Abstract
New 9-(alkyl/aryl)-4-fluoro-6-oxo[1,2,5]thiadiazolo[3,4-h]quinoline-5-carboxylic acids and their esters were designed and synthesized. A detailed discussion of the reactions utilized in the preparation of the intermediate and target compounds is reported. All the newly synthesized compounds were fully characterized using all the physico-chemical means needed. All the intermediates and the final esters and acids were tested against bacterial and fungal strains. The acids 25a and 25c proved to be very active against Gram positive and Gram negative bacteria with MIC 0.15–3 µg/mL. The structure–activity relationship of antibacterial thiadiazoloquinolones shows that compounds 25a and 25c are twice less potent than the corresponding cyclopropyl derivative 16. Therefore, the cyclopropyl moiety on N-9 seems to be the most suitable substituent.
Introduction
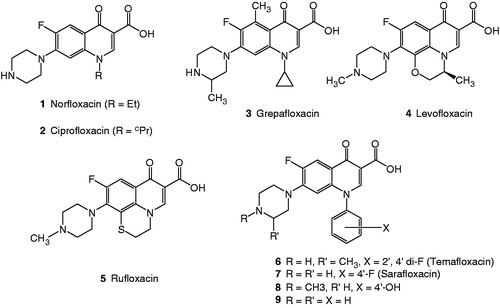
Norfloxacin (1) and ciprofloxacin (2) were among the first quinolones marketed as chemotherapeutic agents for the treatment of systemic bacterial infectionsCitation1–3. Several new members of the quinolone family have emerged with enhanced activities such as grepafloxacin (3), levofloxacin (4), rufloxacin (5), and compounds 6–9 (). Much has been learned about how molecular modifications of the core quinolone structure affect the antibacterial profile. The structure–activity relationship of the quinolones has been the subject of extensive reviewsCitation4–16.
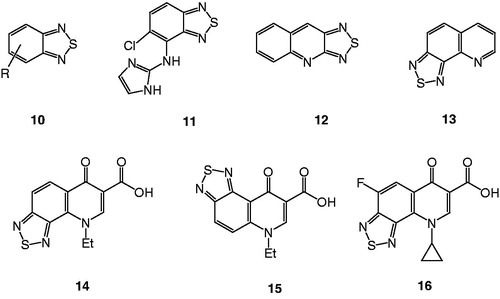
In our continuous effort to develop new chemotherapeutics with enhanced activities against resistant bacterial strainsCitation17,Citation18, we introduced a new class of tricyclic compounds, namely thiadiazoloquinolonesCitation19. In our methodology, we took advantage of many known benzothiadiazoles () with diverse biopharmaceutical activitiesCitation20–26 and we hybridized the thiadiazole moiety with the fluoroquinolone structure to introduce the new compound 9-cyclopropyl-4-fluoro-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline carboxylic acid (16). The latter compound was tested against several clinical isolate bacterial strains and did prove to be quite active compared to the drugs used in the marketCitation19.
Several drugs used in the market show a variation at N-1, e.g. cyclopropyl, ethyl, 2,4-difluorophenyl and many others. Our strategy for the synthesis of compound 16, banked on the introduction of the fluorine atom, for its crucial advantage, and also on the replacement of the ethyl with the cyclopropyl moiety, with respect to thiadiazoloquinolones 14 and 15.
The result of that study was very interesting, the strategy proved its success via enhancement of the activity compared to the previous thiadiazoloquinolones 14 and 15, and also the potency of compound 16 was comparable to the drug ciprofloxacin.Citation19
Herein, we report on the synthesis and the antibacterial activities of new compounds based on the same strategy by fixing the fluorine atom and replacing the cyclopropyl with different moieties, namely ethyl, phenyl, p-fluorophenyl and p-methoxyphenyl, which are based on the structure of important fluoroquinolone agents.
Materials and methods
All the chemicals and solvents used in this study were purchased from Acros. Melting points were measured by Fischer-Johns Melting Point Apparatus and were uncorrected. 1H NMR and 13C NMR spectra were measured on a Bruker DPX-300 instrument. Chemical shifts (δ) are expressed in ppm with reference to TMS as internal standard. High resolution mass spectra (HRMS) were measured in positive ion mode by electrospray ionization (ESI) on a Bruker instrument APEX IV 2008. The samples were dissolved in acetonitrile, diluted in spray solution (methanol/water 1:1 v/v + 0.1% formic acid) and injected using a syringe pump with a flow rate of 2 μL/min. External calibration was conducted using Arginine cluster in a mass range m/z 175–871. For all HRMS data, mass error: 0.00–0.50.
Chemistry
General procedure for the preparation of compounds
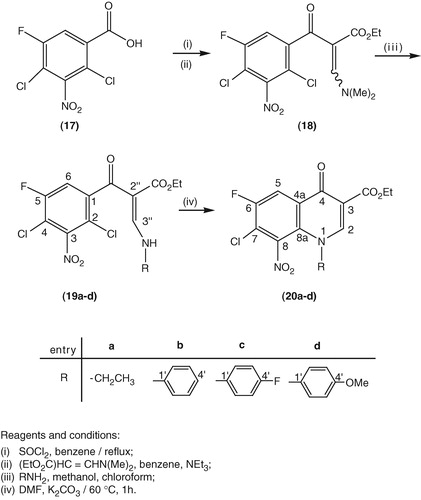
Ethyl 3-(N,N-dimethylamino)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl)acrylate (18). It was prepared using reported methods starting from 2,4-dichloro-5-fluoro-3-nitrobenzoic acid 17 ().Citation19
Preparation of ethyl 3-N-(aryl/alkyl)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl)acrylates (19a–d). A stirred solution of (18) (∼38 mmol) in chloroform (50 mL) and methanol (10 mL), cooled to 8–10 °C, was treated dropwise with the appropriate aryl or alkylamine (1.5 eq) dissolved in MeOH (3 mL). The resulting mixture was further stirred for additional 10–15 min at 5–10 °C. Methanol (90 mL) was then added and the reaction mixture was stirred at rt for 1–2 h. The precipitated white solid product was filtered, washed with cold ethanol (10–20 mL) and air dried. The resultant product was used for the next step without further purification. For identification purpose, a small amount was purified on TLC plates eluting with 2% MeOH/CHCl3 (50:1, v/v).
