Abstract
A series of asymmetric indole curcumin analogs were synthesized and evaluated as possible inhibiters of pro-inflammatory enzymes such as COX-2, pro-inflammatory cytokines as TNF-α and IL-6, trypsin and β-glucuronidase. They were also tested for antioxidant activities. The results showed that compounds 5e and 5h were found to be the most potent inhibitors of COX-2 (83.33%, 82.50%) and β-glucuronidase (67.80%, 64.12%). All the synthesized compounds exhibited promising activity against IL-6 in a range of 71–100% at 10 µM concentration. Compounds 5f, 5h, 5e, 5c and 5d showed significant inhibition against TNF-α (28–51%) and IL-6 (87–98%) with low toxicity (45–51%) against CCK-8 cells. With few exceptions, all other compounds were found to be good to excellent inhibitors of IL-6 and moderate inhibitors of TNF-α; however, the toxicity profiles of these compounds need to be ameliorated in further optimization studies. Amongst the tested compounds, 5c, 5b, 5j and 5g were found to possess excellent reducing activity and 5b, 5c and 5h were moderate DPPH (1,1-diphenyl-2-picryl hydrazine) radical scavengers.
Introduction
The pro-inflammatory enzymes such as cyclooxygenase-2 (COX-2), trypsin and β-glucuronidase and pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) have been described as important targets for the design and development of novel and safe anti-inflammatory agents. Among the pro-inflammatory enzymes, trypsin is a member of the serine proteases family. These proteases are involved in initiation of inflammation; serine protease inhibition has been considered as one of the targets for design of anti-inflammatory drugsCitation1. The lysosomes of the polymorphonuclear neutrophils are rich in β-glucuronidase. This enzyme is attributed as one of the mediators for initiating the process of inflammationCitation2. However, COX-2 enzyme also plays an important role in the transformation of the arachidonic acid to prostaglandins and thromboxane: an important step for the recruitment of inflammationCitation3. The COX-1 is housekeeping enzyme having constructive influence; however, overexpression of COX-2 leads to more production of prostaglandins responsible for causing inflammation and it also participates in the propagation of cancerCitation4. This is consistent with the idea that inhibition of COX-1 underlies the gastrointestinal side effects of non-steroidal anti-inflammatory drug (NSAIDs) and that NSAIDs selectivity toward inhibition of COX-1 over COX-2 correlates with their ability to cause gastrointestinal side effectsCitation5–7. This is the reason why selective COX-2 inhibition has become the important cellular target of a number of chemical entities for the treatment of inflammatory diseases and chemotherapy of cancer.
The pro-inflammatory cytokines, IL-6 and TNF-α are implicated in the pathogenesis of various inflammatory disorders such as rheumatoid arthritis (RA), inflammatory bowel disease, osteoarthritis, psoriasis, endotoxemia and/or toxic shock syndromeCitation8–16. Apart from pro-inflammatory attributes these cytokines have a wide array of functions for maintaining the normal cellular physiology. For example, TNF-α can induce apoptosis and secretion of cytokines such as IL-1, IL-6 and IL-10; it can also activate T cells and other inflammatory cells. However, an overabundance of TNF-α and IL-6 is attributed to the development of various human ailments including inflammatory disorders. Targeting the inhibition of cytokines, in particular TNF-α, has been successful in several clinical trials for the treatment of RA. Nevertheless, the TNF-α inhibition has been identified as one of the attractive targets for the design and development of anti-inflammatory agentsCitation17.
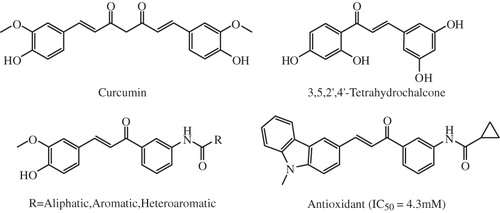
A large body of literature has been accumulated in the recent past describing the importance of curcumin as one of the lead molecules for the design and development of array of therapeutic agents including anti-inflammatory, anticancer, antioxidant, antiviral and antimicrobialsCitation18. Several analogs of curcumin have been synthesized and evaluated for multifunctional pharmacological application in a variety of diseases such as liver fibrosis, inflammation, cardiovascular diseases and cancer ()Citation19–21. The role of curcumin derivatives as antitumor agent, especially targeting the tumor anti-angiogenesis is well describedCitation22. Several curcuminoid pyrazoles have been synthesized and described as new therapeutic agents in inflammatory bowl diseases targeting the matrix metalloproteinasesCitation23. A series of mono-carbonyl five-carbon linker curcumin analogs were synthesized and were found to inactivate pro-inflammatory cytokines such as TNF-α and IL-6: important targets for the design of novel anti-inflammatory agents and tested as anticancer and anti-angiogenic agentsCitation24. More recently, curcumin and its hydrocurcumin analogs were found to be potent DNA hypomethylating agents: an important event that has proven to be effective in restoring gene expression and normal patterns of differentiation and apoptosis in malignant cellsCitation25.
