Abstract
Studies on the mechanisms of saliva secretion have indicated that carbonic anhydrase (CA) is expressed in mammalian salivary glands. The enzyme is present in the saliva as the only known secretory isoenzyme, CAVI; its activity has been related to the modulation of taste and caries development. Unlike mammals, in birds, saliva is produced by the so-called minor salivary glands, mostly concentrated in the tongue. The involvement of CA has never been explored in avian salivary secretion. Thus, we aimed here to ascertain the enzyme occurrence in the quail lingual glands by a parallel investigation of the distributional patterns of CA activity sites, as visualized by histochemistry, and the immunohistochemical patterns of cytosolic CAII and secretory CAVI. The comparative evaluation of our findings does not rule out that some CA isoforms, associated to basolateral borders of the secretory cells and antigenically different from cytosolic CAII and secretory CAVI, may be involved in the salivary secretion in the quail lingual glands.
Introduction
The involvement of carbonic anhydrase (CA) activity in the saliva production and secretion has been widely explored in mammalian salivary glands which express the only secretory CA isoenzyme, CAVICitation1. Its occurrence was detected also in the lingual serous (von Ebner’s) glands of rats and humans, with a putative role in taste reception and taste receptor cell apoptosisCitation2. Little is known, conversely, about the nature and mechanisms of salivary secretion in non-mammalian vertebrates, especially birds. Numerous small salivary glands are found in the avian oral cavity. These are best developed in birds that eat a dry diet, such as seed and insects, and least developed in birds that eat a moist diet, such as fish. Classical studies have described associations between morphofunctional and developmental features of poultry salivary glands in relationship with the type of dietCitation3,Citation4. However, other investigations have suggested that the major function of mucus from lingual and palatine glands is not only lubrication and moistening of food, but also non-immune protection of buccal mucosae, irrespective of the food ingestedCitation5,Citation6. The available data on mechanisms and functions of mucus secretion in birds are mostly based on the identification and characterization of its glycosidic components which were found to be extremely heterogeneous and heterogeneously distributed within secretory units of lingual glands as well as within each secretory cellCitation7–9.
In this context, the study of CA expression in avian salivary glands might contribute new data, with a possible interest also from a phylogenetical point of view. Thus, we investigated here the CA occurrence in the lingual glands of quail by performing both the histochemical demonstration of the CA activity sites and the immunohistochemical identification of CAII and CAVI isoenzymes.
Methods
Five adult quails (Coturnix coturnix japonica) were anaesthetized with ether and killed by decapitation, according to the recommendation of the Italian Ethical Committee. Anterior and posterior tongue portions were removed immediately after sacrifice and processed for either histochemical or immunohistochemical investigation.
Histochemistry
Tissues were fixed in 4% paraformaldehyde and 0.5% glutaraldehyde solution in 0.13 M Millonig’s buffer (0.16 M NaH2PO4 and 0.63 M NaOH), pH 7.3, for 6 h at 4 °C. After washing in the buffer, small fragments of tissue were dehydrated and embedded in the hydrophilic JB-4 resin (Polysciences, Warrington, PA) according to the manufacturer’s instructions. Semi-thin sections (2 µm thick) were collected floating in Millonig’s buffer and processed for CA activity demonstrationCitation10, as previously detailedCitation11. Sections were then counterstained with toluidine blue and mounted in Eukitt. (Electron Microscopy Sciences, Hatfield, PA).
Control sections were treated by adding 1 × 10−6 M acetazolamide, a specific CA activity inhibitor, to the incubation medium or by omitting the sodium bicarbonate substrate.
Immunochemical analyses
Antibodies
The immunohistochemical investigation was performed using the monoclonal antibody 2A2-1, raised against the chicken CAII (from P.J. Linser, Whitney Lab., Florida University, St. Augustine, FL). Furthermore, a monoclonal antibody raised against rat CAVI (from Y. Ogawa, Department of Oral and Maxillofacial Pathology, Osaka University, Osaka, Japan) was applied as a preliminary assay to explore an eventual immunoreactivity in the quail lingual glands.
Western blotting
In order to check the cross-reactivity of the monoclonal anti-chicken CAII in quail tissues, a Western blot assay was performed on kidneys from either chicken and quail.