Ethyl 3-(N-ethylamino)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl)acrylate (19a). Yield 95%; m.p. 154 °C; 1H NMR δ ppm (CDCl3): 1.01 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.35 (t, J = 7.1 Hz, 3H, NCH2CH3), 3.52 (q, J = 7.1 Hz, 2H, NCH2CH3), 3.98 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.11 (d, 3JH–F = 8.0 Hz, 1H, H-6), 8.19 (d, J = 14.4 Hz, 1H, N–C(3″)-H), 10.99 (br d, J = 14.4 Hz, 1H, exchangeable N–H); 13C NMR δ ppm (CDCl3): 13.9 (OCH2CH3), 15.6 (NCH2CH3), 40.5 (NCH2CH3), 60.1 (OCH2CH3), 99.8 (C-2″), 114.1 (d, 2JC–F = 23.3 Hz, C-4), 115.8 (d, 2JC–F = 23.3 Hz, C-6), 117.7 (d, 3JC–F = 4.4 Hz, C-1), 144.0 (d, 3JC–F = 6.2 Hz, C-3), 148.6 (C-2), 156.7 (d, 1JC–F = 253.4 Hz, C-5), 161.2 (N–C-3″), 165.9 (CO2Et), 188.3 (C=O ketone); HRMS (ESI): calcd. for C14H13Cl2FN2O5Na [M + Na]+: 401.00832, found: 401.00778.
Ethyl 3-(N-phenylamino)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl)acrylate (19b). Yield 95%; m.p. 150 °C; 1H NMR δ ppm (CDCl3): 1.07 (t, J = 7.1 Hz, 3H, OCH2CH3), 4.07 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.19 (d, 3JH–F = 8.0 Hz, 1H, H-6), 7.24 (d, J = 7.6 Hz, 1H, H-4′), 7.28 (dd, J = 7.8, 7.6, Hz, 2H, H-3′/H-5′), 7.44 (d, J = 7.8 Hz, 2H, H-2′/H-6′), 8.67 (d, J = 13.8 Hz, 1H, N–C(3″)-H), 12.65 (br d, J = 13.8 Hz, 1H, exchangeable N–H); 13C NMR δ ppm (CDCl3): 13.9 (OCH2CH3), 60.7 (OCH2CH3), 102.2 (C-2″), 114.3 (d, 2JC–F = 23.3 Hz, C-4), 115.9 (d, 2JC–F = 22.7 Hz, C-6), 118.0 (C-1), 118.5 (C-3′/C-5′), 127.0 (C-4′), 130.1 (C-2′/C-6′), 138.1 (C-1′), 143.5 (C-3), 148.6 (C-2), 154.2 (N–C-3″), 156.7 (d, 1JC–F = 254 Hz, C-5), 165.6 (CO2Et), 189.3 (C=O ketone); HRMS (ESI): Calcd for C18H13Cl2FN2O5Na [M + Na]+: 449.00832, found: 449.00778.
Ethyl 3-(N-4-fluorophenylamino)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl)acrylate (19c). Yield 91%; m.p. 130 °C; 1H NMR δ ppm (CDCl3): 1.05 (t, J = 7.1 Hz, 3H, OCH2CH3), 4.05 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.09 (d, 3JH–F = 8.0 Hz, 1H, H-6), 7.15 (d, J = 8 Hz, 2H, H-2′/C-6′), 7.25 (dd, J H–H = 8 Hz, 3J H–F = 8 Hz, 2H, H-3′/H-5′), 8.57 (d, J = 11.9 Hz, 1H, N–C(3″)-H), 12.66 (br d, J = 11.9 Hz, 1H, exchangeable N–H); 13C NMR δ ppm (CDCl3): 13.8 (OCH2CH3), 60.6 (OCH2CH3), 102.2 (C-2″), 114.3 (d, 2JC–F = 23.3 Hz, C-4), 115.9 (d, 2JC–F = 23.6 Hz, C-6), 117.0 (d, 2JC–F = 23.3 Hz, C-3′/C-5′), 117.8 (C-1), 120.3 (d, 3JC–F = 8.3 Hz, C-2′/C-6′), 134.5 (C-1′), 143.4 (C-3), 148.6 (C-2), 154.6 (N–C-3″), 156.7 (d, 1JC–F = 254.1 Hz, C-5), 161.2 (d, 1JC–F = 245.8 Hz, C-4′), 165.5 (CO2Et), 189.3 (C=O ketone); HRMS (ESI): Calcd for C18H12Cl2F2N2O5Na [M + Na]+: 466.99890, found: 466.99835.
Ethyl 3-(N-4-methoxyphenylamino)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl)acrylate (19d). Yield 97%; m.p. 162 °C; 1H NMR δ ppm (CDCl3): 1.05 (t, J = 7.1 Hz, 3H, OCH2CH3), 3.81 (s, 3H, OCH3), 4.04 (q, J = 7.1 Hz, 2H, OCH2CH3), 6.93 (d, J = 8.9 Hz, 2H, H-3′/H-5′), 7.15 (d, 3JH–F = 8.0 Hz, 1H, H-6), 7.21 (d, J = 8.9 Hz, 2H, H-2′/H-6′), 8.56 (d, J = 13.9 Hz, 1H, N–C(3″)-H), 12.7 (br d, J = 13.9 Hz, 1H, exchangeable N–H); 13C NMR δ ppm (CDCl3): 13.9 (OCH2CH3), 55.7 (OCH3), 60.5 (OCH2CH3), 101.5 (C-2″), 114.3 (d, 2JC–F = 23.3 Hz, C-4), 115.2 (C-3′/C-5′), 115.9 (d, 2JC–F = 23.4 Hz, C-6), 117.8 (C-1), 120.0 (C-2′/C-6′), 131.4 (C-1′), 143.6 (d, 3JC–F = 6.3 Hz, C-3), 148.6 (C-2), 154.3 (C-3″), 156.7 (d, 1JC–F = 253.7 Hz, C-5), 158.6 (C-4′), 165.6 (CO2Et), 188.8 (C=O Ketone); HRMS (ESI): Calcd for [M + H]+: 457.03694, found: 457.03694.
Preparation of ethyl 7-chloro-1-(aryl/alkyl)-6 fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylates (20a–d). A solution of the particular precursor 19a–d (30 mmol) in DMF (50 mL) and potassium carbonate (90 mmol) was heated at 60 °C. The progress of the cyclization reaction was monitored by TLC (eluent: EtOAc: N–Hexane, 1:1/v:v). The reaction was complete within 80 to 90 min. The reaction mixture was then poured slowly onto crushed ice (100 g) under vigorous stirring, the precipitated pale yellow solid product was collected, washed with water, triturated with cold ethanol, and air dried to produce the pure product.
