Abstract
In this study, 14 different 2-[(1-methyl-1H-tetrazole-5-yl)thio]-1-(phenyl)ethanone derivatives (1–14) were synthesized. The structures of the obtained compounds were elucidated using IR, 1H-NMR, 13C-NMR and FAB+-MS spectral data and elemental analyses results. The compounds were screened for their anticandidal activity using the microbroth dilution method and for their cytotoxic effects using the MTT assay against NIH/3T3 cells. Some of the compounds were found to be potent anticandidal agents with weak cytotoxicities.
Introduction
Over the past two decades, the incidence of fungal infections has increased dramatically in an alarming way. This is mainly due to the development of drug resistance of fungal pathogens and also an increasing population of immunocompromised patients (e.g. HIV-infected patients). Candida infections occur that range from non-life-threatening mucocutaneous illnesses to invasive processes that may involve virtually any organ. It may become life-threatening in patients undergoing anticancer chemotherapy, organ transplants or long treatment with antimicrobial agents and in patients with AIDS because of suppressed immune system. Such a broad range of infections and development of resistance to currently available antifungal agents require an equally broad range of diagnostic and therapeutic strategiesCitation1. As is known, not only is the biochemical similarity of the human cell and fungi forms a handicap for selective activity and toxicity, but also, easily gained resistance is the main problem encountered in developing safe and efficient antifungalsCitation2–4.
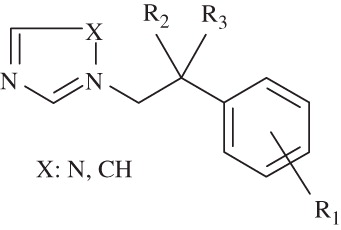
In antimycotic pharmacotherapy, azoles have maintained a key role in the treatment of fungal infections and today the azole scaffold is still considered a viable lead structure for the synthesis of more efficacious and broad spectrum antifungal agentsCitation5. Azoles which include imidazole or triazole ring effect by inhibiting the synthesis of sterols in fungi which is necessary for fungal cell membraneCitation6. In antifungal chemotherapy; ketoconazole, itraconazole, fluconazole and miconazole which are well-known azole antifungals proved to be important drugs for combating fungal infections and currently remain the drug of choice in the treatmentCitation7. A critical structure survey of azole class of antifungals revealed that most of them owe this activity to (phenethyl)azole moiety that seems to be the pharmacophore ()Citation8.
In recent years, number of publications and patents on the preparation, properties and applications of tetrazole derivatives which are members of well-known azoles has been increased with respect to other heterocyclic systems. First, development of the tetrazole chemistry has been largely associated with ring flexibility, stability which provides easily to different binding modes and toxicity decreasing propertiesCitation9. Second reason is the wide-scale application of these compounds in medicine and biochemistry, especially based on isosteric properties. Tetrazoles are non-classical isosteres of the carboxylate groupCitation10–14 and also as an isostere for imidazole ringCitation15. The term “nonclassical isosterism” derives from the concept that functional groups having similar physicochemical properties can be interchangeable, while the biological activity of the initial and the new compounds will be similar and it is a fundamental tactical approach useful to address a number of aspects associated with the design and development of drug candidates. For new molecules, it could be provided improving potency, enhancing selectivity, altering physical properties, reducing or redirecting metabolism, eliminating or modifying toxicophores, and acquiring novel intellectual property with using isoster groupsCitation16,Citation17.
Tetrazole and its derivatives possess very interesting pharmacological and biological properties and are reported to exhibit variety of biological activitiesCitation18–29. A number of remarkable studies have been reported that newly synthesized tetrazole derivatives had higher anticandidal activity than standard antifungal drugs, and this result was attributed to the isosteric properties of tetrazolesCitation30,Citation31. Currently, there are numerous approaches to the preparation of five-substituted tetrazoles in the literature, and their number is still increasing because these heterocycles can be found in medicinal chemistryCitation32. There is also a particular interest for the synthesis of five-substituted thiotetrazoles as these thiotetrazoles are having powerful activating property than the corresponding five-substituted tetrazoles used for synthesis of DNA and RNACitation33. In the literature, there are a lot of studies including five-substituted thiotetrazoles with different biological activities which are also bearing similar skeleton to the title compoundsCitation34–43.
