Abstract
An α-carbonic anhydrase (CA, EC 4.2.1.1) was purified and characterized kinetically from erythrocytes of the sturgeon Acipenser gueldenstaedti, an endangered species. The sturgeon enzyme (AgCA) showed kinetic parameters for the CO2 hydration reaction comparable with those of the human erythrocytes enzyme hCA II, being a highly active enzyme, whereas its esterase activity with 4-nitrophenyl acetate as substrate was lower. Sulphonamide inhibitors (acetazolamide, sulphanilamide) strongly inhibited AgCA, whereas metal ions (Ag+, Zn2+, Cu2+ and Co2+) were weak, millimolar inhibitors. Several widely used pesticides (2,4-dichlorophenol, dithiocarbamates, parathion and carbaryl) were also assayed as inhibitors of this enzyme. The dithiocarbamates were low micromolar AgCA inhibitors (IC50 of 16–18 µM), whereas the other pesticides inhibited the enzyme with IC50s in the range of 102–398 µM. The wide use of dithiocarbamate pesticides may be one of the factors enhancing the vulnerability of this sturgeon species to pollutants.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are metalloenzymes catalysing the reversible interconversion between CO2 and HCO3− with generation of protonsCitation1,Citation2. In vertebrates, including fish, CO2 diffuses from the tissues where it has been generated down its concentration gradient into the erythrocytes, where it is converted to HCO3− and H+ by the CA hydrase enzymatic activity, essential to many physiological processes such as pH homeostasis, respiration, electrolyte secretion and so onCitation1–3. The generated protons are largely buffered by haemoglobin in which most of the bicarbonate anions are passively transported out of the cell in exchange for plasma chloride, assisted by anion exchangers (AEs) such as AE1–AE3Citation3. Several CAs in fact are known to form metabolons with some AE isoforms, such as for example the CA II–AE1 complex from mammalian red blood cells (RBCs)Citation3, the resulting membrane-impermeable HCO3− being thus transported into the plasma by the Cl−/HCO3− exchanger. The concerted action of the CAs and AE1 is thus increasing blood CO2-carrying capacityCitation1–3. Furthermore, the CO2/HCO3− system constitutes one of the most important physiological buffers for acid–base regulationCitation1–3. CAs play a crucial role in the excretion of metabolic CO2 in all vertebrates, including fishCitation3–7. In mammals, at least 16 CA isoenzymes have been identified to dateCitation8. These isoenzymes differ in their catalytic activity, subcellular and tissue distribution and sensitivity to various classes of inhibitors. Some of them are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), membrane-bound (CA IV, CA IX, CA XII, CA XIV and CA XV), mitochondrial (CA VA and CA VB) and secreted (CA VI). Three of them, CA VIII, CA X and CA XI, are non-catalyticCitation8. CA inhibitors (CAIs) of the sulphonamide type are widely used pharmacological agents as diuretics, for the management of glaucoma, obesity, epilepsy and as antitumour agents/diagnostic toolsCitation8.
At least 26 species of sturgeons are known so far, and they belong to the Acipenseridae family, one of the oldest existent fish familiesCitation9. Sturgeons are spread in subtropical, temperate and sub-Arctic rivers, lakes and coastlines of Eurasia and North America, being harvested for caviarCitation9. Sturgeons are, however, slowly growing fish and mature late in life, being particularly sensitive to environmental pollution. Many of them are in decline or on the brink of extinction due to overfishing, pollution and other factors less well-understood at this momentCitation9.
Pesticides of various chemical structures are widely and massively used worldwide. Most of them show toxic effects not only for the organisms against which they are used but also for other species, such as fish, birds, mammals and so on. Pesticides are present in low concentrations in many environmental niches, and may cause deleterious effects even many years after their use has been stopped (e.g. the action of DDT on the calcification of eggs in birds)Citation10. Small amounts of pesticides can cause sublethal damage to many organisms, but little is known about how their affects on specific enzymes in fish such as the sturgeon investigated here. There are only very few literature reports regarding the interaction of pollutants with fish enzymes critical for physiological processes, such as the CAsCitation2,Citation10,Citation11.