Taking the above circumstances into consideration we endeavored to synthesize a series of asymmetric indole curcumin analogs (AICAs) and evaluate the same for inhibition of major pro-inflammatory enzymes such as COX-2, trypsin and β-glucuronidase and pro-inflammatory cytokines such as TNF-α and IL-6, along with their antioxidant potential.
Experimental
General
Chemicals were purchased from Aldrich Chemical Co. (Milwaukee, WI). Thin layer chromotography (TLC) was performed on an aluminum-backed silica plate with visualization by UV-light. Melting points were determined with a digital thermometer. IR spectra were recorded on a FT-IR spectrophotometer (Shimadzu 8300, Kyoto, Japan) and 1H NMR spectra were recorded on 300 MHz instrument (Bruker Avance DRX 300, Rheinstetten, Germany) in CDCl3 using tetramethylsilane as an internal standard and chemical shifts are reported in δ units. Mass spectra were obtained with a Shimadzu LCMS-2010EV (Kyoto, Japan).
General procedure for the synthesis of N-(3-acetyl phenyl)benzamides
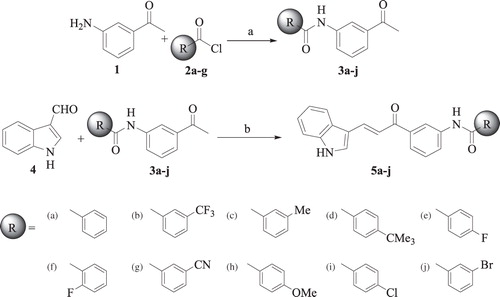
1-(3-aminophenyl) ethanone (1 g, 7.40 mmol) was suspended in 20 ml 5% of sodium hydroxide solution in a well corked two necked round bottom flask and to it was added 2 ml of various substituted benzoyl chlorides, 0.5 ml at a time, with constant shaking and stirring vigorously for 10 min, reaction mixture was heated under reflux on water both at 70 °C and at 80 °C for 20 min until the odor of the benzoyl chloride disappeared. Solid benzoyl derivative was filtered and recrystallized from petroleum ether and ethyl acetate to obtain an N-(3-acetyl phenyl)benzamide derivative.
General procedure for the preparation of AICAs
N-(3-acetyl phenyl)benzamide (219 mg, 1 mmol) was dissolved in 10 ml of ethanol and to this was added 4 ml (40%) aqueous NaOH and stirred for 10 min. To this reaction mixture indole-3-carboxaldehyde was added and stirred at 25 °C for 24 h. After completion of reaction (TLC), the reaction mixture was poured over crushed ice (25 g) and acidified with 10% aqueous HCl followed by basification with saturated Na2CO3 solution, extracted with chloroform and organic layer was washed with water, dried (anhydrous Na2SO4) and concentrated. Purification was carried out using silica gel column and a mixture of 0.5–1% MeOH + 1% liquor ammonia in chloroform as eluent to obtain AICAs in pure form.
The spectral data of some representative compounds are as follows:
N-(3-1H-indole-3-yl)acryloyl)phenyl)benzamide (5a)
Yellow solid, m.p. 182–185 °C; 1H NMR (CDCl3, 300 MHz): 11.26 (bs, 1H, NH), 9.25 (bs, 1H, CONH), 8.23 (m, 2H, ArH), 7.82 (d, 1H, H-β), 7.56 (m, 5H, ArH), 7.23 (m, 3H, ArH), 7.02 (m, 3H, ArH), 7.06 (d, 1H, H-α), 6.96 (s, 1H, ArH); Citation13C NMR (100 MHz, CDCl3): 190.2, 173.8, 148.1, 142.4, 140.5, 137.7, 136.2, 134.0, 134.5, 133.0, 132.8, 131.5, 130.1, 129.6, 128.0, 126.4, 125.2, 121.4, 115.7, 107.5; MS: m/z 367 (M + 1).