In this study, we planned to synthesize five-disubstituted thiotetrazole derivatives due to their isosteric and toxicity decreasing properties and we used a modified pharmacophore residue to be responsible for the anticandidal activity.
Experimental
Chemistry
All chemicals were purchased from Sigma-Aldrich Chemical Co (Sigma-Aldrich Corp., St. Louis, MO). All melting points (m.p.) were determined by Electrothermal 9100 digital melting point apparatus (Electrothermal, Essex, UK) and are uncorrected. All the reactions were monitored by thin-layer chromatography (TLC) using Silica Gel 60 F254 TLC plates (Merck KGaA, Darmstadt, Germany). Spectroscopic data were recorded with the following instruments: IR, Shimadzu 8400S spectrophotometer (Shimadzu, Tokyo, Japan); 1H-NMR, Bruker 500 MHz spectrometer (Bruker Bioscience, Billerica, MA); MS-FAB, VG Quattro Mass spectrometer (Fisons Instruments Vertriebs GmbH, Mainz, Germany) and elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyzer (Perkin Elmer, Norwalk, CT).
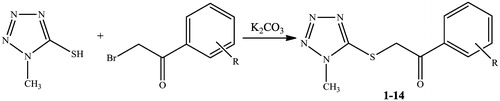
General procedure for the synthesis of the compounds (1–14)
A mixture of 1-methyl-1H-tetrazole-5-thiol (4.31 mmol, 0.5 g), the appropriate phenyl acetyl bromide derivative (4.31 mmol) and K2CO3 (5.17 mmol, 0.7 g) in aceton were stirred for 1–3 h. The mixture was evaporated until dryness. The residue was washed with water and recrystallized from ethanol.
1-(2-Chlorophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (1)
IR (KBr) νmax (cm−1): 1672 (C=O), 1597 (C=N).
1H-NMR (500 MHz, dimethylsulfoxide, DMSO-d6): δ 4.01 (3H, s, NCH3), 4.97 (2H, s, CH2), 7.73–7.50 (1H, m, Ar-H), 7.61 (2H, d, J = 3.7 Hz, Ar-H), 7.86 (1H, d, J = 7.6 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.85, 43.86, 128.70, 131.33, 131.54, 131.95, 134.43, 137.52, 154.48 and 196.14.
MS (FAB) [M + 1]+: m/z 269.
For C10H9ClN4OS calculated: 44.70% C, 3.38% H and 20.85% N; found 44.74% C, 3.40% H and 20.83% N.
1-(3-Methylphenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (2)
IR (KBr) νmax (cm−1): 1677 (C=O), 1583 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 2.41 (3H, s, CH3), 4.01 (3H, s, CH3), 5.1 (2H, s, CH2), 7.47 (1H, t, J1 = 7.6 Hz, J2 = 7 Hz, Ar-H), 7.53 (H, d, J = 7.7, Ar-H), 7.85 (H, d, J = 7.8, Ar-H), 7.87 (H, s, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 21.42, 34.35, 42.17, 126.89, 130.0, 130.08, 135.85, 136.35, 139.65, 154.68 and 194.33.
MS (FAB) [M + 1]+: m/z 249.
For C11H12N4OS calculated: 53.21% C, 4.87% H and 22.56% N; found 53.74% C, 4.90% H and 22.61% N.
1-(3-Chlorophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (3)
IR (KBr) νmax (cm−1): 1687 (C=O), 1585 (C=O).
1H-NMR (500 MHz, DMSO-d6): δ 4.01 (3H, s, CH3), 5.1 (2H, s, CH2), 7.63 (1H, t, J1 = 7.8 Hz, J2 = 7.9 Hz, Ar-H), 7.8 (1H, d, J = 9.2 Hz, Ar-H), 8.0 (1H, d, J = 8.0 Hz, Ar-H), 8.6 (1H, s, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.38, 41.90, 128.33, 129.39, 132.17, 134.89, 135.07, 138.18, 154.52 and 193.47.