Samples (0.1 ± 0.02 g) of kidney were homogenized in a Mixer Mill MM300 (Qiagen, Hilden, Germany) with 1000 µl of 0.1 M phosphate-buffered saline (PBS), pH 7.4, 0.1% IGEPAL CA-630, 1 mM of CaCl2, 1 mM of MgCl2, 0.1% NaN3, 1 mM of phenyl-methyl-sulphonil-fluoride, aprotinin and 1 mM of sodium orthovanadate. After two centrifugations at 13 000 rpm (10 min at 4 °C), aliquots of the supernatant were used for protein assay against a standard of bovine serum albumin (BSA) using a BIO-RAD protein assay (BIO-RAD, Munich, Germany). Equal amounts of protein (40 µg) were separated by 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane by electroblotting in Towbin buffer (BIO-RAD, Munich, Germany). Transblotted membranes were first washed in 0.05% Tween-20 (Sigma, St. Louis, MO) in PBS and then incubated with CAII monoclonal antibody diluted 1:500 in PBS. The immunochemistry product was visualized using as HRP antibody (donkey anti-mouse IgG cat. no. sc-2314, Santa Cruz Biotechnology, Heidelberg, Germany) followed by a chemiluminescence detection system (Lite Ablot® plus, cat. EMP 011005, EuroClone, Milano, Italy).
Immunohistochemistry
Tongue portions were fixed in Bouin solution for 6 h at room temperature, dehydrated in graded ethanols, cleared in xylene and embedded in paraffin. Tissue samples from rat submandibular glands, processed as above, were used as positive controls for CAVI immunostaining. Sections (5 µm thick) were rehydrated and subjected to the immunohistochemical staining using the immmunoperoxidase procedure. After inactivation of the endogenous peroxidase and blocking of endogenous avidin-binding activity, sections were incubated for 20 min with normal rabbit serum, diluted 1:5 with 1% BSA (Sigma, St. Louis, MO) in 0.05M PBS, pH 7.6. Incubation of sections with either anti-chicken CAII monoclonal antibody or anti-rat CAVI monoclonal antibody was performed at dilution 1:100 in PBS containing 1% BSA overnight at room temperature. Sections of rat submandibular glands were incubated with anti-rat CAVI antibody in the same conditions as above. After washing in PBS, biotinylated anti-mouse IgGs were applied for 45 min, followed by rinsing in PBS and incubation with avidin–biotin–peroxidase complex for 45 min. The CAII- and CAVI-immunoreactive sites were visualized with 3,3′-diaminobenzidine as a yellow–brown precipitate. All the reagents used were from Vector Laboratories (Burlingame, CA). Some of the immunostained sections were counterstained with methylene blue. Finally, sections were dehydrated and mounted in Eukitt. Controls for the specificity of the immunohistochemical stainings were performed by replacing the primary antibody with PBS plus 1% BSA or preimmune rabbit serum.
Results
The microscopic observation fully reproduced the morphological features of the quail lingual glands, reported previously by several authorsCitation12,Citation13. They comprise the anterior glands, with a rostral and a caudal portion, and the posterior glands. Both the glands are located in the lamina propria and are surrounded by a connective tissue capsule. The rostral glands exhibit seromucous cells whereas the caudal and posterior glands consist only of mucous cells.
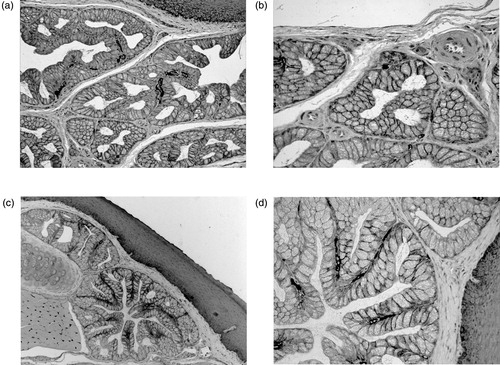
The histochemical staining, indicative of CA activity, could be detected at the basolateral border of some scattered cells in the secretory tubules of the anterior rostral portion (). A similar distribution of the reactivity was found, at the same cellular sites, in the tubulo-acini and tubules of the anterior caudal portion and in the posterior gland (). A marked staining was also evident in the capillary endothelia of both glands and in the basal layer of the squamous-stratified epithelium ().
