Abstract
Objective: A non-synonymous single nucleotide polymorphism (nsSNP, rs2233434, Val194Ala) in the NFKBIE (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, epsilon) gene is known to be a rheumatoid arthritis (RA) susceptibility polymorphism in the Japanese RA population and could be closely associated with nuclear factor kappaB (NF-κB) activity. Inflammation caused by RA is sometimes associated with changes in expression levels of MTX (methotrexate) pathway-related genes. It is of interest to examine whether the NFKBIE gene had any influences on the mode of MTX action.
Methods: Both knockdown of NFKBIE gene expression and overexpression of wild-type NFKBIE and Val194Ala mutation were performed. A transfected human RA synovial cell line was cultured and then gene expressions in the MTX pathway were measured. In addition, we measured the uptake and efflux of MTX derivatives under the NFKBIE knockdown condition.
Results: Knockdown of NFKBIE reduced the mRNA for SLC19A1, a main MTX membrane transporter, and the intracellular accumulations of MTX derivatives. Moreover, our experiments also confirmed that overexpression of Val194Ala mutant NFKBIE decreased the SLC19A1 mRNA when compared to that of wild-type NFKBIE.
Conclusions: We suggest that the impairment of NFKBIE gene function can reduce the uptake of MTX into cells, suggesting that the gene is an important factor for the RA outcome.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease that affects many tissues and organs, which is especially characterized by persistent synovial tissue inflammation, leading to the progressive joint disorder. In most RA patients, the activation of nuclear factor kappaB (NF-κB), a hallmark of inflammatory responses, plays an important role in the expression of cytokines in the synovial tissue [Citation1–3].
Methotrexate (MTX) is the most commonly used anchor drug for treating RA. MTX is a folate analog, and its anti-rheumatic mechanism has been discussed; the effect of MTX in vivo may be mediated by reducing cell proliferation, increasing the rate of apoptosis of T cells, and endogenous adenosine release; altering the expression of cellular adhesion molecules; and influencing the production of cytokines, humoral responses, and bone formation [Citation4].
A non-synonymous single nucleotide polymorphism (nsSNP, rs2233434, Val194Ala) in the NFKBIE (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, epsilon) gene has been reported to be an RA susceptibility polymorphisms using a large-scaled genome-wide association study (GWAS) meta-analysis in Japanese RA patients [Citation5]. NFKBIE encodes I kappaB-epsilon (IκBɛ), a member of the Inhibitor of kappaB (IκB) family and its mRNA is widely expressed in different human tissues. Additionally, the gene is known to inhibit the nuclear translocation of NF-κB by binding to NF-κB proteins [Citation6,Citation7]. For this reason, the risk haplotype of this nsSNP in NFKBIE has been reported to show an enhancement of NF-κB activity in transfected cells compared with the wild-type construct [Citation8], following changes in IκBɛ structure. In a study on the use of human peripheral blood cells, Blits et al. observed that the expressions of MTX pathway genes were increased in MTX-naive RA patients compared to those of healthy controls [Citation9]. Additionally, from the study using synovial tissues obtained from RA patients, Stamp et al. noted a negative correlation between the ESR (erythrocyte sedimentation rate) and expressions levels of some MTX pathway genes [Citation10]. These findings led us to wonder whether MTX pathway genes are influenced by the NFKBIE gene.
In the present study, we hypothesized that NFKBIE may play a role in regulating the expression levels of MTX pathway genes (i.e. drug membrane transporters and MTX-metabolizing enzymes). To test our hypothesis, we examined the effect of knockdown of NFKBIE or overexpression of wild-type NFKBIE and the mutant (Val194Ala) NFKBIE on the gene expression levels of “drug membrane transporters” that are closely related to the intracellular accumulations of MTX in a human RA synovial cell line. We also performed an investigation of “MTX-metabolizing enzyme” expression in the similar culture conditions. Knockdown of NFKBIE expression resulted in down-regulation of SLC19A1 (solute carrier family 19 (folate transporter), member 1) expression and intracellular accumulations of MTX derivatives. These results suggest that impairing the effect of the NFKBIE gene may reduce cellular uptake of MTX by down-regulating SLC19A1 expression.
Materials and methods
Cell culture
MH7A, a human RA synovial cell line, was purchased from Riken Cell Bank (Tsukuba, Japan). MH7A is established by transfection with the SV40 T antigen, and the cells are fibroblast-like synoviocytes [Citation11]. Cells were cultured in RPMI 1640 medium (Sigma-Aldrich, Tokyo, Japan) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Tokyo, Japan) in a humidified incubator at 37°C under 5% CO2. For experiments, MH7A was trypsinized and passaged to 24-well dishes.
Chemicals
MTX, TNF-α, and IL-1β were purchased from Sigma-Aldrich. Stock solutions of MTX (dissolved in 0.1 N NaOH and then diluted in PBS), TNF-α, and IL-1β (diluted in PBS) were stored at −20°C. For these drugs, working concentrations were prepared by diluting stock solutions in a culture medium immediately before use.
