfalls in the first Delphi process, in which consensus was only achieved on two items (). Given that Doppler assessment influences the sonographic evaluation of overall activity of synovitis more significantly than does gray-scale assessment [Citation26], at least these two pitfalls should be well known to sonographers who scan joints for this purpose.
False-positive due to intra-articular normal vessels can be avoided to some extent by knowing where in the joint normal vessels are usually identified and by not considering Doppler signals outside synovial hypertrophy as pathological blood flow due to synovitis/tenosynovitis. As advanced technology constantly increases sensitivity to detect very slow blood flow in normal joints, whether very mild Doppler signals inside synovial hypertrophy should be considered as pathological has become an important issue of debate. False-positive due to reverberation can be avoided by (1) scanning with a region of interest for the Doppler mode covering the superficial area not to overlook blood vessels, (2) scanning with multiple imaging planes to distinguish whether the Doppler signals are only present or increased below the blood vessels (Supplementary Figure and Videos 22 and 23), and (3) finding an optimal imaging plane on which blood vessels are not present above the structure if possible.
Although we provide representative still and video images that illustrate major factors related to false-positives in the sonographic evaluation of synovial inflammation, these images and videos do not cover all pitfalls that are characteristic or specific to each joint region. However, the structured list of factors related to false-positives determined by consensus in our study () can be utilized as framework to further identify and classify joint-specific pitfalls.
In conclusion, our study provides a set of representative images that can help sonographers to understand possible false-positives in the evaluation of synovial/tenosynovial inflammation and also provides framework to develop joint-region-specific pitfall atlases. Increased awareness of false-positive pitfalls by our study would improve the specificity of ultrasound-detected inflammation in synovial tissues and further maximize the benefits of musculoskeletal ultrasound in the management of rheumatic conditions.
Supplementary material available online
US_Synovitis_Falsepositives_SupplementaryFigure_30Apr2015.pdf
Download PDF (2 MB)imor_a_1091123_sm9205.avi
Download Microsoft Video (AVI) (19.7 MB)imor_a_1091123_sm9265.avi
Download Microsoft Video (AVI) (25.1 MB)imor_a_1091123_sm9277.avi
Download Microsoft Video (AVI) (11.3 MB)imor_a_1091123_sm9281.avi
Download Microsoft Video (AVI) (9.9 MB)imor_a_1091123_sm9282.avi
Download Microsoft Video (AVI) (6.6 MB)imor_a_1091123_sm9283.avi
Download Microsoft Video (AVI) (3.8 MB)imor_a_1091123_sm9288.avi
Download Microsoft Video (AVI) (13.1 MB)imor_a_1091123_sm9292.avi
Download Microsoft Video (AVI) (7.4 MB)imor_a_1091123_sm9351.avi
Download Microsoft Video (AVI) (8 MB)imor_a_1091123_sm9603.avi
Download Microsoft Video (AVI) (17.9 MB)imor_a_1091123_sm9703.avi
Download Microsoft Video (AVI) (24.7 MB)imor_a_1091123_sm9730.avi
Download Microsoft Video (AVI) (12.7 MB)imor_a_1091123_sm9780.avi
Download Microsoft Video (AVI) (6 MB)imor_a_1091123_sm9804.avi
Download Microsoft Video (AVI) (12.2 MB)imor_a_1091123_sm9826.avi
Download Microsoft Video (AVI) (7.1 MB)imor_a_1091123_sm9845.avi
Download Microsoft Video (AVI) (19 MB)imor_a_1091123_sm9846.avi
Download Microsoft Video (AVI) (3.3 MB)imor_a_1091123_sm9853.avi
Download Microsoft Video (AVI) (18.7 MB)imor_a_1091123_sm9855.avi
Download Microsoft Video (AVI) (2.8 MB)imor_a_1091123_sm9864.avi
Download Microsoft Video (AVI) (2.4 MB)imor_a_1091123_sm9888.avi
Download Microsoft Video (AVI) (5.2 MB)imor_a_1091123_sm9897.avi
Download Microsoft Video (AVI) (28.9 MB)Acknowledgments
We thank Mr. Gentetsu Hirayama and the Japan College of Rheumatology office for their contribution to the data management.
Conflict of interest
This work was supported in part by a Health Labour Sciences Research Grant on Allergic Disease and Immunology from the Ministry of Health, Labor and Welfare of Japan.
References
- Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D'Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–7.
- Wakefield RJ, Green MJ, Marzo-Ortega H, Conaghan PG, Gibbon WW, McGonagle D, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63:382–5.
- Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis. 2005;64:375–81.
- Ogishima H, Tsuboi H, Umeda N, Horikoshi M, Kondo Y, Sugihara M, et al. Analysis of subclinical synovitis detected by ultrasonography and low-field magnetic resonance imaging in patients with rheumatoid arthritis. Mod Rheumatol. 2014;24:60–8.