Ethyl 7-chloro-1-ethyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate (20a).Yield 97%; m.p. 150 °C; 1H NMR δ ppm (CDCl3): 1.39 (t, J = 7.2 Hz, 3H, OCH2CH3), 1.43 (t, J = 7.2 Hz, 3H, NCH2CH3), 4.04 (q, J = 7.2 Hz, 2H, NCH2CH3), 4.40 (q, J = 7.2 Hz, 2H, OCH2CH3), 8.43 (s, 1H, H-2), 8.47 (d, 3JH–F = 8.34 Hz, 1H, H-5); 13C NMR δ ppm (CDCl3): 14.7 (OCH2CH3), 16.1 (NCH2CH3), 50.3 (NCH2Me), 60.9 (OCH2Me), 112.1 (C-3), 115.8 (d, 2JC–F = 22.8 Hz, C-5), 120.7 (d, 2JC–F = 23.9 Hz, C-7), 128.8 (C-4a), 130.9 (d, 4JC–F = 5.7 Hz, C-8a), 140.5 (C-8), 152.6 (C-2), 154.0 (d, 1JC–F = 249 Hz, C-6), 164.0 (CO2Et), 170.4 (C-4) (C=O ketone); HRMS (ESI): calcd. for C14H12ClFN2O5 [M + H]+: 343.04970, found: 343.04915.
Ethyl 7-chloro-6-fluoro-8-nitro-4-oxo-1-phenyl-1,4-dihydroquinoline-3-carboxylate (20b). Yield 97%; m.p. 194 °C; 1H NMR δ ppm (CDCl3): 1.36 (t, J = 7.1 Hz, 3H, OCH2CH3), 4.36 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.33 (d, J = 7.7 Hz, 2H, H-2′/H-6′), 7.48 (dd, J = 7.7, 7.3 Hz, 2H, H-3′/H-5′), 7.56 (d, J = 7.3 Hz, 1H, H-4′), 8.37 (s, 1H, H-2), 8.44 (d, 3JH–F = 8.3 Hz, 1H, H-5); 13C NMR δ ppm (CDCl3): δ 14.4 (OCH2CH3), 61.6 (OCH2Me), 112.0 (C-3), 115.4 (d, 2JC–F = 22.9 Hz, C-5), 121.8 (d, 2JC–F = 23.9 Hz, C-7), 127.6 (C-3′/C-5′), 128.8 (d, 3JC–F = 3 Hz, C-4a), 129.8 (C-8a), 129.9 (C-2′/C-6′), 131.0 (C-4′), 139.5 (C-1′), 141.5 (C-8), 152.0 (C-2), 154.8 (d, 1JC–F = 253 Hz, C-6), 164.1 (CO2Et), 171.0 (C-4) (C=O ketone); HRMS (ESI): Calcd for C18H12ClFN2O5Na [M + Na]+: 413.03165, found: 413.03110.
Ethyl 7-chloro-6-fluoro-1-(4-fluorophenyl)-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate (20c). Yield 97%; m.p. 226 °C; 1H NMR δ ppm (CDCl3): 1.36 (t, J = 7.1 Hz, 3H, OCH2CH3), 4.36 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.18 (dd, J = 8.9 Hz, Citation2JH–F = 7.9 Hz, 2H, H-3′/H-5′), 7.35(dd, JH–H = 8.9 Hz, Citation3JH–F = 4.5 Hz, 2H, H-2′/H-6′), 8.31 (s, 1H, H-2), 8.43 (d, Citation3JH–F = 8.2 Hz, 1H, H-5); Citation13C NMR δ ppm (CDCl3): 14.4 (OCH2CH3), 61.7 (OCH2Me), 112.2 (C-3), 115.4 (d, Citation2JC–F = 22.8 Hz, C-5), 117.1 = (d, Citation2JC–F = 23.2 Hz, C-3′/C-5′), 122.0 (d, Citation2JC–F = 23.7 Hz, C-7), 128.7 (C-4a), 129.7 (C-8a), 129.8 (d, Citation3JC–F = 9.2 Hz, C-2′/C-6′), 135.2 (d, Citation3JC–F = 3.4 Hz, C-8), 135.5 (C-1′), 151.9 (C-2), 154.8 (d, Citation1JC–F = 253 Hz, C-6), 163.5 (d, Citation1JC–F = 253 Hz, C-4′), 164 (CO2Et), 170.9 (C-4)(C=O); HRMS (ESI): Calcd for C18H11Cl2F2N2O5Na [M + Na]+: 431.02223, found: 431.02168.
Ethyl 7-chloro-6-fluoro-1-(4-methoxyphenyl)-8-nitro-4-oxo-1,4-dihydroquinoline-3- carboxylate (20d). Yield 96%; m.p. 176 °C; 1H NMR δ ppm (CDCl3): 1.33 (t, J = 7.1 Hz, 3H, OCH2CH3), 3.86 (s, 3H, OCH3), 4.32 (q, J = 7.1 Hz, 2H, OCH2CH3), 6.91 (d, J = 8.8 Hz, 2H, H-2′/H-6′), 7.24 (d, J = 8.8 Hz, 2H, H-3′/H-5′), 8.31 (s, 1H, H-2), 8.38 (d, Citation3JH–F = 8.1 Hz, 1H, H-5).Citation13C NMR δ ppm (CDCl3): 14.4 (OCH2CH3), 55.8 (OCH3), 61.5 (OCH2CH3), 111.7 (C-3), 114.7 (C-3′/C-5′), 115.2 (d, Citation2JC–F = 23 Hz, C-5), 121.7 (d, Citation2JC–F = 23.5 Hz, C-7),128.6 (C-4a), 129.1 (C-2′/C-6′), 129.7 (C-8a), 132.0 (d, Citation3JC–F = 3.4 Hz, C-8), 141.2 (C-1′), 152.4 (C-2), 154.6 (d, Citation1JC–F = 252.8 Hz, C-6), 161.1 (C-4′), 164.1 (CO2Et), 171.0 (C-4)(C=O). HRMS (ESI): Calcd for C19H14ClFN2O6Na [M + Na]+: 421.06027, found: 421.05972.