Small interfering RNA (siRNA) transfection
To knockdown endogenous NFKBIE, cells were transfected with 20 pmol ON-TARGETplus NFKBIE siRNA (Dharmacon, Tokyo, Japan) using Lipofectamine 2000 (Invitrogen, Tokyo, Japan). Non-targeting control siRNA (Dharmacon) was used as a transfection control and knockdown efficiency was examined by reverse transcription quantitative real-time PCR (qRT-PCR). Cells were plated at 0.75 × 105 cells/well and cultured for 24 h; then the cells were transfected for 24 h. Subsequently, they were incubated with or without 0.01, 0.1, 1, 5, and 10 μM MTX for 24 h in the presence or absence of 20 U/mL IL-1β and 20 U/mL TNF-α.
Measurement of the uptake and efflux of MTX derivatives
After washing the cells with PBS, the cells were incubated in the presence of 5 μM fluorescein methotrexate (Life Technologies, Tokyo, Japan), an MTX derivative, for 5 h. In another experiment, pulse chase analysis of the efflux of the MTX derivative was performed. Two groups of transiently transfected cells were incubated with 5 μM MTX derivatives for 5 h and then washed with PBS, followed by chase for 0 and 6 h exclusively in RPMI supplemented with 10% FBS. On the basis of these results, we studied differences in the efflux of the MTX derivative between control and NFKBIE knockdown cells. Before analysis, cells were treated with trypsin (Wako, Osaka, Japan) to obtain a single cell suspension. Intracellular accumulation of MTX derivatives was measured with a Cell Lab Quanta SC (Beckman Coulter) flow cytometer and mean fluorescence intensities was calculated on the basis of our arbitrary drawing regions on the histogram. As a result, at least 6000 cells were evaluated.
Plasmid constructs and preparations
The full coding IκBɛ clone was constructed by fusing two overlapping cDNAs for residue 26-807 from HEK293, for residue 793-1528 from MH7A with appropriate primer pairs (HEK293 forward: 5′-ATG AAT CAA CGA AGG AGT GAG TCA AGG CC-3′, HEK293 reverse: 5′-GTG TCT CCG TCC TCG GAG ATG TAA GTG AGT GC-3′; MH7A forward: 5′-CGA GGA CGG AGA CAC GCT GGT CCA CCT GGC AGT GA-3′, MH7A reverse: 5′-TCA GTC GGT ACA CAG CAG CAG TTT CCC TG-3′) and KOD-Plus-Neo (TOYOBO). PCR products were inserted into the pFLAG-CMV5 vector, which was generated by replacing the Myc-tag of pMyc_CMV5 (a gift from Dr. David W. Russell in UT Southwestern Medical Center, Dallas, TX) with a Flag-tag, between HindIII and BamHI using the 5 × In-Fusion HD Enzyme Premix (Clontech, Shiga, Japan). The 581T > C (Val194Ala) mutation was generated using the 5 × In-Fusion HD Enzyme Premix with the wild-type NFKBIE construct as a template and the following primers (forward: 5′-CGG GAC CCG CCA AGG AAC CAC AGG AGA A-3′; reverse: 5′-CCT TGG CGG GTC CCG GAG GAT GGG TGC A-3′). Both wild-type and mutated constructs were sequenced to verify that only the desired constructs were present.
To overexpress wild-type or Val194Ala mutant NFKBIE, cells were plated at 1.50 × 105 cells/well and cultured for 24 h. Cells were transfected with 0.8 μg of plasmid DNA using Lipofectamine 2000 and empty pFLAG-CMV5 plasmid was used as the transfection control (Mock) and then incubated for 18 h. After changing the medium, the cells were incubated with or without 0.01, 0.1, 1, 5, and 10 μM MTX for 24 h in the presence or absence of both 20 U/mL IL-1β and 20 U/mL TNF-α.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions was used as a template for one-step qRT-PCR with One Step SYBR® PrimeScript™ RT-PCR Kit II (Perfect Real Time) (TaKaRa, Shiga, Japan). Amplification of the cDNA was performed using specific oligonucleotide primers () with Applied Biosystems 7500 Real-Time PCR system according to the supplier’s recommendations. The program used for qRT-PCR amplification included a 10-s inactivation of reverse transcriptase at 95 °C, a 5-s denaturation step at 95°C, a 10-s annealing step at 58°C, a 34-s extension step at 72°C (for 35 cycles), and a dissociation step (15 s at 95°C, 60 s at 60°C, and 15 s at 95°C). To determine the specificity of each primer set, we performed melting curve analysis after the completion of PCR amplification. The accumulated levels of fluorescence were analyzed by the fit-point method after the melting curve analysis. The expression levels of mRNA were calculated using the 2−ΔΔCT method as previously described [Citation12]. NFKBIE, SLC19A1, ABCC1 (ATP-binding cassette, sub-family C (CFTR/MRP), member 1), ABCC5 (ATP-binding cassette, sub-family C (CFTR/MRP), member 5), ABCG2 (ATP-binding cassette, sub-family G (WHITE), member 2), FPGS (folylpolyglutamate synthase), GGH (gamma-glutamyl hydrolase (conjugase, folylpolygammaglutamyl hydrolase)), DHFR (dihydrofolate reductase), TYMS (thymidylate synthetase), MTHFR (methylenetetrahydrofolate reductase (NAD(P)H)), and ATIC (5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase) gene mRNA levels were normalized with housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) in each sample.