MS (FAB) [M + 1]+: m/z 269.
For C10H9ClN4OS calculated: 44.70% C, 3.38% H and 20.85% N; found 44.74% C, 3.39% H and 21.01% N.
1-(3-Nitrophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (4)
IR (KBr) νmax (cm−1): 1671 (C=O), 1573 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 4.02 (3H, s, CH3), 5.2 (2H, s, CH2), 7.9 (1H, t, J1 = 7.9 Hz, J2 = 8 Hz, Ar-H), 8.48 (1H, d, J = 8.55 Hz, Ar-H), 8.55 (1H, d, J = 8.2 Hz, Ar-H), 8.74 (1H, s, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.42, 41.98, 124.05, 129.30, 132.01, 135.92, 137.58, 149.43, 154.45 and 193.24.
MS (FAB) [M + 1]+: m/z 280.
For C10H9N5O3S calculated: 43.01% C, 3.25% H and 25.08% N; found 43.04% C, 3.29% H and 25.03% N.
1-(4-Methylphenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (5)
IR (KBr) νmax (cm−1): 1674 (C=O), 1578 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 2.40 (3H, s, C–CH3), 4.01 (3H, s, CH3), 5.09 (2H, s, CH2), 7.39 (2H, d, J = 8.0 Hz, Ar-H), 7.95 (2H, d, J = 8.22 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 21.84, 34.35, 42.04, 129.82, 130.68, 133.83, 145.89, 154.71 and 193.77.
MS (FAB) [M + 1]+: m/z 249.
For C11H12N4OS calculated: 53.25% C, 4.87% H and 22.56% N; found 53.24% C, 4.83% H and 22.60% N.
1-(4-Methoxyphenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (6)
IR (KBr) νmax (cm−1): 1689 (C=O), 1573 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 3.87 (3H, s, O–CH3), 4.01 (3H, s, CH3), 5.07 (2H, s, CH2), 7.10 (2H, d, J = 8.86 Hz, Ar-H), 8.03 (2H, d, J = 8.83 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.35, 41.90, 56.49, 115.28, 129.13, 132.15, 165.19 and 192.57.
MS (FAB) [M + 1]+: m/z 265.
For C11H12N4O2S calculated: 49.99% C, 4.58% H and 21.20% N; found 49.95% C, 4.53% H and 21.17% N.
1-(4-Chlorophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (7)
IR (KBr) νmax (cm−1): 1680 (C=O), 1577 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 4.02 (3H, s, CH3), 5.11 (2H, s, CH2), 7.67 (2H, d, J = 8.41 Hz, Ar-H), 8.06 (2H, d, J = 8.44 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.37, 41.95, 130.27, 131.64, 135.06, 140.19, 154.58 and 193.46.
MS (FAB) [M + 1]+: m/z 269.
For C10H9ClN4OS calculated: 44.70% C, 3.38% H and 20.85% N; found 44.72% C, 3.38% H and 21.00% N.
1-(4-Florophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (8)
IR (KBr) νmax (cm−1): 1683 (C=O), 1579 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 4.08 (3H, s, CH3), 5.1 (2H, s, CH2), 7.42 (2H, d, J = 8.02 Hz, Ar-H), 8.15 (2H, d, J = 8.04 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.37, 41.93, 117.06, 117.24, 132.80, 132.87, 154.62, 165.88, 167.89 and 192.97.
MS (FAB) [M + 1]+: m/z 253.
For C10H9FN4OS calculated: 47.61% C, 3.60% H and 22.21% N; found 47.64% C, 3.58% H and 22.19% N.
1-(4-Bromophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (9)
IR (KBr) νmax (cm−1): 1684 (C=O), 1580 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 4.00 (3H, s, CH3), 5.1 (2H, s, CH2), 7.80 (2H, d, J = 8.60 Hz, Ar-H), 7.97 (2H, d, J = 8.6 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.36, 41.92, 129.37, 130.33, 131.78, 133.25, 154.65 and 193.69.