In this study, we report the purification, characterization and inhibition of a CA from the endangered sturgeon species, Acipenser gueldenstaedti. We isolated this enzyme from RBCs of this fish and determined its catalytic activity for the physiological reaction and as esterase, with 4-nitrophenyl acetate (4-NPA) as substrate and inhibition effects with sulphonamides, pesticides and metal ions (i.e. some common pollutants). These findings may be useful for comparing CAs from various vertebrate species, their susceptibility to inhibition with various classes of compounds and also for understanding the toxic effects of some pesticides or heavy metal ions in endangered fish species. Some physiological aspects of CA-mediated processes in this type of less-investigated vertebrate are in fact poorly understood, since most of the CA pharmacological/environmental research have been done with the human and rodent enzymesCitation8.
Material and methods
Materials
All chemicals used in this study were the highest grade purity available and were used without further purification, being obtained from Sigma (Milan, Italy) and E. Merck (Darmstadt, Germany). Sepharose 4B activated by CNBr, protein assay reagents and chemicals for electrophoresis were obtained from Sigma-Aldrich Chemie (Taufkirchen, Germany). 4-Aminobenzene sulphonamide 1, acetazolamide 2 and l-tyrosine were from E. Merck. The herbicide, fungicides and insecticides 3–7 were purchased from Bayer (Türk Kimya San. Ltd., Istanbul, Turkey).
Fish samples
A. gueldenstaedti erythrocytes were obtained from fish grown in the Fishery Sciences Department at Karadeniz Technical University, Trabzon, Turkey. Twelve fish, between 10 and 45 kg and 120 to 180 months in age have been used in our experiments. The difference in the weights and ages of these fish groups did not significantly influence the amount/quality of isolated enzyme. The maintenance and feeding conditions were those of a fish farm. The fish were anaesthetized with 500 ppm benzocaine (98% ethyl 4-aminobenzoate; Sigma-Aldrich Chemie). Blood samples were taken from the caudal vessels using heparinized syringes in <1 min and transferred to heparinized tubes held on ice until centrifugation. The haemolysate from RBC was obtained according to the method reported by Kolayli and KehaCitation11. Erythrocytes were isolated by centrifugation for 10 min at 3000 g, washed three times with 1% NaCl, lysed in distilled water and stored at −15°C until needed.
Purification of CA by affinity chromatography
The RBC haemolysate was applied to a Sepharose 4B-l-tyrosine sulphanilamide affinity column equilibrated with 25 mM Tris–HCl/0.1 M Na2SO4 (pH 8.7). CA was eluted with 1.0 M NaCl/25 mM Na2PO4 (pH 6.3) and 0.1 M NaCH3COO/0.5 M NaClO4 (pH 5.6) as described earlier for human CAs (hCAs)Citation12. All procedures were performed at 4°C and all activities were calculated as average from at least three assays. The 280 nm absorbance of the protein in the column effluents was determined spectrophotometrically. CO2 hydrase activities in the eluents were then determined and the active fractions were collected. The purified sample was dialyzed, the obtained solution was lyophilized and the resulted enzyme was frozen.
Esterase activity assay
The CA activity was assayed by the esterase method, following the change in absorbance at 348 nm of 4-NPA hydrolyzed to 4-nitrophenylate ion in the presence of the enzyme, over a period of 3–15 min at 30°C, using a UV–vis spectrophotometer (ATI-Unicam, Cambridge, UK) according to the Verpoorte procedureCitation13. The total volume of the enzymatic assay was of 3.0 mL and contained 1.4 mL of 0.05 M Tris–SO4 buffer (pH 9.0); 1.0 mL of 3 mM 4-NPA in acetone, 0.5 mL of distilled, deionized water and 0.1 mL of enzyme solution, at a concentration of 0.1 µM. The non-catalysed 4-NPA hydrolysis in this buffer was always subtracted from the enzymatic measurements reported in this article. To determine inhibitory effects of acetazolamide, sulphanilamide, metal ions (Zn2+, Cu2+, Co2+ and Ag+) and pesticides 3–7 on sturgeon erythrocyte CA activity, the esterase method has also been used (14). Zinc, copper, cobalt and silver salts were dissolved in water. All pesticides were dissolved in dimethyl sulphoxide (DMSO) and inhibitory effect was measured by the hydrase activity. The inhibition effect of DMSO was determined as blank and subtracted from actual values. IC50 values were obtained from activity (%)–metal ion concentration plots.