Table 1. Primers for quantitative real-time PCR (qPCR).
Total cell and viable cell counting
All cells in one well were collected after trypsinization. The cell proliferation and viability were evaluated by counting the cells and 0.4% trypan blue (Wako) staining using the TC20 automated cell counter (Bio-Rad Laboratories) according to the manufacturer's instructions. To evaluate the number of total cells and live cells, we mixed the cell suspension 1:1 with 0.4% trypan blue solution and pipetted 10 μL of this mixture onto a counting slide specific for the TC20 automated cell counter.
Statistical analysis
All of the data were compared using Student’s t-test.
Results
Effects of NFKBIE knockdown by siRNA in drug membrane transporters
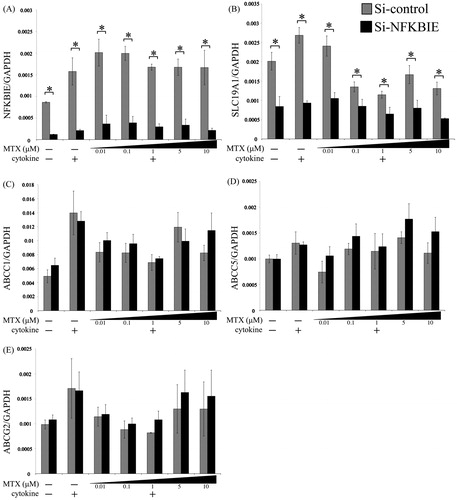
To investigate the effects of NFKBIE knockdown on the expressions of drug membrane transporters we confirmed expression by qRT-PCR. The NFKBIE expression level in the NFKBIE knockdown group was almost 12–20% of the control expression level under many conditions (). NFKBIE knockdown could reduce the SLC19A1 expression (), whereas there was no obvious alteration in the expressions of the ABC transporters ().
Figure 1. Effects of NFKBIE knockdown on the expression levels of SLC19A1 and ABC transporters. Quantitative gene expressions of NFKBIE (A), SLC19A1 (B), ABCC1 (C), ABCC5 (D), and ABCG2 (E) genes in control (gray) and NFKBIE knockdown (black) MH7A treated with or without 0.01, 0.1, 1, 5, and 10 μM methotrexate (MTX) application for 24 h in the presence or absence of 20 U/mL IL-1β and 20 U/mL TNF-α were measured by qRT-PCR. Statistical significance was analyzed using the unpaired t-test (*p < 0.05). The results are presented as the mean ± standard error of the mean (SEM) of at least three independent experiments.
Uptake and efflux of MTX derivatives under the knockdown condition of NFKBIE by siRNA
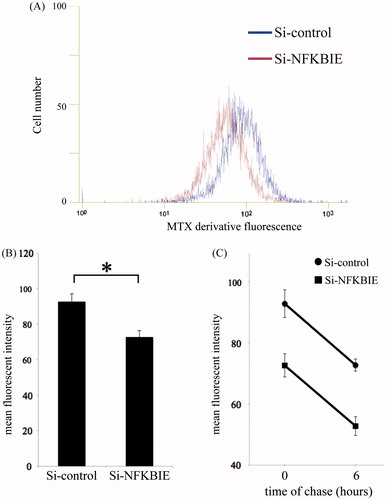
Because several research studies have estimated that both the uptake and accumulation of MTX derivatives in mammalian cells are strongly affected by differences in the SLC19A1 expression [Citation13–18], we examined both the uptake and efflux of MTX derivatives and measured functional SLC19A1 expressed in transfectant MH7A in an inflammatory milieu that mimics RA joints using flow cytometry. To determine whether knockdown of NFKBIE by siRNA affects the accumulation of MTX, MH7A was transfected and pre-treated with cytokines; then MTX derivative uptake into cells was examined. As shown in , knockdown of NFKBIE reduced the accumulation of MTX derivatives. But, we found no significant differences in the efflux of MTX derivatives between the control and NFKBIE knockdown ().
Figure 2. Uptake and efflux of a methotrexate (MTX) derivative under knockdown of NFKBIE by siRNA in MH7A. MH7A cells were cultured for 24 h, and then transfected for 24 h. They were further incubated with 20 U/mL IL-1β and 20 U/mL TNF-α for 24 h. After washing the cells with PBS, the cells were incubated in the presence of 5 μM of MTX derivatives for 5 h. The uptake of MTX derivatives was quantified by measuring the fluorescence emission for each sample. (A) The representative histogram of control (blue) and knockdown of NFKBIE (dark red). The mean fluorescent intensity (MFI) per cell, expressed in arbitrary units, was then determined using a flow cytometer. (B) The summary results. In addition to these experiments, after incubating with MTX derivatives (5 μM) for 5 h and washing cells with PBS, cells were chased for 6 h exclusively in RPMI supplemented with 10% FBS. Subsequently, the MFI per cell was measured at 0 and 6 h. On the basis of these results, differences in the efflux of MTX derivatives between control and NFKBIE knockdown were studied (C). Each column and its corresponding vertical lines represent the mean and the standard error of the mean (SEM) of triplicate cultures. Statistical significance was analyzed with the unpaired t-test (*p < 0.05).