MS (FAB) [M + 1]+: m/z 314.
For C10H9BrN4OS calculated: 38.85% C, 2.90% H and 17.89% N; found 38.82% C, 2.98% H and 17.90% N.
1-(4-Nitrophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (10)
IR (KBr) νmax (cm−1): 1669 (C=O), 1570 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 4.02 (3H, s, CH3), 5.18 (2H, s, CH2), 8.3 (2H, d, J = 8.95 Hz, Ar-H), 8.4 (2H, d, J = 8.87 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.40, 42.18, 125.17, 131.16, 141.06, 151.65, 154.47 and 193.81.
MS (FAB) [M + 1]+: m/z 280.
For C10H9N5O3S calculated: 43.01% C, 3.25% H and 25.08% N; found 43.05% C, 3.27% H and 25.04% N.
1-(2,4-Dimethylphenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (11)
IR (KBr) νmax (cm−1): 1685 (C=O), 1582 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 2.34 (3H, s, C–CH3), 2.37 (3H, s, C–CH3), 3.99 (3H, s, N–CH3), 4.98 (2H, s, CH2), 7.17 (2H, s, Ar-H), 7.20 (1H, d, J = 8.00 Hz, Ar-H), 7.88 (1H, d, J = 7.91, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 21.46, 26.95, 34.31, 43.82, 127.75, 131.11, 133.81, 133.96, 139.58, 143.85, 154.78 and 196.73.
MS (FAB) [M + 1]+: m/z 263.
For C12H14N4OS calculated: 54.94% C, 5.38% H and 21.36% N; found 54.96% C, 5.37% H and 21.34% N.
1-(2,4-Dichlorophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (12)
IR (KBr) νmax (cm−1): 1677 (C=O), 1574 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 3.99 (3H, s, CH3), 4.96 (2H, s, CH2), 7.65 (1H, dd, J1 = 2.04 Hz, J = 10.51 Hz, Ar-H), 7.81 (1H, d, J = 2.00 Hz, Ar-H), 7.92 (1H, d, J = 8.5 Hz, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.37, 43.83, 128.94, 131.56, 132.80, 133.01, 136.16, 138.38, 154.42 and 192.64.
MS (FAB) [M + 1]+: m/z 304.
For C10H8Cl2N4OS calculated: 39.62% C, 2.66% H and 18.48% N; found 39.65% C, 2.67% H and 18.51% N.
1-(2,5-Dichlorophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (13)
IR (KBr) νmax (cm−1): 1684 (C=O), 1590 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 4.01 (3H, s, CH3), 4.96 (2H, s, CH2), 7.64 (1H, d, J = 8.60 Hz, Ar-H), 7.68 (1H, dd, J1 = 2.05 Hz, J2 = 8.52 Ar-H), 7.97 (1H, s, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.38, 43.73, 130.32, 130.74, 133.32, 133.68, 134.01, 138.96, 154.32 and 192.14.
MS (FAB) [M + 1]+: m/z 304.
For C10H8Cl2N4OS calculated: 39.62% C, 2.66% H and 18.48% N; found 39.67% C, 2.68% H and 18.52% N.
1-(3,4-Dichlorophenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone (14)
IR (KBr) νmax (cm−1): 1669 (C=O), 1571 (C=N).
1H-NMR (500 MHz, DMSO-d6): δ 4.01 (3H, s, CH3), 5.11 (2H, s, CH2), 7.88 (1H, d, J = 8.46 Hz, Ar-H), 8.02 (1H, d, J = 9.05 Hz, Ar-H), 8.29 (1H, s, Ar-H).
13C-NMR (125 MHz, DMSO-d6): δ 34.40, 41.78, 129.69, 131.68, 132.52, 133.21, 136.53, 138.06, 154.45 and 192.82.
MS (FAB) [M + 1]+: m/z 304.
For C10H8Cl2N4OS calculated: 39.62% C, 2.66% H and 18.48% N; found 39.60% C, 2.63% H and 18.53% N.