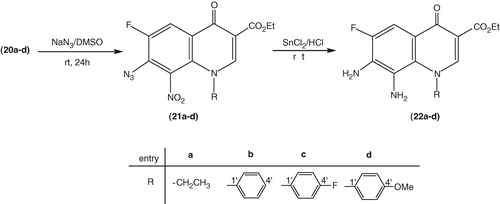
Preparation of ethyl 7-azido-1-(aryl/alkyl)-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylates (21a–d). A warm (∼35 to 40 °C) solution of soduim azide (7.8 g, 120 mmol) in dimethylsulfoxide (DMSO, 50 mL) was added to a solution of the appropriate ethyl 7-chloro-1-aryl(or alkyl)-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoxaline-3-carboxylate (20 mmol) (20a–d) in DMSO (50 mL). The resulting mixture acquired yellow turbidity within few minutes, and was further stirred at (∼35 to 40 °C) for 16–20 h. Thereafter, the reaction mixture was diluted with cold water (250 mL), and the precipitated solid product was collected under suction, washed with cold water and air dried.
Ethyl 7-azido-1-ethyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate (21a). Yield 90%; m.p. 150 °C; 1H NMR δ ppm (CDCl3): 1.39 (m, 6H, NCH2CH3, OCH2CH3), 3.98 (q, J = 7.2 Hz, 2H, NCH2CH3), 4.38 (q, J = 7.2 Hz, 2H, OCH2CH3), 8.37 (s, 1H, H-2), 8.39 (d, Citation3JH–F = 11.2 Hz, 1H, H-5);Citation13C NMR δ ppm (CDCl3): 14.4 (OCH2CH3), 16.2 (NCH2CH3), 50.5 (NCH2CH3), 61.5 (OCH2CH3), 112.3 (C-3), 115.9 (d, Citation2JC–F = 20.9 Hz, C-5), 128.2 (C-4a), 128.8 (d, 2JC–F = 11.1 Hz, C-7), 127.7 (d, 4JC–F = 5.7 Hz, C-8a), 135.2 (C-8), 151.3 (C-2), 154.9 (d, Citation1JC–F = 250.2 Hz, C-6), 164.5 (CO2Et), 171.0 (C-4)(C=O); HRMS (ESI): Calcd for C14H12FN5O5Na [M + Na]+: 372.07202, found: 372.07147.
Ethyl 7-azido-6-fluoro-8-nitro-4-oxo-1-phenyl-1,4-dihydroquinoline-3-carboxylate (21b). Yield 92%; m.p. 160 °C; 1H NMR δ ppm (CDCl3): 1.35 (t, J = 7.1 Hz, 3H, OCH2CH3), 4.35 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.30 (d, J = 7.6 Hz, 2H, H-2′/H-6′), 7.45 (dd, J = 7.6, 7.3 Hz, 2H, H-3′/H-5′),7.55 (d, J = 7.3 Hz, 1H, H-4′), 8.31 (d, Citation3JH–F = 9.4 Hz, 1H, H-5), 8.37 (s, 1H, H-2). Citation13C NMR δ ppm (CDCl3): 14.4 (OCH2CH3), 61.5 (OCH2CH3), 112.2 (C-3), 115.4 (d, Citation2JC–F = 21.2 Hz, C-5), 118.1 (C-4a), 127.5 = (C-3′/C-5′), 128.9 (C-8a), 129.8 (C-2′/C-6′), 130.5 (C-4′), 132.4 (d, Citation2JC–F = 23.9 Hz, C-7), 141.5 (C-8), 139.6 (C-1′), 151.7 (C-2), 153.1 (d, Citation1JC–F = 251 Hz, C-6), 164.2 (CO2Et), 171.0 (C-4)(C=O). HRMS (ESI): calcd. for C18H12FN5O5Na [M + Na]+: 420.07202, found: 420.07147.
Ethyl 7-azido-6-fluoro-1-(4-fluorophenyl)-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate (21c). Yield 91%; m.p. 172 °C; 1H NMR δ ppm (CDCl3): 1.36(t, J = 7.1 Hz, 3H, OCH2CH3), 4.36 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.14 (dd, J = 8.9 Hz, 4JH–F = 2.2 Hz, 2H, H-2′/H-6′), 7.31 (dd, J = 8.9 Hz, Citation3JH–F = 4.5 Hz, 2H, H-3′/H-5′), 8.34 (d, Citation3JH–F = 11.2 Hz, 1H, H-5), 8.38 (s, 1H, H-2); Citation13C NMR δ ppm (CDCl3): 14.3 (OCH2CH3), 61.6 (OCH2CH3), 112.2 (C-3), 116.8 (d, Citation2JC–F = 22.8 Hz, C-5), 117.1 = (d, Citation2JC–F = 22.5 Hz, C-3′/C-5′), 126.5 (C-4a), 129.7 (d, Citation3JC–F = 7.2 Hz, C-2′/C-6′), 129.9 (d, Citation2JC–F = 23.7 Hz, C-7), 132.6 (C-8a), 135.2 (d, 3JC–F = 3.2 Hz, C-8), 135.5 (C-1′), 151.7 (C-2), 154.7 (d, Citation1JC–F = 247 Hz, C-6), 163.5 (d, Citation1JC–F = 252.2 Hz, C-4′), 164 (CO2Et), 171.0 (C-4)(C=O). HRMS (ESI): Calcd for C18H11F2N5O5Na [M + Na]+: 438.06259, found: 438.06205.
Ethyl 7-azido-6-fluoro-1-(4-methoxyphenyl)-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate (21d). Yield 94 %; m.p. 170 °C; 1H NMR δ ppm (CDCl3): 1.35 (t, J = 7.1 Hz, 3H, OCH2CH3), 3.86 (s, 3H, OCH3), 4.34 (q, J = 7.1 Hz, 2H, OCH2CH3), 6.90 (d, J = 8.9 Hz, 2H, H-2′/H-6′), 7.21 (d, J = 8.9 Hz, 2H, H-3′/H-5′), 8.28 (d, Citation3JH–F = 6.5 Hz, 1H, H-5) 8.38 (s, 1H, H-2); Citation13C NMR δ ppm (CDCl3): 14.3 (OCH2CH3), 55.7 (OCH3), 61.4 (OCH2CH3), 112.0 (C-3), 114.6 (d, Citation2JC–F = 21.5 Hz, C-5), 117.9 (C-3′/C-5′), 126.6 (d, Citation2JC–F = 23.5 Hz, C-7),128.6 (C-4a), 129.0 (C-2′/C-6′), 129.3 (C-8a), 132.1 (d, Citation3JC–F = 3.4 Hz, C-8), 132.3 (C-1′), 152.2 (C-2), 154.6 (d, Citation1JC–F = 252.8 Hz, C-6), 161.0 (C-4′), 164.4 (CO2Et), 171.1 (C-4)(C=O). HRMS (ESI): Calcd for C19H14FN5O6 [M + H]+: 428.10064, found: 428.10009.