N-(3-1H-indole-3-yl)acryloyl)phenyl)-3-trifluoromethylbenzamide (5b)
Yellow solid, m.p. 162–165 °C; 1H NMR (CDCl3, 300 MHz): 11.47 (bs, 1H, NH), 9.47 (bs, 1H, CONH), 8.38 (m, 2H, ArH), 7.86 (d, 1H, H-β), 7.80 (m, 2H, ArH), 7.78 (m, 2H, ArH), 7.32 (m, 3H, ArH), 7.24 (m, 3H, ArH), 7.09 (d, 1H, H-α), 6.98 (s, 1H, ArH); Citation13C NMR (100 MHz, CDCl3): 191.8, 175.0, 148.6, 143.2, 143.0, 141.5, 141.3, 135.8, 134.7, 134.4, 134.0, 133.7, 133.5, 133.0, 128.6, 128.1, 127.2, 127.0, 124.9, 124.4, 123.2, 120.8, 114.7, 108.5; MS: m/z 435 (M + 1).
N-(3-1H-indole-3-yl)acryloyl)phenyl)-3-methylbenzamide (5c)
Yellow solid, m.p. 132–135 °C; 1H NMR (CDCl3, 300 MHz): 11.02 (bs, 1H, NH), 9.14 (bs, 1H, CONH), 8.17 (m, 2H, ArH), 7.68 (d, 1H, H-β), 7.59 (m, 2H, ArH), 7.57 (m, 2H, ArH), 7.21 (m, 3H, ArH), 7.11 (m, 3H, ArH), 6.99 (d, 1H, H-α), 6.92 (s, 1H, ArH), 2.14 (s, 3H, CH3); Citation13C NMR (100 MHz, CDCl3): 187.2, 169.9, 145.2, 141.7, 140.6, 139.4, 138.5, 135.4, 134.2, 132.3, 132.2, 131.5, 130.3, 130.2, 127.9, 127.8, 127.0, 126.5, 123.8, 123.1, 120.4, 113.5, 106.2, 18.6; MS: m/z 381 (M + 1).
N-(3-(3-1H-indole-3-yl)acryloyl)phenyl)-4-flurobenzamide (5e)
Yellow solid, m.p. 157–160 °C; 1H NMR (CDCl3, 300 MHz): 11.40 (bs, 1H, NH), 9.22 (bs, 1H, CONH), 8.35 (d, 2H, ArH), 7.87 (d, 1H, H-β), 7.63 (d, 2H, ArH), 7.66 (m, 2H, ArH), 7.28 (m, 3H, ArH), 7.18 (m, 3H, ArH), 7.08 (d, 1H, H-α), 6.97 (s, 1H, ArH); Citation13C NMR (100 MHz, CDCl3): 189.0, 170.4, 161.8, 147.5, 144.9, 142.7, 142.0, 135.3, 135.0, 134.9, 134.7, 133.8, 131.5, 130.6, 129.4, 127.0, 125.9, 125.1, 120.0, 114.7, 106.8; MS: m/z 385 (M + 1).