Biology
Anticandidal activity
The antifungal properties of compounds 1–14 were evaluated by the broth microdilution method according to the modified National Committee for Clinical Laboratory Standards (NCCLS) M27-A2 standard procedureCitation44. Tested candida strains were Candida albicans (isolate, obtained from Department of Microbiology, Faculty of Medicine, Osmangazi University, Eskisehir, Turkey), C. albicans (ATCC 90028), C. glabrata (isolate-1 obtained from Department of Microbiology, Faculty of Medicine, Osmangazi University, Eskisehir, Turkey), C. tropicalis (NRRL Y-12968), C. krusei (NRRL Y-7179), C. parapsilosis (NRRL Y-12696), C. albicans (NRRL Y-12983), C. glabrata (isolate-2 obtained from Department of Microbiology, Faculty of Medicine, Osmangazi University, Eskisehir, Turkey). Ketoconazole was used as positive control.
Stock solutions were prepared in dimethylsulfoxide (DMSO, Carlo-Erba, Val de Reuil, France). Overnight grown Candida suspensions in Mueller–Hinton Broth were standardized to 106 CFU/ml using suspension turbidity detector (BioSan, Riga, Latvia) adjusted to McFarland no. 0.5. Different from the NCCLS method, 100 µL of each Candida suspension was added into the wells. Sterile distilled water and medium served as a positive growth control. The first well without turbidity was assigned as the minimum inhibitory concentration (MIC, mg/mL). After incubation at 37 °C for 18–24 h, antifungal activity was detected by spraying of 0.5% TTC (triphenyl tetrazolium chloride, Merck) aqueous solution. MIC was defined as the lowest concentration of compounds that inhibited visible growth, as indicated by the TTC staining.
Cytotoxicity
The cytotoxic activities of the tested compounds were determined by cell proliferation analysis using the standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assayCitation45,Citation46. Mouse embryonic fibroblast (NIH/3T3) cells were cultured in 96-well flat-bottom plates at 37 °C for 24 h (2 × 104 cells per well). All the compounds were dissolved in DMSO individually and added to culture wells at varying concentrations (0.5–500 µg/mL), the highest final DMSO concentration was under 0.1%. After 24 h of drug incubation at 37 °C, 20 µL MTT solution (5 mg/mL MTT powder in PBS) was added to each well. Then 3 h incubation period was maintained in the same conditions. Purple formazan occurred at the end of the process, which is the reduction product of MTT agent by the mitochondrial dehydrogenase enzyme of intact cells. Formazan crystals were dissolved in 100 µL DMSO and the absorbance was read by ELISA reader (OD570nm). The percentage of viable cells was calculated based on the medium control.
Results and discussion
Chemistry
In this study, we aimed to synthesize a series of tetrazole-based compounds by a facile, convenient, one-pot synthetic route. 1-(4-Substituted phenyl)-2-[(1-methyl-1H-tetrazol-5-yl)thio]ethanone compounds were synthesized by reacting 1-methyl-1H-tetrazole-5-thiol with some phenylacetyl bromide derivatives. The synthetic protocol of the compounds and synthesized molecules are shown in and .
Table 1. Some characteristics of the synthesized compounds.
The structure elucidation of the compounds was determined by IR, 1H-NMR, 13C-NMR, FAB+-MS spectral data and elemental analyses results. In the IR spectra of all compounds, characteristic carbonyl stretching band was observed at about 1669–1689 cm−1 region due to the C=O vibration and also the C=N vibration of the compounds was seen at 1570–1597 cm−1 region as expected.
The 1H-NMR spectral data were also consistent with the assigned structures. In the 500 MHz 1H-NMR spectrum of compounds, the N–CH3 protons resonated at 3.99–4.08 ppm as a singlet and also S–CH2 protons were observed at 4.96–5.20 ppm. In aromatic field, the signals of characteristic aromatic protons were observed at expected regions. In the 13C-NMR spectra of the compounds, the signal of characteristic carbonyl carbon appeared at a range of 192.14–196.73 ppm. Also N-CH3 and S-CH2 signals were seen at 34.31–34.85 ppm and 41.78–43.86 ppm, respectively. In the MS spectra, the electron spraying technique with positive polarity mode was applied and M + 1 peaks were detected as base peak. All compounds gave satisfactory elemental analysis results.