- Yoshimi R, Hama M, Takase K, Ihata A, Kishimoto D, Terauchi K, et al. Ultrasonography is a potent tool for the prediction of progressive joint destruction during clinical remission of rheumatoid arthritis. Mod Rheumatol. 2013;23:456–65.
- Horikoshi M, Suzuki T, Sugihara M, Kondo Y, Tsuboi H, Uehara T, et al. Comparison of low-field dedicated extremity magnetic resonance imaging with articular ultrasonography in patients with rheumatoid arthritis. Mod Rheumatol. 2010;20:556–60.
- Terslev L, Torp-Pedersen S, Savnik A, von der Recke P, Qvistgaard E, Danneskiold-Samsoe B, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study. Arthritis Rheum. 2003;48:2434–41.
- Kawashiri SY, Suzuki T, Nakashima Y, Horai Y, Okada A, Nishino A, et al. Synovial inflammation assessed by ultrasonography correlates with MRI-proven osteitis in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53:1452–6.
- Takase K, Ohno S, Takeno M, Hama M, Kirino Y, Ihata A, et al. Simultaneous evaluation of long-lasting knee synovitis in patients undergoing arthroplasty by power Doppler ultrasonography and contrast-enhanced MRI in comparison with histopathology. Clin Exp Rheumatol. 2012;30:85–92.
- Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–8.
- Freeston JE, Wakefield RJ, Conaghan PG, Hensor EM, Stewart SP, Emery P. A diagnostic algorithm for persistence of very early inflammatory arthritis: the utility of power Doppler ultrasound when added to conventional assessment tools. Ann Rheum Dis. 2010;69:417–19.
- Filer A, de Pablo P, Allen G, Nightingale P, Jordan A, Jobanputra P, et al. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann Rheum Dis. 2011;70:500–7.
- Nakagomi D, Ikeda K, Okubo A, Iwamoto T, Sanayama Y, Takahashi K, et al. Ultrasound can improve the accuracy of the 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis to predict the requirement for methotrexate treatment. Arthritis Rheum. 2013;65:890–8.
- Kawashiri SY, Suzuki T, Okada A, Yamasaki S, Tamai M, Nakamura H, et al. Musculoskeletal ultrasonography assists the diagnostic performance of the 2010 classification criteria for rheumatoid arthritis. Mod Rheumatol. 2013;23:36–43.
- Colebatch AN, Edwards CJ, Ostergaard M, van der Heijde D, Balint PV, D'Agostino MA, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–14.
- Fukae J, Shimizu M, Kon Y, Tanimura K, Matsuhashi M, Kamishima T, et al. Screening for rheumatoid arthritis with finger joint power Doppler ultrasonography: quantification of conventional power Doppler ultrasonographic scoring. Mod Rheumatol. 2009;19:502–6.
- Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57:116–24.
- Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67.
- Naredo E, Moller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:2248–56.
- Fukae J, Isobe M, Kitano A, Henmi M, Sakamoto F, Narita A, et al. Radiographic prognosis of finger joint damage predicted by early alteration in synovial vascularity in patients with rheumatoid arthritis: Potential utility of power doppler sonography in clinical practice. Arthritis Care Res (Hoboken). 2011;63:1247–53.
- Ikeda K, Nakagomi D, Sanayama Y, Yamagata M, Okubo A, Iwamoto T, et al. Correlation of radiographic progression with the cumulative activity of synovitis estimated by power Doppler ultrasound in rheumatoid arthritis: difference between patients treated with methotrexate and those treated with biological agents. J Rheumatol. 2013;40:1967–76.
- Kirino Y, Hama M, Takase-Minegishi K, Kunishita Y, Kishimoto D, Yoshimi R, et al. Predicting joint destruction in rheumatoid arthritis with power Doppler, anti-citrullinated peptide antibody, and joint swelling. Mod Rheumatol. 2015:1–7.
- Iwamoto T, Ikeda K, Hosokawa J, Yamagata M, Tanaka S, Norimoto A, et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission. Arthritis Care Res (Hoboken). 2014;66:1576–81.
- Ogasawara M, Murayama G, Yamada Y, Nemoto T, Kageyama M, Toyama S, et al. Autofeedback from ultrasound images provides rapid improvement in palpation skills for identifying joint swelling in rheumatoid arthritis. J Rheumatol. 2012;39:1207–14.
- Hama M, Takase K, Ihata A, Ohno S, Ueda A, Takeno M, et al. Challenges to expanding the clinical application of musculoskeletal ultrasonography (MSUS) among rheumatologists: from a second survey in Japan. Mod Rheumatol. 2012;22:202–8.
- Ikeda K, Seto Y, Narita A, Kawakami A, Kawahito Y, Ito H, et al. Ultrasound assessment of synovial pathologic features in rheumatoid arthritis using comprehensive multiplane images of the second metacarpophalangeal joint: identification of the components that are reliable and influential on the global assessment of the whole joint. Arthritis Rheumatol. 2014;66:523–32.