COX-2 Inhibition microtiter assay
The assay was performed by using Colorimetric COX (human ovine) Inhibitor Screening Assay KitCitation26. Briefly, the reaction mixture of 100% initial activity wells contained 160 μl of assay buffer, 150 μl of heme and 10 μl of COX-2 enzyme solution. While the reaction mixture of inhibitor wells comprised of 150 μl of assay buffer, 10 μl of heme, 10 μl of enzyme COX-2 and 10 μl of the test samples (10 μM). The plates were carefully shaken for 5 s and were incubated for 5 min at 25 °C. After 5 min incubation, 20 μl of the colorimetric substrate solution was added, followed by the addition of 20 μl of arachidonic acid to all the wells. The plates were shaken gently for few seconds and again incubated for 5 min at 25 °C. The absorbance of all the wells was read at 590 nm using Thermo make Automatic Ex-Microplate Reader (M 51118170) (Thermo, Ventaa, Finland). The COX-2 inhibition activity (%) was calculated using the following formulaCitation12:
where
Trypsin inhibition assay
The trypsin inhibition assay was carried out by employing a previously reported methodCitation27. The method is based on the measurement of inhibition of trypsin induced hydrolysis of bovine serum albumin (BSA). Trypsin (0.075 mg/ml) was initially incubated with 10 μM individual concentrations of test sample of 0.1 ml for 20 min. The substrate BSA (6 g/100 ml, in 0.1 M phosphate buffer, pH 7.6) was added after 20 min. The reaction mixture was incubated for 25 min at 37 °C. The reaction was terminated by the addition 3 ml of CCl3COOH (5%, w/v). The acid soluble fractions were obtained by centrifuging the contents at 5000 RPM for 15 min. The amount of protein in the acid soluble fractions was estimated by a method of Lowry et al.Citation27. Acetyl salicylic acid (10 μM) was used as a reference drug.
β-glucuronidase inhibition assay
The effect of the AICAs on activity of β-glucuronidase was studied using a method described by Demetrios et al.Citation28. 10 μM concentrations of test sample (0.1 ml) in 0.1 M acetate buffer pH 7.4 for 5 min at 37 °C were preincubated with 0.8 ml of 2.5 mM p-nitrophenyl-β-D-glucopyranosiduronic acid and 0.1 ml of β-glucuronidase was added. The mixture was incubated for 30 min. Reaction was terminated by addition of 2 ml of 0.5 N NaOH. The reaction mixtures were observed spectrophotometrically at 410 nm. Salicylic acid (10 μM) was used as a reference compound.
Assay for TNF-α and IL-6 inhibition
Pro-inflammatory cytokine production by lipopolysaccharide (LPS) in THP-1 cells was measured according to the method described elsewhereCitation29. Briefly, THP-1 cells were cultured in RPMI 1640 culture medium containing 100 U/ml penicillin and 100 mg/ml streptomycin containing 10% fetal bovine serum. Cells were differentiated with phorbol myristate acetate. Following cell plating, the test compound (10 μM) or vehicle using 0.5% DMSO was added to each well and the plate was incubated for 30 min at 37 °C. Finally, LPS (Escherichia coli 0127:B8) was added, at a final concentration of 1 mg/ml. Plates were incubated at 37 °C for 24 h, 5% CO2. Supernatants were harvested and assayed for TNF-α and IL-6 by ELISA as described by the manufacturer (BD Biosciences). The cells were simultaneously evaluated for cytotoxicityCitation30 using CCK-8. Percent inhibition of cytokine release compared to the control was calculated.
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay
The DPPH radical scavenging assay was performed as describedCitation31. The reaction mixture contained 10 μM concentrations of individual test sample (in absolute ethanol) and DPPH radical (10−4 M in absolute ethanol) solution. The contents of the reaction mixture were observed spectrophotometrically at 517 nm after 20 min. Gallic acid was used a reference drug (86.30%).
Determination of the reducing power of AICAs
The assay was carried out as per reported methodCitation32. The principle of the method is that the antioxidants reduce Fe3+ of K3Fe(CN)6 to Fe2+, the reducing power of AICAs was determined by the decrease in absorption of K3Fe(CN)6 at 420 nm. The reaction mixture contained 0.5 ml solution of individual AICAs (10 μM) in 3 ml of 1 mM potassium ferricyanide solution and the absorbance was measured at 420 nm after 10 min incubation time. Ascorbic acid (52.93%) was used as a reference compound.
Results and discussion
Chemistry
The compounds N-(3-acetyl phenyl) benzamides (3a–j) were prepared by the reaction of 3-amino acetophenone with a variety of benzoyl chlorides (2a–j) in the presence of sodium hydroxide in ethanol at 80 °C, whereas the Claisen–Schmidt condensation of N-(3-acetyl phenyl) benzamides (3a–j) with indole-3-carboxyaldehyde 4 using sodium hydroxide in ethanol at room temperature afforded the target AICAs (5a–j) in quantitative yield (). All the synthesized compounds were characterized by IR, 1H NMR, Citation13C NMR and mass spectrometry analysis.