Biology
The target compounds 1–14 were screened for their in vitro anticandidal activity against eight Candida species, including standard strains and clinical isolates. MIC is defined as the concentration of the compound required to give complete inhibition of bacterial growth and MICs of the synthesized compounds along with the reference drug ketoconazole.
The results provided in indicate that most of the prepared compounds displayed broad antifungal spectrum with MIC values ranging from 8 to 375 µg/mL against all the tested strains and some of them exhibited comparable or even better efficiency in comparison with the reference drug ketoconazole. Among all evaluated strains, the highest inhibitory activity was observed against C. albicans (ATCC 90028). Among all the compounds, 1, 2, 6, 12, 13 and 14 had the highest anticandidal activity against all species compared with ketoconazole. Compounds 1, 2, 6 and 13 were more potent than ketoconazole against C. albicans (ATCC 900028) whereas 1, 2 and 13 compounds were more potent against C. albicans (clinical isolate). Compound 12 inhibited C. albicans (ATCC 900028) and C. albicans (clinical isolate) at concentrations of 30 and 60 µg/mL, respectively, when ketoconazole inhibited both of Candida spp. with a concentration of 30 µg/mL.
Table 2. Antimicrobial activities of the compounds (µg/mL).
Compounds 2 and 14 showed higher activity against C. glabrata (clinical isolate-1 obtained from Department of Microbiology, Faculty of Medicine, Osmangazi University, Eskisehir, Turkey) whereas 5 and 6 had similar activity to ketoconazole against C. glabrata (clinical isolate-1). Compound 6 was the most effective compound against C. krusei when 2 was against C. glabrata (clinical isolate-2 obtained from Department of Microbiology, Faculty of Medicine, Osmangazi University, Eskisehir, Turkey). Compounds 2 and 14 were potent against C. albicans (NRRL Y-12983). All the compounds were observed moderate anticandidal activity against C. tropicalis (NRRL Y-12968) and C. parapsilosis (NRRL Y-12696).
Compounds were also studied for their cytotoxic properties using the MTT assay. The IC50 (μg/mL) values of the compounds against NIH/3T3 cells are shown in . The biological study indicated that compound 9 possessed the highest cytotoxicity with a value of about 15 μg/mL, whereas compound 1 exhibited the lowest cytotoxicity with a value of about 526 μg/mL against NIH/3T3 cells.
Table 3. In vitro cytotoxicity of the compounds.
Conclusion
In this study, we report the synthesis, spectral studies and biological evaluation of a series of tetrazole derivatives (1–14). The structures proposed to the synthesized compounds (1–14) are well supported by spectroscopic data and elemental analysis. Compounds 1, 2, 6, 12, 13 and 14 exhibited the highest anticandidal activity. Candida albicans (Clinical isolate), C. albicans (ATCC 90028) and C. glabrata (Clinical isolate) are the most susceptible fungi to all the compounds. Compounds 1, 3, 5, 12 and 13 had the lowest cytotoxicity against NIH/3T3 cells. In comparison with the results of cytotoxicity and anticandidal activity tests, it can be claimed that compounds 1, 12 and 13 possibly have anticandidal activity because of their selective anticandidal effect; in addition, chloro substitution on benzene ring causes increase in anticandidal activity.
Declaration of interest
The authors report no conflicts of interest.