Preparation of ethyl 7,8-diamino-1-(aryl/alkyl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylates (22a–d). Anhydrous stannous chloride (5 g, 26 mmol) was added portionwise to a stirred ice-cooled (4–8 °C) solution of the appropriate ethyl 7-azido-1-alkyl or 1-aryl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate (21a--d) () (6.3 mmol) in concentrated hydrochloric acid (36%, 50 mL). The reaction mixture was further stirred at room temperature (24 h), and the reaction progress was monitored by TLC. Upon completion of the reaction, the reaction mixture was diluted with ice-cold water (50 mL), basified with 40% cold aqueous sodium hydroxide solution to pH ∼ 13 and set aside for a few minutes. The precipitated solid product was collected by suction filtration and recrystallized from 90% ethanol.
Ethyl 7,8-diamino-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (22a). Yield 85%; m.p. (dec) 347 °C; 1H NMR δ ppm (CDCl3): 1.08 (t, J = 7.0 Hz, 3H, OCH2CH3), 1.23 (t, J = 7.0 Hz, 3H, NCH2CH3), 4.16 (q, J = 7.0 Hz, 2H, NCH2CH3), 4.63 (q, J = 7.0 Hz, 2H, OCH2CH3), 4.73 (br s, 2H, C(8)–NH2), 5.53 (br s, 2H, C(7)–NH2),7.32 (d, Citation3JH–F = 11.3 Hz, 1H, H-5), 8.38 (s, 1H, H-2).Citation13C NMR δ ppm (CDCl3):14.8 (OCH2CH3), 16.0 (NCH2CH3), 50.8 (NCH2CH3), 60.0 (OCH2CH3), 101.9 (d, Citation2JC–F = 20.6 Hz, C-5), 108.7 (C-3), 120.7 (C-4a), 124.7 (d, Citation3JC–F = 5.1 Hz, C-8), 127.7 (C-8a), 131.2 (d, Citation3JC–F = 15.1 Hz, C-7), 150.0 (d, Citation1JC–F = 236.6 Hz, C-6), 151.8 (C-2), 165.4 (CO2Et), 173.1 (C-4)(C=O); IR (KBr) v: br 3472.33, 3387.38, 1710.58, 1682.94, 1611.55, 1555.40, 1460.96, 1202.94 cm−1; HRMS (ESI): calcd. for C14H16FN3O3 [M + H]+: 294.12539, found: 294.12485.
Ethyl 7,8-diamino-6-fluoro-1-phenyl-4-oxo-1,4-dihydroquinoline-3-carboxylate (22b). Yield 80%; m.p. 240 °C; 1H NMR, δ ppm (DMSO-d6): 1.19 (t, J = 7.0 Hz, 3H, OCH2CH3), 4.14 (q, J = 7.0 Hz, 2H, OCH2CH3), 3.87 (br s, 2H, C(8)-NH2), 5.39 (br s, 2H, C(7)-NH2),7.32 (dd, JH–H = 8.6 Hz, JH–F = 2.3 Hz, 2H, H-2′/H-6′), 7.38 (dd, Citation3JH–H = 8.6 Hz, JH–F = 4.3 Hz, 2H, H-3′/H-5′), 7.56 (d, Citation3JH–F = 8.3 Hz, 1H, H-5), 8.15 (s, 1H, H-2); Citation13C NMR, δ ppm (DMSO-d6): 14.7 (OCH2CH3), 60.3 (OCH2Me), 112.2 (C-3), 115.4 (d, Citation3JC–F = 22.8 Hz, C-5), 117.1 = (d, Citation2JC–F = 23.2 Hz, C-3′/C-5′), 122.0 (d, Citation2JC–F = 23.7 Hz, C-7),128.6 (C-4a), 129.8 (d, Citation3JC–F = 7.2 Hz, C-2′/C-6′), 129.9 (C-8a), 135.2 (d, Citation3JC–F = 3.4 Hz, C-8), 135.5 (C-1′), 151.9 (C-2), 154.8 (d, 1JC–F = 253 Hz, C-6), 163.5 (d, Citation1JC–F = 253 Hz, C-4′), 164 (CO2Et), 170.9 (C-4); HRMS (ESI): Calcd for [M + H]+: 306.12540, found: 306.12475.
Ethyl 7,8-diamino-6-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (22c). Yield 83%; m.p. 274 °C; 1H NMR, δ ppm (DMSO-d6): 1.19 (t, J = 7.0 Hz, 3H, OCH2CH3), 4.14 (q, J = 7.0 Hz, 2H, OCH2CH3), 3.87 (br s, 2H, C(8)-NH2), 5.39 (br s, 2H, C(7)-NH2),7.32 (dd, J = 8.6 Hz, Citation4JH–F = 2.3 Hz, 2H, H-2′/H-6′), 7.38 (dd, J = 8.6 Hz, Citation3JH–F = 4.3 Hz, 2H, H-3′/H-5′), 7.56 (d, Citation3JH–F = 8.3 Hz, 1H, H-5), 8.15 (s, 1H, H-2); Citation13C NMR, δ ppm (DMSO-d6): 14.7 (OCH2CH3), 60.3 (OCH2CH3), 101.1 (d, Citation2JC–F = 21 Hz, C-5), 109.0 (C-3), 116.8 (d, Citation2JC–F = 23.0 Hz, C-3′/C-5′), 120.9 (d, Citation3JC–F = 8.3 Hz, C-4a), 124.4 (d, Citation3JC–F = 6.1 Hz, C-8), 125.8 (C-8a), 128.6 (d, Citation3JC–F = 8.9 Hz, C-2′/C-6′), 129.7 (d, Citation2JC–F = 16.1 Hz, C-7), 140.2 (d, Citation4JC–F = 2.9 Hz, C-1′), 150.0 (d, Citation1JC–F = 236 Hz, C-6), 151.2 (C-2), 162 (d, Citation1JC–F = 244 Hz, C-4′), 164.9 (CO2Et), 172.7 (C-4)(C=O); HRMS (ESI): calcd. for C18H15F2N3O3Na [M + Na]+: 382.09792, found: 382.09737.