Figure 1. Histochemical demonstration of CA activity by Hansson’s method followed by counterstaining with toluidine blue. Anterior gland of quail tongue: (a) a dark staining, indicative of CA activity, is detectable in the secretory units as well as in capillary endothelia and basal cells of the stratified squamous epithelium and (b) at higher magnification, the reaction product is clearly recognizable at the basolateral borders of sparse secretory cells. Posterior gland of quail tongue: (c) the secretory mucous cells show marked deposits of the CA reaction product that, at higher magnification (d), appears to be strictly associated to the basolateral cell membranes. Staining is also present at the basal cell layer of the stratified squamous epithelium. Original magnification: 10× (a, c); 20× (b, d).
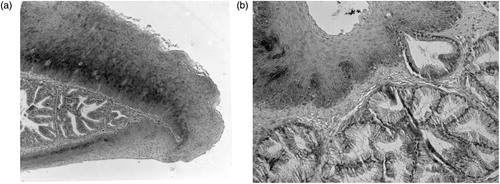
In Western blot of quail kidneys, the monoclonal antibody against chicken CAII recognized a single protein band, identified approximately at 29 kDa, which was the same as that detected in the chicken kidneys (), thus validating its application on quail tissues. When applied to sections of the quail tongue, the anti-CAII produced no immunostaining in secretory cells of the anterior and posterior lingual glands. CAII antibody abundantly stained only the cytoplasm of the basal cells in the dorsal stratified squamous epithelium (). A lack of immunoreactivity in the quail tongue was found also using anti-CAVI. In contrast to this finding, sections from rat submandibular glands, stained for positive control, yielded a specific reaction in serous acinar cells (not shown). Control sections incubated with either preimmune serum or PBS remained unstained (not shown).
Figure 2. Western blot of kidney extracts from chicken (lane A) and quail (lane B) stained with anti-chicken CAII. Lane ST, molecular weight standards.
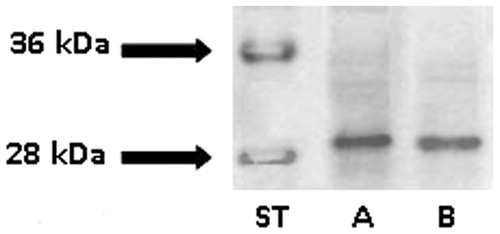
Figure 3. Immunohistochemical localization of CAII isoenzyme in the quail lingual glands. Counterstaining with methylene blue: (a) in the anterior glands, secretory cells show no immunoreactivity that is, instead, widely present in the basal cells of the dorsal stratified epithelium and (b) the secretory units of the posterior glands lack CAII immunostaining. A secretory acinus can be seen opening into the ventral surface epithelium whose basal cells show weak immunoreactivity. Original magnification: 10 × (a); 20 × (b).
Discussion
By the application of two distinct methodologies, this study was aimed to ascertain whether CA is expressed in the quail lingual glands, also known as minor salivary glands. The question arises when considering that CA is present in the homologous organs of mammalian species, where its enzymatic activity has been related to the secretion and function of saliva. In particular, the rat and human minor salivary glands, the von Ebner’s glands, proved to express CAVICitation2, the only known secretory CA isoenzyme, first identified in mammalian major salivary glandsCitation14–16. The enzyme showed to be secreted to the bottom of the trenches surrounding the lingual circumvallate and foliate papillae. Based on the data available, it was suggested that locally secreted CAVI is implicated in the paracrine modulation of taste functionCitation2.
In this study on the minor salivary glands of quail, findings from histochemistry indicated that CA activity is expressed in the secretory units of both anterior and posterior glands. Its localization is tightly restricted to the basolateral border of the epithelial cells forming the tubules and tubuloalveoli of the branched glands. The distribution of the enzyme activity sites proved to be wider in the rostral anterior gland than in the caudal and posterior glands. In order to identify the specific isoenzyme responsible for the histochemical staining, we compared the distributional patterns of CA activity with those produced by immunohistochemistry. However, despite evidence for CA reactive sites, the present immunohistochemical findings failed to visualize the occurrence, in the lingual gland secretory cells, of the cytosolic CAII isoenzyme, known to be widely expressed in avian tissuesCitation17,Citation18. The reliability of such a result is based on the cross-reactivity that the anti-chicken CAII showed in quail tissues by the Western blotting analysis () as well as on recent works which documented 100% similarity between quail and chicken amplicons for CAIICitation18.