Biological evaluation
All the synthesized AICAs 5a–j were tested for their in vitro anti-inflammatory activity against pro-inflammatory enzymes, namely COX-2, trypsin and β-glucuronidase at 10 μM concentration and the results are summarized in . Among all the tested compounds, 5e and 5h were found to be better inhibitors of COX-2 (83.33% and 82.50%) than the acetylsalicylate reference while other compounds were in the same range or with lower activities. Compounds 5e, 5h and 5i showed moderate inhibitory activity against trypsin and 5c, 5e, 5h and 5i showed significant activity against β-glucuronidase whereas remaining compounds showed poor inhibitory activity against both trypsin and β-glucuronidase. Compound 5a (without functional groups) showed very poor inhibition of COX-2, trypsin and β-glucuronidase; however, substitution of electrophilic groups at p-position of phenyl ring (5e, 5h) exhibited increase in inhibitory activity against COX-2, trypsin and β-glucuronidase.
Table 1. COX-2, trypsin and β-glucuronidase inhibitory activities (%) of AICAs (at 10 μM).
All the compounds 5a–j were also tested for their in vitro inhibitory activity against pro-inflammatory cytokines, namely TNF-α and IL-6. Some of the synthesized AICAs were observed to be promising leads, possessing excellent IL-6 inhibitory activity as compared to dexamethasone, a commercial anti-inflammatory agent ().
Table 2. TNF-α and IL-6 inhibitory activities and cytotoxicity of AICAs.
Compound 5a and 5b showed excellent inhibition against both TNF-α and IL-6, at 10 μM as compared to the dexamethasone used as a standard as well as curcumin used as a parent compound. Compounds 5e, 5c, 5d and 5h showed more than 90% inhibition against IL-6, compounds 5f, 5j, 5i and 5g also showed significant inhibition against IL-6 (87--71%) and compounds 5g, 5f, 5e, 5j and 5h were found to be moderate inhibitors (59–45%) of TNF-α. The cytotoxicity profile indicates that the compound 5g (63%), 5b (62%), 5a (57%) and 5i (53%) were found to be slightly toxic, while all other compounds were non-toxic.
On the other hand, all the synthesized compounds were also evaluated for DPPH radical scavenging and reducing activity and results are summarized in . Mostly, all the compounds have shown significant DPPH radical scavenging and reducing activity. The compound 5b (52.69%) was observed to be an effective scavenger of DPPH radicals while 5c (78.68%), 5j (71.82%), 5g (70.62%) and 5b (62.12%) were identified as most effective reducing agents.
Table 3. DPPH radical scavenging and reducing activities (RA) of AICAs (at 10 μM).
While discussing the structure activity relationship it was observed that the presence of lipophilic groups such as fluoro and methoxy at 4-position of benzoyl group seems to be the most compatible structural configuration for the inhibition of COX-2, trypsin and β-glucuronidase and for possessing significant antioxidant activity. In general, it was also observed that the antioxidant activity of the tested AICAs can be correlated with the extent of pro-inflammatory enzyme inhibition to a greater extent. The substitution at 4-position of benzoyl group seems to be important for COX-2 inhibition as unsubstituted benzoyl group showed very poor COX-2 inhibition. Also substitution of fluorine at 2-position significantly reduces the inhibition of trypsin and β-glucuronidase. The substitution at 3-position seems to be pivotal for the antioxidant activity. Whereas the presence of trifluoromethyl group at 3-position and fluorine at 4-position seems to be the significant compatible structural configuration for the inhibition of TNF-α and IL-6.
Conclusion
In conclusion, a new series of AICAs were synthesized and evaluated for anti-inflammatory activities (COX-2, trypsin, β-glucuronidase, TNF-α and IL-6) along with antioxidant activity. The results of the present investigations indicate the importance of these new compounds as potential candidates of anti-inflammatory and antioxidant agents. From the activity results of the tested compounds, 5e and 5h showed excellent inhibition of COX-2 and β-glucuronidase, 5f, 5h, 5e, 5c and 5d showed promising activity against TNF-α and IL-6 with low cytotoxicity and moderate antioxidant activity. The results of the other compounds such as 5a and 5b as inhibitors of TNF-α and IL-6 are definitely encouraging, but the cytotoxicity of these compounds limits the therapeutic applications. All the compounds were also found to possess significant reducing activity and moderate DPPH radical scavenging activity.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
H.V.C. is grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, for the financial support.