Hydrase activity assay
CA activity was also assayed following the hydration of CO2 to bicarbonate and protons according to the Wilbur–Anderson methodCitation14 for measuring active enzyme samples from the eluent, during the purification procedures.
Kinetic and inhibition stopped-flow studies
The CO2 hydrase activity of the new enzyme has also been determined by a stopped-flow methodCitation15. An Applied Photophysics (Oxford, UK) stopped-flow instrument has been used for assaying the CA-catalysed CO2 hydration activity. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM HEPES (pH 7.5 for the α-CAs) as buffer, and 20 mM Na2SO4 (for maintaining the ionic strength constant), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 sec. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The non-catalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (10 mM) were prepared in distilled–deionized water and dilutions up to 0.01 µM were done thereafter with distilled–deionized water. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E–I complex. The inhibition constants were obtained by nonlinear least-square methods using PRISM 3, and represent the mean from at least three different determinationsCitation15.
Protein determination
The absorbance at 280 nm was used to monitor the protein in the column effluents. Quantitative protein determination was achieved by absorbance measurements at 595 nm according to Bradford method with bovine serum albumin as a standardCitation16.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a Bio-Rad Mini Gel system. The protein samples were fully denatured by boiling with β-mercaptoethanol and SDS and separated in a 12% polyacrylamide resolving gel and 4% stacking gel. Proteins were stained with Coomassie Blue and molecular weights were estimated by comparison with the molecular weight markers.
Results
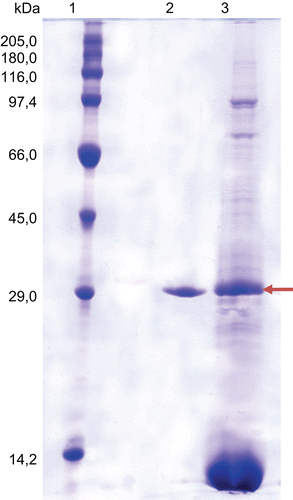
The purification of the fish CA was carried out by one-step chromatography on l-tyrosine sulphonamide coupled to Sepharose 4B ()Citation17,Citation18. The fish CA was purified with a specific activity of 26973 EU/mg protein (539-fold) with a yield of 29% (). shows the SDS-PAGE obtained for the raw and purified enzyme. As hCA II, this protein has molecular weight of 29 kDa.
Table 1. Purification of sturgeon erythrocytes carbonic anhydrase (AgCA) by affinity chromatography.
Figure 1. Elution graph of CA from sturgeon fish erythrocytes with 0.1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6) (flow rate: 20 mL, fraction volume: 3 mL).
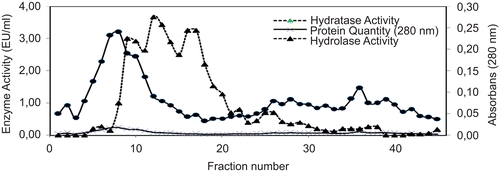
Figure 2. SDS-PAGE bands of CA: 1: standard proteins α-lactalbumin, bovine milk (14.2); bCA, bovine erythrocytes CA (29.0): ovalbumin, chicken egg (45.0 kDa); albumin, bovine serum (66.0 kDa); phosphorylase B, rabbit muscle (97.4 kDa); β-galactosidase, Escherichia coli (116.0 kDa); α2-macroglobulin (80.0 kDa): myosin, rabbit muscle (205.0 kDa), 2: purified sturgeon enzyme, 3: sturgeon erythrocyte haemolysate.