Effects of NFKBIE Val194Ala overexpression on drug membrane transporters
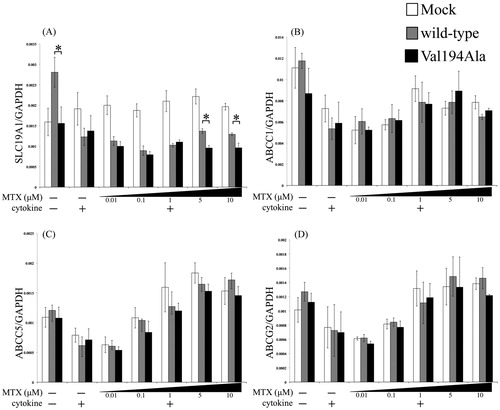
We transiently overexpressed either wild-type, Val194Ala or Mock into MH7A and incubated as described above. Small but statistically significant differences in the SLC19A1 expression levels at a significant MTX level (>5μM) were noted; the wild-type overexpression group exhibited more SLC19A1 expression than the Val194Ala overexpression group (). In addition, this graph shows that overexpression of the wild-type gene had significantly more upregulated expression of SLC19A1 than NFKBIE Val194Ala under no cytokines condition (). There were no statistical changes between wild-type and Val194Ala in the expressions of ABC transporters ().
Figure 3. Effects of overexpression of Mock, wild-type NFKBIE, and Val194Ala mutant NFKBIE on the expressions of SLC19A1 and the ABC transporters. Quantitative gene expressions of SLC19A1 (A), ABCC1 (B), ABCC5 (C), and ABCG2 (D) genes in Mock (white), overexpression of wild-type NFKBIE (gray), and Val194Ala mutant NFKBIE (black). Those cells were treated with or without 0.01, 0.1, 1, 5, and 10 μM methotrexate (MTX) application for 24 h in the presence or absence of 20 U/mL IL-1β and 20 U/mL TNF-α. The expression levels of each gene were measured by qRT-PCR. Statistical significance was analyzed using the unpaired t-test (*p < 0.05). The results are presented as the mean ± standard error of the mean (SEM) of at least three independent experiments. *p < 0.05 versus wild-type.
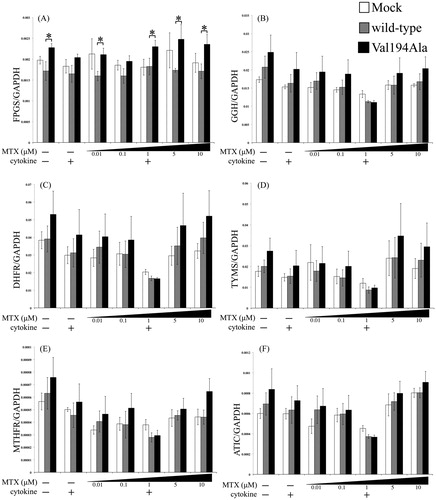
MTX-metabolizing enzymes expressions under knockdown and overexpression conditions
The expression levels of MTX-metabolizing enzymes including FPGS, GGH, TYMS, DHFR, MTHFR, and ATIC were observed under different conditions ( and ). Our data indicate that the expressions of MTX-metabolizing enzymes except for FPGS had no significant associations either between the control and NFKBIE knockdown, or between wild-type and Val194Ala NFKBIE overexpression ( and ). In FPGS expression, we observed that there was a general tendency towards a difference between the overexpression of the wild-type and Val194Ala gene in the overexpression study alone ().
Figure 4. Effects of NFKBIE knockdown on the expressions of MTX-metabolizing enzymes. Quantitative gene expressions of FPGS (A), GGH (B), DHFR (C), TYMS (D), MTHFR (E), and ATIC (F) genes in control (gray) and NFKBIE knockdown (black) MH7A. Those cells were treated with or without 0.01, 0.1, 1, 5, and 10 μM methotrexate (MTX) application for 24 h in the presence or absence of 20 U/mL IL-1β and 20 U/mL TNF-α. The expression levels of each gene were measured by qRT-PCR. Statistical significance was analyzed by the unpaired t-test (*p < 0.05). The results are presented as the mean ± standard error of the mean (SEM) of at least three independent experiments.