Ethyl 7,8-diamino-6-fluoro-1-(4-methoxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (22d). Yield 89%; m.p. 274 °C; 1H NMR, δ ppm (DMSO-d6): 1.20 (t, J = 7.1 Hz, 3H, OCH2CH3), 3.75 (br s, 2H, C(8)-NH2), 3.81 (s, 3H, OCH3), 4.14 (q, J = 7.1 Hz, 2H, OCH2CH3), 5.29 (br s, 2H, C(7)-NH2), 7.09 (d, J = 8.9 Hz, 2H, H-2′/H-6′), 7.32 (d, Citation3JH–F = 11.3 Hz, 1H, H-5), 7.48 (d, J = 8.9 Hz, 2H, H-3′/H-5′), 8.11 (s, 1H, H-2). Citation13C NMR, δ ppm (DMSO-d6): 14.8 (OCH2CH3), 56.1 (OCH3), 60.1 (OCH2CH3), 100.9 (d, Citation2JC–F = 20.9 Hz, C-5), 108.5 (C-3), 115.4 (C-3′/C-5′), 120.1 (d, Citation3JC–F = 7.2 Hz, C-4a), 124.5 (d, Citation3JC–F = 6.1 Hz, C-8), 125.7 (C-8a), 127.8 (C-2′/C-6′), 129.3 (d, Citation2JC–F = 16.2 Hz, C-7), 136.6 (C-1′), 150.0 (d, Citation1JC–F = 235.5 Hz, C-6), 151.2 (C-2), 159.8 (C-4′), 164.9 (CO2Et), 172.7 (C-4). IR (cm−1, KBr): br 3294.77, 2980.30, 1716.58, 1674.64, 1616.90, 1508.57, 1461.32 cm−1; HRMS (ESI): Calcd for C19H18FN3O4 [M + H]+: 372.13596, found: 372.13541.
Preparation of ethyl(methyl) 9-(aryl/alkyl)-4 fluoro-6-oxo-6,9-dihydro-[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylates (23a,b,d/24b–d). To a stirred suspension of the particular diamine compound (22a–d) () (3 mmol) in dry benzene (25 mL), was added purified thionylchloride (6 mL) and the resulting mixture was heated at 80–85 °C for 8 h. Benzene and excess thionyl chloride were distilled off in vacuum and the residue was cooled, dissolved in CHCl3 and washed with water (2 × 30 mL). The organic layer was separated, dried (anhydrous MgSO4) and the solvent CHCl3 was then evaporated to dryness under reduced pressure. The residual product was recrystallized from dichloromethane/methanol. In some cases, methanol was used to destroy the excess thionyl chloride within the reaction. Upon using this workup process transesterfication occurs and the resultant product was either methyl ester instead of ethyl ester or a mixture of both esters.
Methyl 9-ethyl-4-fluoro-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylate (23a). Yield 80%, m.p. 325 °C. 1H NMR, δ ppm (D2O): 1.42 (t, J = 6.9 Hz, 3H, NCH2CH3), 3.76 (s, 3H, CO2CH3), 5.05 (q, J = 6.9 Hz, 2H, NCH2CH3), 8.49 (d, Citation3JH–F = 10.2 Hz, 1H, H-5), 8.86 (s, 1H, H-2); Citation13C NMR, δ ppm (D2O): 16.5 (NCH2CH3), 52.1 (OCH3), 52.1 (NCH2CH3), 109.1 (d, Citation2JC–F = 19.7 Hz, C-5), 114.1 (C-7), 127.5 (d, Citation3JC–F = 5.8 Hz, C-5a), 130.4 (Hz, C-9a), 148.4 (d, Citation2JC–F = 10.5 Hz, C-3a), 148.8 (d, Citation3JC–F = 1.6 Hz, C-9b), 149.5 (C-8), 150.0 (d, Citation1JC–F = 256 Hz, C-4), 165.1 (CO2Me), 171.3 (C-6)(C=O); HRMS (ESI): Calcd for C14H12FN3O3S [M + H]+: 308.05052, found: 308.04982.
Methyl 4-fluoro-6-oxo-9-phenyl-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinol-ine-7-carboxylate (23b). Yield 40% m.p. 300 °C; 1H NMR, δ ppm (DMSO-d6): 3.74 (s, 3H, CO2CH3), 7.61 (m, 5H, H-2′/H-3′/H-4′/H-5′/H-6′), 8.09 (d, Citation3JH–F = 10.8 Hz, H-5), 8.43 (s, 1H, H-8); Citation13C NMR, δ ppm (DMSO-d6): 52.2 (CO2CH3), 108.9 (d, Citation2JC–F = 18.7 Hz, C-5), 113.9 (C-7), 127.6 (C-5a), 127.7 (C-3′/C-5′), 130.1 (C-2′/C-6′), 130.2 (C-4′), 130.9 (C-9a), 143.6 (C-1′), 148.5 (C-3a), 148.9 (C-9b), 149.3 (C-8), 150.0 (d, Citation1JC–F = 214 Hz, C-4), 164.7 (CO2Et), 171.7 (C-6); HRMS (ESI): Calcd for C17H10FN3O3SNa [M + Na]+: 378.03246, found: 378.03209.
Methyl 4-fluoro-9-(4-methoxyphenyl)-6-oxo-6,9-dihydro[1,2,5]thiadiazolo [3, 4-h]quinoline-7-carboxylate (23d). Yield 40%; m.p. 281 °C; 1H NMR, δ ppm (CDCl3):δ 3.93 (s, 6H, OCH3, CO2CH3), 7.04 (d, J = 8.9 Hz, 2H, H-2′/H-6′), 7.29 (d, J = 8.9 Hz, 2H, H-3′/H-5′), 8.32 (d, Citation3JH–F = 10.3 Hz, 1H, H-5), 8.52 (s, 1H, H-8); Citation13C NMR, δ ppm (CDCl3): 52.5 (CO2CH3), 55.8 (OCH3), 109.7 (d, Citation2JC–F = 19.6 Hz, C-5), 113.8 (C-7), 114.9 (C-3′/C-5′), 128.2 (C-2′/C-6′), 128.7 (d, Citation3JC–F = 5.7 Hz, C-5a), 130.5 (C-9a), 135.9 (C-1′), 148.5 (C-3a), 148.8 (C-9b), 149.6 (C-8), 150.5 (d, Citation1JC–F = 261 Hz, C-4), 160.5 (C-4′), 165.5 (CO2Et), 172.3 (C-6); HRMS (ESI): Calcd for C18H12FN3O4SNa [M + Na]+: 408.04302, found: 408.04225.