Kinetic parameters for sturgeon CA with 4-NPA and CO2 as substrates were determined by the Lineweaver–Burk procedure, and are shown in . The kcat value for 4-NPA hydrolysis was of 20.3 sec−1 (pseudo-first-order kinetic), whereas that for the CO2 hydration was of 1.2 × 106 sec−1, showing that this enzyme, similar to other α-CAs, is a very effective catalyst for the CO2 hydration reaction and a rather weak esteraseCitation8,Citation19.
Table 2. Kinetic parameters for 4-nitrophenyl acetate (4-NPA) hydrolysis and CO2 hydration reactions catalysed by the sturgeon enzyme AgCA and human isoforms hCA I and hCA II.
shows the in vitro effects of sulphonamides, pesticides and metal ions on the sturgeon CA activity.
Table 3. In vitro inhibition of some metal ions, sulphonamides and pesticides, against sturgeon erythrocytes enzyme AgCA, obtained by the esterase method with 4-nitrophenyl acetate (4-NPA) as substrateCitation13.
Discussion
CAs are ubiquitous enzymes, catalysing a physiologically crucial reaction, the reversible hydration of CO2 to HCO3− and H+8,19–23. These enzymes, as multiple isoforms in most organisms studied so farCitation8, are involved in a wide range of physiological processes, being present in high amounts in most tissues, for example, in erythrocytesCitation8. In mammals, these enzymes are involved in processes connected with respiration and transport of CO2/HCO3−, pH and CO2 homeostasis, electrolyte secretion in a variety of tissues/organs, biosynthetic reactions (such as gluconeogenesis, lipogenesis and ureagenesis), bone resorption, calcification, tumourigenicity and many other physiological or pathological processes (thoroughly studied in many vertebrates), whereas in algae, plants and some bacteria they play an important role in photosynthesis and other biosynthetic reactionsCitation8,Citation19–23. The classical CAIs are the primary sulphonamides, RSO2NH2, which are in clinical use for >50 years as diuretics and as systemically acting anti-glaucoma drugsCitation8. In addition to the established role of these CAIs as diuretics and anti-glaucoma agents, it has recently emerged that they have potential as anticonvulsant, anti-obesity, anticancer, anti-pain and anti-infective drugsCitation8,Citation19. Other compounds, such as the complexing anions (cyanide, azide, thiocyanate, etc.) or some heavy metal ions also act as CAIs, but with less potency compared with sulphonamides and their isosteresCitation8,Citation19–25.
In this study, we purified the first sturgeon CA from RBCs, in a single step, by using affinity chromatography. The purified enzyme showed very good catalytic activity for the physiological reaction, possessing kcat and KM values rather similar to those of its human counterpart, hCA II, which is one of the best catalysts known in natureCitation8. Furthermore, this enzyme has been inhibited by the classical sulphonamide inhibitor acetazolamide 1, with a KI of 50 nM, higher than the corresponding value for hCA II (KI of 12 nM) but much lower than the corresponding value for another blood isoform found in humans, hCA I, which showed a KI of 250 nM ()Citation8. Soyut and BeydemirCitation17 purified a fish enzyme from the rainbow trout liver and achieved a purification of 2260-fold with a specific activity of 4318 EU × mg−1 and recovery of 38% using the same technique.
AgCA has a molecular weight of 29 kDa, being thus similar to hCA I and hCA II (). The molecular weight of CAs from several vertebrate species has been reported to be around 29 kDa, not only for the human isoforms hCA I and hCA II, but also for the rainbow trout liver, enzymeCitation17 and dog erythrocyte CACitation25, among others.
Data of show that AgCA was weakly inhibited by divalent or monovalent heavy metal ions, with IC50s in the range of 1.7–5.2 mM. The most inhibitory cation was Ag(I) and the least inhibitory one Cu(II). Thus, it is probable that heavy metal ions do not constitute a great danger for this fish, or at least they do not inhibit significantly the CA found in the RBCs of this sturgeon.