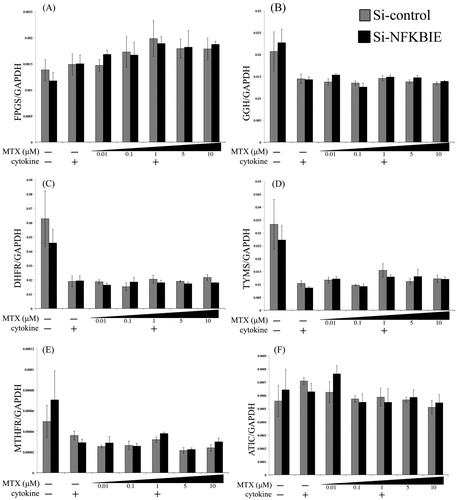
Figure 5. Effects of overexpression of Mock, wild-type NFKBIE, and Val194Ala mutant NFKBIE on MTX-metabolizing enzyme expressions. Quantitative gene expressions of FPGS (A), GGH (B), DHFR (C), TYMS (D), MTHFR (E), and ATIC (F) genes in Mock (white), overexpression of wild-type NFKBIE (gray) and Val194Ala mutant NFKBIE (black). Those cells were treated with or without 0.01, 0.1, 1, 5, and 10 μM methotrexate (MTX) application for 24 h in the presence or absence of 20 U/mL IL-1β and 20 U/mL TNF-α. The expression levels of each gene were measured by qRT-PCR. Statistical significance was analyzed using the unpaired t-test (*p < 0.05). The results are presented as the mean ± standard error of the mean (SEM) of at least three independent experiments. *p < 0.05 versus wild-type.
Discussion
We have shown that, in a human RA synovial cell line, knockdown of NFKBIE expression results in the down-regulation of SLC19A1 but does not influence ABC transporters or MTX-metabolizing enzymes. In addition, we have demonstrated that NFKBIE knockdown of MH7A results in lower intracellular accumulation of MTX derivatives than control MH7A. Furthermore, overexpression of the Val194Ala mutant NFKBIE resulted in decreased SLC19A1 levels when compared to that of wild-type NFKBIE both above a specified MTX concentration (more than 5 μM) with cytokines and at a cytokine-free environment. ABC transporters and MTX-metabolizing enzymes expressions except for FPGS, lacked a significant difference between the overexpressed wild-type and Val194Ala NFKBIE constructs. In FPGS gene expression, there was a tendency of slight upregulation in the Val194Ala overexpression group compared to the wild-type overexpression group.
IκBɛ is known to be expressed in human synovial cells [Citation19]. In IκBɛ-deficient mice, the expression of inflammatory cytokines, such as IL-6, IL-1β, IL-1α, and IL-1Ra, was constitutively increased compared to that in wild-type mice; also, there was increased B cell proliferation and survival [Citation20,Citation21]. On the other hand, overexpression of IκBɛ with the nsSNP (rs2233433; Val194Ala) vector had an enhancement of NF-κB activity compared with the wild-type construct [Citation8].
SLC19A1, a reduced folate carrier, is the most dominant folate transporter with high affinity for reduced folates and the antifolate drug MTX, but it has very low affinity for folic acid [Citation22–24]. MTX enters the mammalian cells largely via SLC19A1 and it effluxes from cells via ATP-binding cassette (ABC) transporters, which is to say that cellular uptake of MTX via SLC19A1 is considered as the initial step for exerting its effect [Citation25,Citation26]. Once entering the cells mainly via SLC19A1, MTX is polyglutamated by FPGS, retained as a polyglutamated form within the cell [Citation27]. Polyglutamated MTX can be reversed to the nonpolyglutamated form by GGH and then extruded from cells via ABC transporters [Citation28]. Monoglutamated MTX and polyglutamated MTX have equal binding affinity for DHFR, but polyglutamated MTX rather exerts a stronger inhibition of DHFR, TYMS, and ATIC [Citation29–31]. DHFR plays a key role in converting dihydrofolate (DHF) to their active form tetrahydrofolate (THF), which is needed for several one-carbon transfer reactions in de novo synthesis of varieties of essential metabolites (e.g. purines, pyrimidines, etc.) [Citation32]. TYMS is an important gene in de novo pyrimidine synthesis and for DNA synthesis and repair [Citation33]. ATIC is an enzyme involved in the de novo purine synthesis pathway that is responsible for the conversion of AICAR into formyl-AICAR (FAICAR) [Citation34]. Intracellular accumulation of AICAR owing mainly to direct inhibition of ATIC by polyglutamated MTX leads to increased intracellular adenosine, a potent anti-inflammatory agent, and its consequent release to the extracellular space [Citation35]. Additionally, MTHFR is thought to be a key enzyme that regulates DNA synthesis and DNA methylation [Citation36]. Although, MTHFR is not directly inhibited by MTX or by polyglutamated MTX, its expression level is recognized to be involved in MTX chemosensitivity [Citation37].