Ethyl 4-fluoro-6-oxo-9-phenyl-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylate (24b). Yield 40%, m.p. 285 °C; 1H NMR, δ ppm (DMSO-d6): 1.25 (t, J = 7.1 Hz, 3H, OCH2CH3), 4.22 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.60 (m, 5H, H-2′/H-3′/H-4′/H-5′/H-6′), 8.11 (d, Citation3JH–F = 10.8 Hz, H-5), 8.41 (s, 1H, H-2); Citation13C NMR, δ ppm (DMSO-d6): 14.7 (OCH2CH3), 60.9 (OCH2CH3), 108.9 (d, Citation2JC–F = 19.2 Hz, C-5), 114.4 (C-7), 127.8 (C-3′/C-5′),128.7 (d, Citation3JC–F = 5.8 Hz, C-5a), 130.1 (C-2′/C-6′), 130.2 (C-4′), 130.9 (d, Citation4JC–F = 3 Hz, C-9a), 139.0 (C-1′), 148.4 (d, Citation2JC–F = 10.5 Hz, C-3a), 148.7 (d, Citation3JC–F = 1.6 Hz, C-9b), 148.9 (C-8), 150.5 (d, Citation1JC–F = 214 Hz, C-4), 164.2 (CO2Et), 171.7 (C-6)(C=O); HRMS (ESI): Calcd for C18H12FN3O3SNa [M + Na]+: 392.04811, found: 392.04934.
Ethyl 4-fluoro-9-(4-fluorophenyl)-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylate (24c). Yield 79%, m.p. 274 °C; 1H NMR, δ ppm (DMSO-d6): 1.38 (t, J = 7.1 Hz, 3H, OCH2CH3), 4.38 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.26 (d, J = 8.3 Hz, 2H, H-2′/H-6′), 7.41 (dd, J = 8.3 Hz, 4JH–F = 4.6 Hz, 2H, H-3′/H-5′), 8.28 (d, Citation3JH–F = 10.2 Hz, 1H, H-5), 8.46 (s, 1H, H-2); Citation13C NMR, δ ppm (DMSO-d6): 14.4 (OCH2CH3), 61.5 (OCH2Me), 109.6 (d, Citation2JC–F = 19.6 Hz, C-5), 114.3 (C-7), 117.0 (d, Citation2JC–F = 23.0 Hz, C-3′/C-5′),128.7 (d, Citation3JC–F = 5.8; Hz, C-5a), 129.2 (d, Citation3JC–F = 9.0 Hz, C-2′/C-6′), 130.1 (d, Citation4JC–F = 3 Hz, C-9a), 139.0 (C-1′), 148.4 (d, Citation2JC–F = 10.5 Hz, C-3a), 148.7 (d, Citation3JC–F = 1.6 Hz, C-9b), 148.9 (C-8), 150.5 (d, Citation1JC–F = 261 Hz, C-4), 163.3 (d, Citation1JC–F = 249 Hz, C-4′), 164.7 (CO2Et), 172.3 (C-6); HRMS (ESI): Calcd for C18H11F2N3O3SNa [M + Na]+: 410.03869, found: 410.04116.
Ethyl 4-fluoro-9-(4-methoxyphenyl)-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylate (24d). Yield 40%; m.p. 237 °C; 1H NMR, δ ppm (DMSO-d6): 1.24 (t, J = 7.1 Hz, 3H, OCH2CH3), 3.85 (s, 3H, OCH3), 4.20 (q, J = 7.1 Hz, 2H, OCH2CH3), 7.09 (d, J = 8.8 Hz, 2H, H-2′/H-6′), 7.54 (d, J = 8.8 Hz, 2H, H-3′/H-5′), 8.04 (d, Citation3JH–F = 10.8 Hz, 1H, H-5), 8.37 (s, 1H, H-2); Citation13C NMR, δ ppm (DMSO-d6): 14.7 (OCH2CH3), 56.1 (OCH3), 61.5 (OCH2CH3), 108.9 (d, Citation2JC–F = 19.1 Hz, C-5), 114.1 (C-7), 115.1 (C-3′/C-5′),127.5 (d, Citation3JC–F = 5.4 Hz, C-5a), 129.0 (C-2′/C-6′), 131.3 (C-9a), 136.6 (C-1′), 148.3 (C-3a), 148.9 (C-9b), 149.4 (C-8), 150.1 (d, Citation1JC–F = 249 Hz, C-4), 160.4 (C-4′), 164.2 (CO2Et), 171.7 (C-6); HRMS (ESI): Calcd for C19H14FN3O4SNa [M + Na]+: 422.05867, found: 422.05809.
Preparation of 9-(aryl/alkyl)-4-fluoro-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acids (25a–d). A vigorously stirred suspension of the ester (23a,b,d/24b–d). (1.5 mmol) in 6 N HCl (15 mL) and ethanol (6 mL) was heated at 80–85 °C under reflux conditions. Progress of the ester hydrolysis was monitored by TLC and was completed within 20–24 h. Thereafter, the reaction mixture was cooled, poured onto crushed ice (30 g) and the resulting heavy pale yellow precipitate was collected, washed with cold water, dried and recrystallized from N,N-dimethylformamide (DMF) to produce a pure compound.
9-Ethyl-4-fluoro-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acid (25a). Yield 95%; m.p. 315 °C; 1H NMR δ ppm (D2O): 1.48 (t, J = 7.0 Hz, 3H, NCH2CH3), 5.26 (q, J = 7.0 Hz, 2H, NCH2CH3), 8.23 (d, Citation3JH–F = 10.4 Hz, 1H, H-5), 8.68 (s, 1H, H-8), 15.14 (br s, 1H, CO2H); Citation13C NMR δ ppm (D2O): 16.4 (NCH2CH3), 53.5 (NCH2CH3), 108.1 (d, Citation2JC–F = 19.6 Hz, C-5), 111.9 (C-7), 125.7 (d, Citation3JC–F = 6.5 Hz, C-5a), 132.2 (C-9a), 148.7 (d, Citation2JC–F = 10.5 Hz, C-3a), 149.1 (d, Citation3JC–F = 3 Hz, C-9b), 149.7 (C-8), 154.4 (d, Citation1JC–F = 259 Hz, C-4), 165.7 (CO2H), 176.3 (C-6); HRMS (ESI): Calcd for C12H8FN3O3S [M − H]−: 292.01921, found: 292.01976.
4-Fluoro-6-oxo-9-phenyl-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acid (25b). Yield 90%; m.p. 301 °C; 1H NMR δ ppm (D2O): 7.64 (m, 5H, H-1′/H-2′/H-3′/H-4′/H-5′), 8.23 (d, Citation3JH–F = 10.7 Hz, 1H, H-5), 8.68 (s, 1H, H-8), 14.91 (br s,1H, CO2H); Citation13C NMR δ ppm (D2O): 107.9 (d, Citation2JC–F = 19.0 Hz, C-5), 111.4 (C-7), 125.7 (Hz, C-5a), 127.5 (C-3′/C-5′), 130.2 (C-2′/C-6′), 130.7 (C-4′), 131.2 (C-9a), 143.4 (C-1′), 148.6 (d, Citation2JC–F = 10.5 Hz, C-3a), 149.0 (C-9b), 149.7 (C-8), 154.3 (d, Citation1JC–F = 259 Hz, C-4), 165.5 (CO2H), 176.8 (C-6); HRMS (ESI): Calcd for C16H8FN3O3S [M − H]−: 340.01921, found: 340.01976.