In addition to sulphonamides, such as acetazolamide 1 and sulphanilamide 2, we have investigated the inhibition of AgCA with some heavy metal ions such as Zn(II), Cu(II), Co(II) and Ag(I) as well as several widely used pesticides: 2,4,-dichlorophenol 3 (the starting material for the preparation of the widely used herbicide 2,4-D), the dithiocarbamates maneb 4 and propineb 5 used as fungicides, the organophosphate insecticide/acaricide parathion 6 and the carbamate insecticide carbaryl 7. Two reasons motivated the investigation of these compounds as AgCA inhibitors. First, due to their extensive use in agriculture, compounds 3–7 might be present in the environment in high enough concentrations to produce toxic effects in fish, and it is critically important to understand whether an enzyme critical for the life of fish, such as CA may be inhibited by some of them and to what extent. The second reason for our study is due to the fact that we showed recently that phenolsCitation26–28 or dithiocarbamates (such as the inorganic trithiocarbonate or N,N-diethyldithiocarbamate) show potent CA inhibitory effects against the human isoforms hCA I, hCA II, hCA III, hCA IV, hCA VII, hCA IX, hCA XII and hCA XIVCitation29,Citation30, constituting novel classes of CAIs. As some of the pesticides 3–7 possess either phenol moieties (3), dithiocarbamate ones (4 and 5) or other potential zinc binding functions for inhibiting metalloenzymes (thiophosphate in 6 or methyl carbamate in 7), investigating the CA inhibitory effects of these compounds may lead to the discovery of novel enzyme inhibitors.
Data of show that acetazolamide 1 is a potent CAI (IC50 of 0.10 µM) also for the esterase activity of this enzyme, whereas sulphanilamide is a weaker CAI, with an IC50 of 4.00 µM. Thus, in contrast to heavy metal ions that are very weak AgCA inhibitors, the aromatic and heterocyclic sulphonamides strongly inhibit this enzyme, as they do with most α-CAs investigated so far in all types of organisms, from bacteria to protozoa, fish and mammalsCitation8.
Among the investigated pesticides, the phenol 3 showed modest inhibition against AgCA (IC50 of 240 µM), similar to parathion 6 and carbaryl 7 (IC50s in the range of 102–398 µM). However, the two dithiocarbamate fungicides 4 and 5 significantly inhibited the activity of this enzyme, with IC50 in the range of 16–18 µM. As the metal ions are millimolar inhibitors (), it is clear that this inhibition is due to the dithiocarbamate functionalities present in these compounds. In fact, the structurally related Et2N-CSSNa was recently shown by us to be a low micromolar or submicromolar CAI against several human isoformsCitation29,Citation30 (with the stopped-flow CO2 hydrase assay that normally gives 1–2 orders of magnitude lower KIs, as the enzyme concentration in the assay system is 5–10 nM. On the contrary, due to the low esterase activity of CAs, for the esterase assay with which these data were generated, low micromolar or submicromolar concentrations of enzyme must be usedCitation28–30.
There are no precise literature data regarding the concentration of these dithiocarbamate pesticides in the environment, but on cultivated plants (such as spinach) treated with them, concentrations as high as 3.65 µg/kg have been reportedCitation31. It is thus rather difficult to speculate whether the inhibition levels evidenced here interfere or not with the life cycle of this fish. It is however established that the juveniles of several fish species are much more sensitive than adult animals to these agents, which in addition may also inhibit their testicular developmentCitation31.
In conclusion, a sturgeon erythrocyte CA was purified in a one-step affinity chromatography procedure. Its kinetic properties show that AgCA is a high activity CA (for the physiological reaction of carbon dioxide hydration), being comparable with the human rapid isoform, hCA II. The susceptibility of AgCA to inhibition with classical sulphonamide inhibitors, sulphanilamide and acetazolamide and some environmental pollutants (phenols, dithiocarbamates and heavy metal ions) was studied by employing the esterase activity of this enzyme. Our study indicates that widely used dithiocarbamate fungicides (of the maneb or propineb type) act as low micromolar inhibitors of the sturgeon enzyme and may be toxic to this endangered fish species.
Acknowledgements
We thank the late Dr. İbrahim OKUMUŞ, for the supply sturgeon red blood cells. We are grateful to Dr. Şükrü Beydemir and Dr. Murat KÜÇÜK for helpful discussions and to Dr. Daniela Vullo for the stopped-flow measurements.