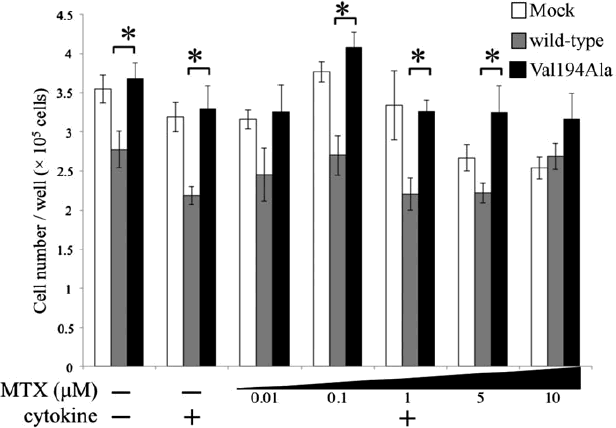
Within the cell, folates, known as essential vitamins that serve as one-carbon donors in many biosynthetic pathways, also undergo polyglutamylation catalyzed by FPGS. As a result, polyglutamylated folates enhanced the cellular retention and thus exert stronger effects [Citation38]. The mRNA level of FPGS is upregulated by cell proliferation and reduced by cell maturation [Citation39,Citation40]. The results obtained in synovial cells demonstrated that activated NF-κB pathways play an important role in cell proliferation [Citation2]. We measured cell proliferation of overexpressing wild-type NFKBIE and Val194Ala NFKBIE by a cell counter. As a result, it was found that cells overexpressing Val194Ala constructs have a tendency for more cell-proliferative activity than those overexpressing wild-type constructs (Appendix 1). This observation is in agreement with our result of FPGS expression in an overexpression study.
The ABC transporter genes including ABCC1, ABCC5, and ABCG2 utilize the energy of ATP hydrolysis to translocate their substrates across biological membranes [Citation24]. We have chosen these ABC transporter genes because, in previous studies, transfectant cells overexpressing ABCC1 and ABCC5 displayed more MTX resistance levels than those of other ABC transporters as well as because ABCG2 is a transporter of both MTX monoglutamates as well as di- and triglutamate conjugates of MTX, which is an important feature differentiating ABCG2 from other transporters [Citation24]. We found little differences in the mRNA expressions of those efflux transporters.
Although, the molecular mechanisms for the reduction of SLC19A1 expression are still unknown, our results strongly suggest that impairment of NFKBIE gene function suppressed SLC19A1 expression. Studies in mouse synovial cells show that SLC19A1 expression was down-regulated by inflammatory components, such as IL-6 [Citation18]. In RA patients, there was a significant negative correlation between SLC19A1 expression and ESR [Citation10]. These studies lead us to believe that SLC19A1 down-regulation is associated with inflammation. MTX itself is closely related to SLC19A1 expression [Citation18,Citation41]. More recently, it has become more obvious that MTX induces proinflammatory cytokines, such as IL-6, which may be one of the reasons MTX has some effects on the expression of SLC19A1 [Citation42,Citation43]. As previously mentioned, IκB-deficient mice have increased expression of some inflammatory cytokines [Citation20,Citation21]. SLC19A1 expression is reportedly upregulated under the folate-restricted condition, but increased folate availability suppresses its expression [Citation44,Citation45]. Jansen et al. identified that, upon growth in a low folate medium, a mutation in the SLC19A1 gene results in increasing affinity of transporters for (anti)folates and that in folate-replete medium, the preferential folate transport results in a markedly expanded intracellular folate pool impairing antifolate metabolism [Citation46]. We demonstrated that in an overexpression study, there were no significant differences in the SLC19A1 expression level between wild-type and Val194Ala at a low MTX concentration (0.01–1 μM) of cytokines. Because the medium used in our study contained far (approximately 100 times) higher levels of folate than the in vivo folate level [Citation46], the dose-effect relationship between MTX and SCL19A1 suppression may also be shifted to the higher concentration side.
In conclusion, the results obtained in this paper support the hypothesis that NFKBIE may play a role in regulating the expression of some MTX pathway genes in a cell culture model which reproduced the synovial cell context in RA joints as accurately as possible by the addition of inflammatory cytokines, and the results may be helpful for further understanding of the MTX-resistant mechanism. This study provides an important contribution because these findings were detected in NFKBIE (rs2233434, Val194Ala), an RA susceptibility gene locus in a large-scaled GWAS meta-analysis for RA in the Japanese population. The present study suggests that impairment of NFKBIE gene function may result in the down-regulation of SLC19A1, reduction of MTX uptake, and MTX-resistance, although further research with additional in vivo studies is also needed.
Acknowledgements
We thank members of the Mitani Laboratory for discussion.
Conflict of interest
KI received honorarium for the lecture and/or unrestricted research grants from AbbVie, Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Bristol-Myers Squibb Co., Chugai Pharmaceutical Co., Eisai Co., Ltd., Hisamitsu Pharmaceutical Co. Inc., Janssen Pharmaceutical K.K., Kaken Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Co., Santen Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co., Ltd. SM received honorarium for the lecture and/or unrestricted research grants from AbbVie, Inc., Asahi Kasei Pharma Corp., Bristol-Myers Squibb Co., Chugai Pharmaceutical Co., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Co., Nakashima Medical Co., Ltd., Santen Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. The sponsors were not involved in the: study design; collection, analysis, and interpretation of data; writing of the paper; and/or decision to submit for publication.
References
- Handel ML, McMorrow LB, Gravallese EM. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–70.