- Gartner M, Mandl P, Radner H, Supp G, Machold KP, Aletaha D, et al. Sonographic joint assessment in rheumatoid arthritis: associations with clinical joint assessment during a state of remission. Arthritis Rheum. 2013;65:2005–14.
- Yoshimi R, Hama M, Minegishi K, Kishimoto D, Watanabe T, Kamiyama R, et al. Ultrasonography predicts achievement of Boolean remission after DAS28-based clinical remission of rheumatoid arthritis. Mod Rheumatol. 2014;24:590–8.
- Ikeda K, Koike T, Wakefield R, Emery P. Is the glass half full or half empty? Comment on the article by Gartner et al. Arthritis Rheumatol. 2014;66:1055–6.
- Egund N, Wingstrand H. Pitfalls in ultrasonography of hip joint synovitis in the child. Acta Radiol. 1989;30:375–9.
- Soini I, Kotaniemi A, Kautiainen H, Kauppi M. US assessment of hip joint synovitis in rheumatic diseases. A comparison with MR imaging. Acta Radiol. 2003;44:72–8.
- Fiocco U, Ferro F, Cozzi L, Vezzu M, Sfriso P, Checchetto C, et al. Contrast medium in power Doppler ultrasound for assessment of synovial vascularity: comparison with arthroscopy. J Rheumatol. 2003;30:2170–6.
- Karim Z, Wakefield RJ, Quinn M, Conaghan PG, Brown AK, Veale DJ, et al. Validation and reproducibility of ultrasonography in the detection of synovitis in the knee: a comparison with arthroscopy and clinical examination. Arthritis Rheum. 2004;50:387–94.
- Terslev L, Torp-Pedersen S, Qvistgaard E, von der Recke P, Bliddal H. Doppler ultrasound findings in healthy wrists and finger joints. Ann Rheum Dis. 2004;63:644–8.
- Ellegaard K, Torp-Pedersen S, Holm CC, Danneskiold-Samsoe B, Bliddal H. Ultrasound in finger joints: findings in normal subjects and pitfalls in the diagnosis of synovial disease. Ultraschall Med. 2007;28:401–8.
- Robertson BL, Jamadar DA, Jacobson JA, Kalume-Brigido M, Caoili EM, Margaliot Z, et al. Extensor retinaculum of the wrist: sonographic characterization and pseudotenosynovitis appearance. Am J Roentgenol. 2007;188:198–202.
- Luukkainen R, Ekman P, Luukkainen P, Koski JM. Ultrasonographic findings in metatarsophalangeal and talocrural joints in healthy persons. Clin Rheumatol. 2009;28:311–13.
- Millot F, Clavel G, Etchepare F, Gandjbakhch F, Grados F, Saraux A, et al. Musculoskeletal ultrasonography in healthy subjects and ultrasound criteria for early arthritis (the ESPOIR cohort). J Rheumatol. 2011;38:613–20.
- Magni-Manzoni S, Scire CA, Ravelli A, Klersy C, Rossi S, Muratore V, et al. Ultrasound-detected synovial abnormalities are frequent in clinically inactive juvenile idiopathic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis. 2013;72:223–8.
- Sant'Ana Petterle G, Natour J, Rodrigues da Luz K, Soares Machado F, dos Santos MF, da Rocha Correa Fernandes A, et al. Usefulness of US to show subclinical joint abnormalities in asymptomatic feet of RA patients compared to healthy controls. Clin Exp Rheumatol. 2013;31:904–12.
- Bruyn GA, Hanova P, Iagnocco A, d'Agostino MA, Moller I, Terslev L, et al. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focussing on the diagnostic reliability. Ann Rheum Dis. 2014;73:1929–34.
- Naredo E, D'Agostino MA, Wakefield RJ, Moller I, Balint PV, Filippucci E, et al. Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann Rheum Dis. 2013;72:1328–34.
- Naredo E, Rodriguez M, Campos C, Rodriguez-Heredia JM, Medina JA, Giner E, et al. Validity, reproducibility, and responsiveness of a twelve-joint simplified power doppler ultrasonographic assessment of joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2008;59:515–22.
- Hiraga M, Ikeda K, Shigeta K, Sato A, Yoshitama T, Hara R, et al. Sonographic measurements of low-echoic synovial area in the dorsal aspect of metatarsophalangeal joints in healthy subjects. Mod Rheumatol. 2015;25:386–92.
- Ten Cate DF, Luime JJ, Hazes JM, Kleinrensink GJ, Jacobs JW. Is the frequent sonographic anechoic area distally in metacarpophalangeal joints a sign of arthritis? Ultrasound Med Biol. 2014;40:2537–41.