Declaration of interest
This study was supported by Research Fund of Karadeniz Technical University (Project No: 2004, 111. 002. 08) and in part by an EU project of the 6th framework programme (DeZnIT, to CTS).
References
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Dogan S. The in vitro effects of some pesticides on carbonic anhydrase activity of Oncorhynchus mykiss and Cyprinus carpio carpio fish. J Hazard Mater 2006;132:171–176.
- Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl(−)/HCO(3)(−) anion exchanger AE1. Biochemistry 2000;39:5527–5533.
- Gülçin I, Beydemir S, Büyükokuroglu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull 2004;27:613–616.
- Lessard J, Val A, Aota S, Randal D. Why is there no carbonic anhydrase activity available to fish plasma? J Exp Biol 1994;31:31–38.
- Hisar O, Beydemir S, Gülçin I, Küfrevioglu OI, Supuran CT. Effects of low molecular weight plasma inhibitors of rainbow trout (Oncorhynchus mykiss) on human erythrocyte carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2005;20:35–39.
- Gervais MR, Tufts BL. Characterization of carbonic anhydrase and anion exchange in the erythrocytes of bowfin (Amia calva), a primitive air-breathing fish. Comp Biochem Phys 1999;23A:343–350.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Bemis WE, Findeis EK, Grande L. An overview of Acipenseriformes. Environ Biol Fish 1997;48:25–71.
- Ceyhun SB, Şentürk M, Erdoğan O, Küfrevioğlu Öİ. In vitro and in vivo effects of some pesticides on carbonic anhydrase enzyme from rainbow trout (Oncorhynchus mykiss) Gills. Pest Biochem Physiol 2010;97:177–181.
- Kolayli S, Keha E. A comparative study of antioxidant enzyme activities in freshwater and seawater-adapted rainbow trout. J Biochem Mol Toxicol 1999;13:334–337.
- Beydemir S, CIftçi M, Ozmen I, Buyukokuroglu ME, Ozdemir H, Küfrevioglu OI. Effects of some medical drugs on enzyme activities of carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol Res 2000;42:187–191.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–154.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–2573.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Soyut H, Beydemir S. Purification and some kinetic properties of carbonic anhydrase from rainbow trout (Oncorhynchus mykiss) liver and metal inhibition. Protein Pept Lett 2008;15:528–535.
- Bülbül M, Hisar O, Beydemir S, Ciftçi M, Küfrevioglu OI. The in vitro and in vivo inhibitory effects of some sulfonamide derivatives on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase activity. J Enzyme Inhib Med Chem 2003;18:371–375.
- Supuran CT. Carbonic anhydrase inhibition/activation: trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr Pharm Des 2010;16:3233–3245.
- Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci usa 2009;106:16233–16238.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Exp Opin Ther Pat 2006;16:1627–1664.
- Sinan S, Gencer N, Turan Y, Aslan O. In vitro inhibition of the carbonic anhydrase from saanen goat (Capra hircus) with pesticides. Pest Biochem Physiol 2007;88:307–311.
- Bayram T, Arslan O, Ugras HI, Cakir U, Ozensoy O. Purification of carbonic anhydrase from dog erythrocytes and investigation of in vitro inhibition by various compounds. J Enzyme Inhib Med Chem 2007;22:739–744.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of mammalian isoforms I–XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg Med Chem 2008;16:7424–7428.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of cytosolic isoforms I, II, III, VII and XIII with less investigated inorganic anions. Bioorg Med Chem Lett 2009;19:1855–1857.
- Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of transmembrane isoforms IX, XII, and XIV with less investigated anions including trithiocarbonate and dithiocarbamate. Bioorg Med Chem Lett 2010;20:1548–1550.
- International Programme of Chemical safety. Available at: http://www.wpro.who.int/NR/rdonlyres/ED05F324-B487-4B7F-A240-16DBA39AD994/0/Environmentalhealthcriteria78dithiocarbamatepesticidesethelynethioureaandpropylenethioureaageneralintroduction.pdf