Likewise, the antibody against rat CAVI could not recognize any antigenically active molecule in the quail lingual glands. The non-coincidence of the results derived from histochemistry and immunohistochemistry, as here performed, leads us to suggest that in avian tissues an additional CA isoenzyme, antigenically distinct from either cytosolic CAII or secretory CAVI, is expressed. In support to this hypothesis, our previous findings documented the presence in different avian tissues of a membrane-associated CA form, in addition to cytosolic CAII and CAIII, the only known isoenzymes in birds. In particular, the intracellular localization of CA staining, as shown here by histochemistry, recalls similar distributional patterns of CA activity previously identified at the cell borders in the quail renal tubulesCitation19 as well as at the apical borders of the villus cavity cells in the chick chorioallantoic membraneCitation20,Citation21. At both sites, neither anti-chicken CAII nor anti-chicken CAIII antibodies yielded any immunoreactivity. Rather, using antisera to human CAIV, a specific immunostaining, fully coincident with the histochemical staining, was found in the villus cavity cells of the chick chorioallantoic membraneCitation20. Although a conclusive characterization of such a membrane-associated CA remains to be achieved, its functional meaning might be consistent with that attributed to mammalian CAIV at the different sites where it proved to be expressedCitation22–25. In the quail lingual glands, a putative membrane-associated CA isoform at the basolateral borders of the secretory cells might serve, analogously to CAIV isoenzyme, to regulate the extracellular pH and, thereby, contribute to maintaining the balance of the oral environment. Together with salivary mucins, CA might function to protect the oral surface and upper digestive and respiratory tracts, hindering proliferation of the pathogenic microbiota.
Finally, in light of the roles attributed to CAVI in rat and human salivaCitation2,Citation26–28, the present result that the quail lingual glands lack a secretory form of CA is likely to be evaluated in relation to the specific structural and functional features of the oral cavity in the different vertebrate classes. Indeed, recent studies on Car6−/− mice have reported that salivary CAVI, that is also a component of the enamel pellicle, functions in caries development, probably by modulating the oral microbiota and/or by the enzymatic production of acid within plaqueCitation28. Moreover, the identification of CAVI in von Ebner’s gland saliva, in the taste pores, and in some cells in the taste buds suggested that locally secreted CAVI may play a paracrine role in taste reception and in the protection of taste receptor cells from apoptosisCitation2. In comparison to mammals, birds, especially quail and chicken, show a very low number of taste buds. The paucity of taste buds in the avian tongue has been related to the fact that, unlike mammals, birds do not break down their food orally; therefore, the food is not in contact with the tongue for long. This would have limited need for taste on the tongue.
In conclusion, based on the above considerations, the presence of a secretory CA isoform in the saliva of mammals may be viewed as an evolutionary achievement linked to specific structural features and functional requirements in the oral cavity, which are not shared by birds.
Declaration of interest
No potential conflicts of interest are reported for any of the authors.
References
- Parkkila S, Kaunisto K, Rajaniemi L, et al. Immunohistochemical localization of carbonic anhydrase isoenzymes VI, II, and I in human parotid and submandibular glands. J Histochem Cytochem 1990;38:941–7
- Leinonen J, Parkkila S, Kaunisto K, et al. Secretion of carbonic anhydrase isoenzyme VI (CA VI) from human and rat lingual serous von Ebner’s glands. J Histochem Cytochem 2001;49:657–62
- Farner DS, Ziswiler V. Digestion and digestive system. In: Farner DS, King JR, eds. Avian biology. London: Academic Press; 1972:343–430
- McLelland J. Digestive system. In: King AS, McLelland J, eds. Form and function in birds. London: Academic Press; 1979:170–81
- Samar ME, Avila RE, Fabro SP, et al. Histochemical study of Magellanic penguin (Spheniscus magellanicus) minor salivary glands during postnatal growth. Anat Rec 1999;254:298–306