References
- Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161–89
- Rees JR, Pinner RW, Hajjeh RA. The epidemiological features of invasive mycotic infections in the San Francisco bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis 1998;27:1138–47
- Polak A. The past, present and future of antimycotic combination therapy. Mycoses 1999;42:355–70
- Fostel JM, Lartey PA. Emerging novel antifungal agents. Drug Discov Today 2000;5:25–32
- Bodey GP. Azole antifungal agents. Clin Infect Dis 1992;14:161–9
- Sheehan DJ, Hitchcock CA, Sıbley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev 1999;12:40–79
- Maertens JA. History of the development of azole derivatives. Clin Microbiol Infect 2004;10:1–9
- Rossello A, Bertini S, Lapucci A, et al. Synthesis, antifungal activity, and molecular modeling studies of new inverted oxime ethers of oxiconazole. J Med Chem 2002;45:4903–12
- Rajasekaran A, Thampi PP. Synthesis and analgesic evaluation of some 5-[β-(10-phenothiazinyl)ethyl]-1-(acyl)-1,2,3,4-tetrazoles. Eur J Med Chem 2004;39:273–9
- Dhayanithi V, Syed SS, Kumaran K, et al. Synthesis of selected 5-thio-substituted tetrazole derivatives and evaluation of their antibacterial and antifungal activities. J Serb Chem Soc 2011;76:165–75
- Kraft A, Osterod F, Frohlich R. Bidirectional association of branched noncovalent complexes of tetrazoles and 1,3,5-tris(4,5-dihydroimidazol-2-yl)benzene in solution. J Org Chem 1999;64:6425–33
- Klaubert DH, Sellstedt JH, Guinosso CJ, et al. 5-Tetrazolecarboxamides and their salts: new orally active antiallergy agents. J Med Chem 1981;24:748–52
- El-Sayed WA, Megeid REA, Abbas HS. Synthesis and antimicrobial activity of new 1-[(tetrazol-5-yl)methyl]indole derivatives, their 1,2,4-triazole thioglycosides and acyclic analogs. Arch Pharm Res 2011;34:1085–96
- Sabbah M, Fontaine F, Grand L, et al. Synthesis and biological evaluation of new N-acyl-homoserine-lactone analogues, based on triazole and tetrazole scaffolds, acting as LuxR-dependent quorum sensing modulators. Bioorg Med Chem 2012;20:4727–36
- Rostom SAF, Ashour HMA, Abd El Razik HA, et al. Azole antimicrobial pharmacophore-based tetrazoles: synthesis and biological evaluation as potential antimicrobial and anticonvulsant agents. Bioorg Med Chem 2009;17:2410–22
- Meanwell NA. Synopsis of some recent tactical application of bioisosteres in drug design. J Med Chem 2011;54:2529–91
- Myznikov LV, Hrabalek A, Koldobskii GI. Drugs in the tetrazole series. Chem Heterocycl Comp 2007;43:1–9
- Adamec J, Waisser K, Kunes J, Kaustova J. A note on the antitubercular activities of 1-aryl-5-benzylsulfanyltetrazoles. Arch Pharm 2005;338:385–9
- Mohite PB, Pandhare RB, Khanage SG, Bhaskar VH. Synthesis and in vitro antimicrobial activity of some novel chalcones containing 5-phenyl tetrazole. Acta Pharma Sci 2010;52:505–10
- Cosgrove CE, Laforge RA. Tetrazole derivatives I. Tetrazole alkamine ethers. J Org Chem 1956;21:197–200
- Haydu SP, Bardley J, Hughes DTD. Inhibitory effect of oral doxantrazole on asthma induced by allergen inhalation. Br Med J 1975;3:283–4
- Toney JH, Fitzgerald PMD, Sharma NG, et al. Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of bacteroides fragiris metallo-β-lactamase. Chem Biol 1998;5:185–96
- Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res 2007;75:18–28
- Khanage SG, Mohite PB, Pandhareb RB, Raju SA. Study of analgesic activity of novel 1,2,4-triazole derivatives bearing pyrazole and tetrazole moiety. J Pharm Res 2011;4:3609–11