- Fujisawa K, Aono H, Hasunuma T, Yamamoto K, Mita S, Nishioka K. Activation of transcription factor NF-kappa B in human synovial cells in response to tumor necrosis factor alpha. Arthritis Rheum. 1996;39:197–203.
- Marok R, Winyard PG, Coumbe A, Kus ML, Gaffney K, Blades S, et al. Activation of the transcription factor nuclear factor-kappaB in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–91.
- Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2008;47:249–55.
- Okada Y, Terao C, Ikari K, Kochi Y, Ohmura K, Suzuki A, et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat Genet. 2012;44:511–16.
- Li Z, Nabel GJ. A new member of the I kappaB protein family, I kappaB epsilon, inhibits RelA (p65)-mediated NF-kappaB transcription. Mol Cell Biol. 1997;17:6184–90.
- Whiteside ST, Epinat JC, Rice NR, Israël A. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel NF-kappa B activity. EMBO J. 1997;16:1413–26.
- Myouzen K, Kochi Y, Okada Y, Terao C, Suzuki A, Ikari K, et al. Functional variants in NFKBIE and RTKN2 involved in activation of the NF-κB pathway are associated with rheumatoid arthritis in Japanese. PLoS Genet. 2012;8:e1002949.
- Blits M, Jansen G, Assaraf YG, van de Wiel MA, Lems WF, Nurmohamed MT, et al. Methotrexate normalizes up-regulated folate pathway genes in rheumatoid arthritis. Arthritis Rheum. 2013;65:2791–802.
- Stamp LK, Hazlett J, Highton J, Hessian PA. Expression of methotrexate transporters and metabolizing enzymes in rheumatoid synovial tissue. J Rheumatol. 2013;40:1519–22.
- Miyazawa K, Mori A, Okudaira H. Establishment and characterization of a novel human rheumatoid fibroblast-like synoviocyte line, MH7A, immortalized with SV40 T antigen. J Biochem. 1998;124:1153–62.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
- Baslund B, Gregers J, Nielsen CH. Reduced folate carrier polymorphism determines methotrexate uptake by B cells and CD4 + T cells. Rheumatology (Oxford). 2008;47:451–3.
- Jolivet J, Faure MP, Wong SC, Taub JW, Matherly LH. Confocal microscopy visualization of antifolate uptake by the reduced folate carrier in human leukaemic cells. Br J Cancer. 1997;76:734–8.
- Nagakubo J, Tomimatsu T, Kitajima M, Takayama H, Aimi N, Horie T. Characteristics of transport of fluoresceinated methotrexate in rat small intestine. Life Sci. 2001;69:739–47.
- Jansen G, van der Heijden J, Oerlemans R, Lems WF, Ifergan I, Scheper RJ, et al. Sulfasalazine is a potent inhibitor of the reduced folate carrier: implications for combination therapies with methotrexate in rheumatoid arthritis. Arthritis Rheum. 2004;50:2130–9.
- Kneuer C, Honscha KU, Honscha W. Rat reduced-folate carrier-1 is localized basolaterally in MDCK kidney epithelial cells and contributes to the secretory transport of methotrexate and fluoresceinated methotrexate. Cell Tissue Res. 2005;320:517–24.
- Hashizume M, Yoshida H, Tanaka K, Suzuki M, Matsumoto I, Sumida T, et al. Interleukin-6 regulates anti-arthritic effect of methotrexate via reduction of SLC19A1 expression in a mouse arthritis model. Arthritis Res Ther. 2012;14:R96.
- Stuhlmeier KM, Pollaschek C. Adenovirus-mediated gene transfer of mutated IkappaB kinase and IkappaBalpha reveal NF-kappaB-dependent as well as NF-kappaB-independent pathways of HAS1 activation. J Biol Chem. 2005;280:42766–73.
- Mémet S, Laouini D, Epinat JC, Whiteside ST, Goudeau B, Philpott D, et al. IkappaBepsilon-deficient mice: reduction of one T cell precursor subspecies and enhanced Ig isotype switching and cytokine synthesis. J Immunol. 1999;163:5994–6005.
- Alves BN, Tsui R, Almaden J, Shokhirev MN, Davis-Turak J, Fujimoto J, et al. IκBɛ is a key regulator of B cell expansion by providing negative feedback on cRel and RelA in a stimulus-specific manner. J Immunol. 2014;192:3121–32.
- Henderson GB, Suresh MR, Vitols KS, Huennekens FM. Transport of folate compounds in L1210 cells: kinetic evidence that folate influx proceeds via the high-affinity transport system for 5-methyltetrahydrofolate and methotrexate. Cancer Res. 1986;46:1639–43.
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–57.
- Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat. 2006;9:227–46.
- Agarwal V, Mittal SK, Misra R. Expression of multidrug resistance-1 protein correlates with disease activity rather than the refractoriness to methotrexate therapy in rheumatoid arthritis. Clin Rheumatol. 2009;28:427–33.
- van der Heijden JW, Oerlemans R, Tak PP, Assaraf YG, Kraan MC, Scheffer GL, et al. Involvement of breast cancer resistance protein expression on rheumatoid arthritis synovial tissue macrophages in resistance to methotrexate and leflunomide. Arthritis Rheum. 2009;60:669–77.