- Samar ME, Ávila RE, Esteban FJ, et al. Histochemical and ultrastructural study of the chicken salivary palatine glands. Acta Histochem 2002;104:199–207
- Bondi AM, Gabrielli MG, Accili D, et al. Confocal evaluation of native and induced lectin binding contributes to discriminate between lingual gland glycocomponents in quail. Histol Histopathol 2000;15:1119–25
- Liman N, Bayram G, Koçak M. Histological and histochemical studies on the lingual, preglottal and laryngeal salivary glands of the Japanese quail (Coturnix coturnix japonica) at the post-hatching period. Anat Histol Embryol 2001;30:367–73
- Capacchietti M, Sabbieti MG, Agas D, et al. Ultrastructure and lectin cytochemistry of secretory cells in lingual glands of the Japanese quail (Coturnix coturnix japonica). Histol Histopathol 2009;24:1087–96
- Hansson HPJ. Histochemical demonstration of carbonic anhydrase activity. Histochemie 1967;11:112–28
- Gabrielli MG, Palatroni P, Vincenzetti S. Renal carbonic anhydrase in the quail Coturnix coturnix japonica: I. activity and distribution in male and female metanephros. Histochem J 1990;22:579–87
- Maya S, Lucy P. Histology of the lingual glands in Japanese quail (Coturnix coturnix japonica). Indian J Poult Sci 2000;35:306–8
- Olmedo LA, Samar ME, Avila RE, et al. Avian minor salivary glands: an ultrastructural study of the secretory granules in mucous and seromucous cells. Acta Odontol Latinoam 2000;13:87–99
- Fernley RT, Wright RD, Coghlan JP. A novel carbonic anhydrase from the ovine parotid gland. FEBS Lett 1979;105:299–302
- Murakami H, Sly WS. Purification and characterization of human salivary carbonic anhydrase. J Biol Chem 1987;262:1382–8
- Parkkila S, Kaunisto K, Rajaniemi L, et al. Immunohistochemical localization of carbonic anhydrase isoenzymes VI, II, and I in human parotid and submandibular glands. J Histochem Cytochem 1990;38:941–7
- Nishita T, Tomita Y, Imanari T, et al. Biochemical and developmental characterization of carbonic anhydrase II from chicken erythrocytes. Acta Vet Scand 2011;53:16
- de Winter P, Sugden D, Baggott GK. Effect of egg turning and incubation time on carbonic anhydrase gene expression in the blastoderm of the Japanese quail (Coturnix c. japonica). Br Poult Sci 2008;49:566–73
- Gabrielli MG, Vincenzetti S, Vita A, Menghi G. Immunohistochemical localization of carbonic anhydrase isoenzymes II and III in quail kidney. Histochem J 1998;30:489–97
- Gabrielli MG. Carbonic anhydrases in chick extraembryonic structures: a role for CA in bicarbonate reabsorption through the chorioallantoic membrane. J Enzyme Inhib Med Chem 2004;19:283–6
- Gabrielli MG, Accili D. The chick chorioallantoic membrane: a model of molecular, structural, and functional adaptation to transepithelial ion transport and barrier function during embryonic development. J Biomed Biotechnol [Online] 2010;2010:940741. Available from: http://www.hindawi.com/journals/jbb/2010/940741/ [last accessed 9 Jan 2012]
- Brown D, Zhu X, Sly WS. Localization of membrane-associated carbonic anhydrase type IV in kidney epithelial cells. Proc Natl Acad Sci USA 1990;87:7457–61
- Svichar N, Esquenazi S, Waheed A, et al. Functional demonstration of surface carbonic anhydrase IV activity on rat astrocytes. Glia 2006;53:241–7
- Scheibe RJ, Mundhenk K, Becker T, et al. Carbonic anhydrases IV and IX: subcellular localization and functional role in mouse skeletal muscle. Am J Physiol Cell Physiol 2008;294:C402–12
- Wandernoth PM, Raubuch M, Mannowetz N, et al. Role of carbonic anhydrase IV in the bicarbonate-mediated activation of murine and human sperm. PLoS One 2010;5:e15061
- Kivela J, Parkkila S, Metteri J, et al. Salivary carbonic anhydrase VI concentration and its relation to basic characteristics of saliva in young men. Acta Physiol Scand 1997;161:221–5
- Henkin RI, Martin BM, Agarwal RP. Decreased parotid saliva gustin/carbonic anhydrase VI secretion: an enzyme disorder manifested by gustatory and olfactory dysfunction. Am J Med Sci 1999;318:380–91
- Culp DJ, Robinson B, Parkkila S, et al. Oral colonization by Streptococcus mutans and caries development is reduced upon deletion of carbonic anhydrase VI expression in saliva. Biochim Biophys Acta 2011;1812:1567–76