- Maxwell JR, Wasdahl DA, Wolfson AC, Stenberg VI. Synthesis of 5-aryl-2H-tetrazoles, 5-aryl-2H-tetrazole-2-acetic acids, and [(4-phenyl-5-aryl-4H-1,2,4-triazol-3-yl)thio]acetic acids as possible superoxide scavengers and antiinflammatory agents. J Med Chem 1984;27:1565–70
- Bhaskar VH, Mohite PB. Synthesis, characterization and evaluation of anticancer activity of some tetrazole derivatives. J Optoelectron Biomed M 2010;2:249–59
- Ueda I, Ishii K, Sinozaki K, Htanaka M. Antiulcer agents. II: synthesis and gastric acid antisecretory activity of N-[3-{3-(piperidinomethyl)phenoxy}propyl]-4-(1-methyl-1H-tetrazol-5-ylthio)butanamide and related compounds. Chem Pharm Bull 1991;39:1430–5
- Bhaskar VH, Mohite PB. Synthesis analgesic, anti-inflammatory and antimicrobial activities of some 1-[5-(substituted phenyl)-4,5-dihydro-1H-pyrazol-3-yl]-5-phenyl-1H-tetrazole. J Optoelectron Biomed M 2011;3:7–16
- Singh H, Chawla AS, Kapoor VK, et al. Progress in medicinal chemistry. In: Ellis GP, West GB, eds. Medicinal chemistry of tetrazoles. Amsterdam: Elsevier/North Holland; 1980:151–73
- Upadhayaya RS, Jain S, Sinha N, et al. Synthesis of novel substituted tetrazoles having antifungal activity. Eur J Med Chem 2004;39:579–92
- Matysiak J, Niewiadomy A, Krajewska-Kułak E, Mącik-Niewiadomy G. Synthesis of some 1-(2,4-dihydroxythiobenzoyl)imidazoles, -imidazolines and -tetrazoles and their potent activity against Candida species. IL Farmaco 2003;58:455–61
- Roh J, Vávrová K, Hrabálek A. Synthesis and functionalization of 5-substituted tetrazoles. Eur J Org Chem 2012;31:6101–18
- Kanakaraju S, Prasanna B, Chandramouli GVP. An efficient one-pot three-component synthesis of novel sulfanyl tetrazoles using ionic liquids. J Chem 2013;2013:1--6
- Segura-Cabrera A, Pérez MAR. Structure-based prediction of Mycobacterium tuberculosis shikimate kinase inhibitors by high-throughput virtual screening. Bioorg Med Chem Lett 2008;18:3152–7
- Webster SP, Binnie M, McConnell KMM, et al. Modulation of 11b-hydroxysteroid dehydrogenase type 1 activity by 1,5-substituted 1H-tetrazoles. Bioorg Med Chem Lett 2010;20:3265–71
- Morita H, Takeda M, Yoshimura T, et al. Novel type elimination reactions of sulfoxides bearing several heteroaromatics: trapping of sulfines with 2,3-dimethyl-1,3-butadiene. J Org Chem 1999;64:6730–7
- Webster SP, Seckl JR, Walker BR. 1,5-substituted-1h-tetrazole 11beta-hydroxysteroid dehydrogenase type 1 inhibitors, Patent GB 2429975 A 20070314, UK; 2007
- Hakonarson H, Gurney ME, Halapi E. Methods of diagnosis and treatment for asthma and other respiratory diseases based on haplotype, Patent US 20060014165 A1 20060119, USA; 2006
- Hakonarson H, Gurney ME, Halapi E. Methods of diagnosis and treatment for asthma and other respiratory diseases based on haplotype association related applications, Patent WO 2005007144 A2 20050127, USA; 2005
- Hudkins RL, Mallamo JP, Hamano M, et al. Selected derivatives of K-252a, Patent US 6306849 B1 20011023, USA; 2001
- Hudkins RL, Mallamo JP, Hamano M, et al. K-252 derivatives as protein kinase C inhibitors, their preparation and formulations containing them, Patent WO 9746565 A1 19971211, USA; 1997
- Nishi T, Uno T, Koga Y, Chu GN. Tetrazole derivatives and antiulcer composition containing them, EP 240015 A2 19871007; 1987
- Goldfarb DS. Method for altering the lifespan of eukaryotic organisms, US 20090163545 A1 20090625, USA; 2009
- Koneman EW, Allen SD, Janda WM, et al. Color atlas and textbook of diagnostic microbiology, mycology. Philadelphia, PA: Lippincott-Williams and Wilkins; 1997:983–1057
- Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63
- Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod 2000;26:288–91