- Stark M, Wichman C, Avivi I, Assaraf YG. Aberrant splicing of folylpolyglutamate synthetase as a novel mechanism of antifolate resistance in leukemia. Blood. 2009;113:4362–9.
- Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012;15:183–210.
- Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146:489–503.
- Whitehead VM. Synthesis of methotrexate polyglutamates in L1210 murine leukemia cells. Cancer Res. 1977;37:408–12.
- Chabner BA, Allegra CJ, Curt GA, Clendeninn NJ, Baram J, Koizumi S, et al. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest. 1985;76:907–12.
- Jensen DE, Black AR, Swick AG, Azizkhan JC. Distinct roles for Sp1 and E2F sites in the growth/cell cycle regulation of the DHFR promoter. J Cell Biochem. 1997;67:24–31.
- Lima A, Seabra V, Bernardes M, Azevedo R, Sousa H, Medeiros R. Role of key TYMS polymorphisms on methotrexate therapeutic outcome in portuguese rheumatoid arthritis patients. PLoS One. 2014;9:e108165.
- Dervieux T, Furst D, Lein DO, Capps R, Smith K, Walsh M, et al. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004;50:2766–74.
- Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4:266–73.
- Gemmati D, Ongaro A, Scapoli GL, Della Porta M, Tognazzo S, Serino ML, et al. Common gene polymorphisms in the metabolic folate and methylation pathway and the risk of acute lymphoblastic leukemia and non-Hodgkin's lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2004;13:787–94.
- Celtikci B, Leclerc D, Lawrance AK, Deng L, Friedman HC, Krupenko NI, et al. Altered expression of methylenetetrahydrofolate reductase modifies response to methotrexate in mice. Pharmacogenet Genomics. 2008;18:577–89.
- Lim U, Wang SS, Hartge P, Cozen W, Kelemen LE, Chanock S, et al. Gene-nutrient interactions among determinants of folate and one-carbon metabolism on the risk of non-Hodgkin lymphoma: NCI-SEER case-control study. Blood. 2007;109:3050–9.
- Barredo J, Moran RG. Determinants of antifolate cytotoxicity: folylpolyglutamate synthetase activity during cellular proliferation and development. Mol Pharmacol. 1992;42:687–94.
- Egan MG, Sirlin S, Rumberger BG, Garrow TA, Shane B, Sirotnak FM. Rapid decline in folylpolyglutamate synthetase activity and gene expression during maturation of HL-60 cells. Nature of the effect, impact on folate compound polyglutamate pools, and evidence for programmed down-regulation during maturation. J Biol Chem. 1995;270:5462–8.
- Ma D, Huang H, Moscow JA. Down-regulation of reduced folate carrier gene (RFC1) expression after exposure to methotrexate in ZR-75-1 breast cancer cells. Biochem Biophys Res Commun. 2000;279:891–7.
- Olsen NJ, Spurlock CF, Aune TM. Methotrexate induces production of IL-1 and IL-6 in the monocytic cell line U937. Arthritis Res Ther. 2014;16:R17.
- Barbisan F, Motta JeR, Trott A, Azzolin V, Dornelles EB, Marcon M, et al. Methotrexate-related response on human peripheral blood mononuclear cells may be modulated by the Ala16Val-SOD2 gene polymorphism. PLoS One. 2014;9:e107299.
- Jansen G, Mauritz RM, Assaraf YG, Sprecher H, Drori S, Kathmann I, et al. Regulation of carrier-mediated transport of folates and antifolates in methotrexate-sensitive and -resistant leukemia cells. Adv Enzyme Regul. 1997;37:59–76.
- Said HM, Chatterjee N, Haq RU, Subramanian VS, Ortiz A, Matherly LH, et al. Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am J Physiol Cell Physiol. 2000;279:C1889–95.
- Jansen G, Mauritz R, Drori S, Sprecher H, Kathmann I, Bunni M, et al. A structurally altered human reduced folate carrier with increased folic acid transport mediates a novel mechanism of antifolate resistance. J Biol Chem. 1998;273:30189–98.
Appendix 1. Effects of overexpression of Mock, wild-type NFKBIE, and Val194Ala mutant NFKBIE on cell proliferation
The effects of in Mock (white), overexpression of wild-type NFKBIE (gray), and Val194Ala mutant NFKBIE (black) on cell proliferation were measured. MH7A cells were plated at 1.50 × 105 cells/well and cultured for 24 h. In turn, cells were transfected for 18 h as explained above. After changing medium, they were incubated with or without 0.01, 0.1, 1, 5, and 10 μM methotrexate (MTX) for 24 h in the presence or absence of both 20 U/mL IL-1β and 20 U/mL TNF-α. All cells in a single well were collected after trypsinization and measured by a TC20 automated cell counter. The number of cells was used to determine the proliferation of transfected MH7A. The results are presented as the mean ± standard error of the mean (SEM) of at least four independent experiments. Statistical significance was analyzed by the unpaired t-test (*p < 0.05). *p < 0.05 versus wild-type.