References
- Bilfinger TV, George BS. The role of protease inhibition with emphasis on the effects of inflammation and vascular immune phenomena. Curr Pharm Design 2002;8:125–33
- Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol 1995;6:385–93
- Jacob A, Wu R, Zhou M, Wang P, et al. Mechanism of the anti-inflammatory effect of curcumin: PPAR-γ activation. PPAR Res 2007;2007:89369
- Bandgar BP, Patil SA, Gacche RN, et al. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg Med Chem Lett 2010;20:730–3
- Seibert K, Zhang Y, Leahy K. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci 1994;91:12013–17
- Lee JL, Mukhtar H, Bickers DR, et al. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol 2003;192:294–306
- Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804
- Dinarello CA. Inflammatory cytokines: interleukin-1 and tumor necrosis factor as effector molecules in autoimmune diseases. Curr Opin Immunol 1991;3:941–8
- Arend WP, Dayer JM. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum 1990;33:305–15
- Dayer JM, Demczuk S. Cytokines and other mediators in rheumatoid arthritis. Springer Semin Immunopathol 1984;3:387–413
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev 1998;50:515–96
- Loyau G, Punol JP. The role of cytokines in the development of osteoarthritis. Scand J Rheumatol 1990;81:8–12
- Kirkham B. Interleukin-1, immune activation pathways, and different mechanisms in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis 1991;50:395–400
- Nickoloff BJ. The immunologic and genetic basis of psoriasis. Arch Dermatol 1999;135:1104–10
- Saklavala J, Davis W, Guesdon F. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos Trans R Soc Lond, Ser B 1996;351:151–7
- Sacca R, Cuff CA, Ruddle NH. Mediators of inflammation. Curr Opin Immunol 1997;9:851–7
- Matsui T, Kondo T, Nishita Y, et al. Highly potent inhibitors of TNF-α production. Part 1: discovery of chemical leads. Bioorg Med Chem Lett 2002;12:903–5
- Pabon HYY. A synthesis of curcumin and related compounds. Rec Trav Chim 1964;83:379–86
- Abuarqoub H, Green CJ, Foresti R. Curcumin reduces cold storage-induced damage in human cardiac myoblasts. Exp Mol Med 2007;39:139–48
- Egan ME, Pearson M, Weiner SA, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004;304:600–2
- Smith WL, Marnett LJ, Dewitt DL. Prostaglandin and thromboxane biosynthesis. Pharmacol Therap 1991;49:153–79
- Claramunt RM, Bouissane L, Cabildo MP, et al. Synthesis and biological evaluation of curcuminoid pyrazoles as new therapeutic agents in inflammatory bowel disease: effect on matrix metalloproteinases. Bioorg Med Chem 2009;17:1290–6
- Garret A, FitzGerald MD, Patrono MD. The coxibs, selective inhibitors of cyclooxygenase-2. Eng J Med 2001;345:433–42
- Liang G, Yang S, Zhou H, et al. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur J Med Chem 2009;44:915–19
- Liu Z, Xie Z, Jones W, et al. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett 2009;19:706–9
- Murias M, Handler N, Erker T, et al. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure–activity relationship. Bioorg Med Chem 2004;12:5571–8
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagents. J Biol Chem 1951;193:265–75
- Demetrios NN, Konstantina CF, Konstantinos EL, Dimitra H-L. Synthesis and biological evaluation of several coumarin-4-carboxamidoxime and 3-(coumarin-4-yl)-1,2,4-oxadiazole derivatives. Eur J Med Chem 1998;33:715–24
- Hwang C, Catanaga M, Granger GA, Gatanaga T. Mechanism of release of soluble forms of tumor necrosis factor/lymphotoxin receptors by phorbol myristate acetate-stimulated human THP-1 cells in vitro. J Immunol 1993;151:5631–8
- Dengler WA, Schulte J, Berger DP, et al. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti Can Drugs 1995;6:522–32
- Bartolome B, Nunez V, Monagas M, et al. In vitro antioxidant activity of red grape skins. Eur Food Res Technol 2003;218:173–7
- Gacche RN, Gond DS, Dhole NA, Dawane BS. Coumarin Schiff-bases: as antioxidant and possibly anti-inflammatory agents. J Enz Inhib Med Chem 2006;21:157–61