4-Fluoro-9-(4-fluorophenyl)-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acid (25c). Yield 93%; m.p. 278 °C; 1H NMR δ ppm (DMSO-d6); 7.44 (d, J = 8.4 Hz, 2H, H-2′/H-6′), 7.72 (dd, J = 8.4 Hz, Citation3JH–F = 4.4 Hz, 2H, H-3′/H-5′), 8.19 (d, Citation3JH–F = 10.4 Hz, 1H, H-5), 8.71 (s, 1H, H-8), 14.9 (br s,1H, CO2H); Citation13C NMR δ ppm (DMSO-d6); 107.9 (d, Citation2JC–F = 19.3 Hz, C-5), 114.3 (C-7), 117.0 (d, Citation2JC–F = 23.0 Hz, C-3′/C-5′),125.7 (d, Citation3JC–F = 6.5 Hz, C-5a), 130.1 (d, Citation3JC–F = 9.0 Hz, C-2′/C-6′), 132.2 (C-9a), 139.6 (C-1′), 148.6 (d, Citation2JC–F = 10.5 Hz, C-3a), 149.0 (d, Citation3JC–F = 3 Hz, C-9b), 149.7 (C-8), 151.3 (d, Citation1JC–F = 259 Hz, C-4), 163.1 (d, Citation1JC–F = 245 Hz, C-4′), 165.6 (CO2H), 176.6 (C-6); HRMS (ESI): Calcd for C16H7F2N3O3SNa [M + Na]+: 382.00739, found: 382.00684.
4-Fluoro-9-(4-methoxyphenyl)-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acid (25d). Yield 92%; m.p. 300 °C; 1H NMR δ ppm (DMSO-d6); 3.86 (OCH3), 7.12 (d, J = 8.9 Hz, 2H, H-2′/H-6′), 7.57 (d, J = 8.9 Hz, H-3′/H-5′), 8.20 (d, Citation3JH–F = 10.4 Hz, 1H, H-5), 8.64 (s, 1H, H-8), 14.86 (br s,1H, CO2H); Citation13C NMR δ ppm (DMSO-d6); 56.1 (OCH3), 107.9 (d, Citation2JC–F = 19.5 Hz, C-5), 111.4 (C-7), 115.2 (C-3′/C-5′), 125.7 (d, Citation3JC–F = 6.5 Hz, C-5a), 128.8 (C-2′/C-6′), 132.2 (C-9a), 136.4 (C-1′), 148.6 (d, Citation2JC–F = 10.5 Hz, C-3a), 149.0 (d, Citation3JC–F = 3 Hz, C-9b), 150.0 (C-8), 150.9 (d, Citation1JC–F = 259 Hz, C-4), 160.7 (C-4′), 165.5 (CO2H), 176.8 (C-6).; IR (KBr) v: 3061.36, 1732.53, 1611.62, 1504.53, 1474.49, 1253.78 cm−1; HRMS (ESI): Calcd for C17H10FN3O4S [M − H]−: 370.02978, found: 370.03033.
Antimicrobial activity
The biological screening of compounds 19–22a–d, 23a, 24b–d and 25a–d was performed in vitro by the two fold broth dilution methodCitation27. The antibacterial activity was tested against Bacillus subtilis ATCC 6633 and Staphylococcus aureus ATCC 6538 Gram positive strains, Escherichia coli ATCC 8739 and Haemophilus influenzae ATCC 19418 Gram negative ones using Mueller Hinton medium. The antifungal properties were evaluated against Candida tropicalis ATCC 1369 and Saccharomyces cerevisiae ATCC 9763 yeasts and Aspergillus niger ATCC 6275 mould in Sabouraud dextrose broth. The compounds at the concentration range of 0.0015–400 μg/mL in 1% DMSO were used in this study, with ciprofloxacin as reference antibacterial substance and miconazole as reference antifungal drug. DMSO was loaded as negative control. Suspensions of the microorganisms were prepared to inoculate 5 × 105 bacteria/mL and 1 × 103 fungi/mL. The minimum inhibitory concentration (MIC, μg/mL) value was taken as the lowest concentration of compound that showed inhibition of microbial growth after 24 h of incubation at 37 °C for bacteria and after 48 h of incubation at 30 °C for fungi. Each experiment has been done in triplicate and repeated at least three times.
Results and discussion
Chemistry
Our synthesis banks on the reported strategy used by us and others in building the quinolone skeleton. shows the synthesis of our N-aryl/alkyl substituted quinolones, in which the benzoic acid derivative 17 was converted to the benzoyl chloride via interaction with thionyl chloride under reflux conditions. The resulting benzoyl chloride was reacted with ethyl 3-(N,N-dimethylamine)acrylate in benzene to produce the intermediate 18 which was reacted with the desired amino compound to get the respective 19a–d as the predominant Z isomers.
Scheme 1. synthesis of ethyl 7-chloro-1-(ethyl/aryl)-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylates (20a–d).
The structures of compounds 19a–d are supported by spectral (NMR and MS) data. The various 1H and Citation13C NMR signals were assigned based on DEPT and 2D NMR (HMQC) experiments. The 1H NMR spectra show the methylene protons (O–CH2CH3) as quartet in the range δ 3.98–4.07 ppm, while the methyl protons (O–CH2CH3) resonate as triplet in the range δ 1.01–1.07 ppm. The H-3″ proton resonates as a doublet signal in the range δ 8.19–8.67 ppm (with J = 11–14 Hz) indicating that the products are the Z-isomers. The H-6 proton of this series is well characterized as a sharp doublet in the range δ 7.09–7.19 ppm, (with 3JH–F = 8.0–8.2 Hz) due to spin–spin coupling interaction with the vicinal fluorine atom.
The Citation13C NMR spectra of compounds 19a–d reveal clearly the presence of the fluorine atom and its effect on the adjacent carbons; thus the following carbons showed through-bonds coupling with the fluorine atom and appear as doublets (C-5, 1J = 253–254 Hz), (C-6, 2J = 22.5–22.7 Hz). The Citation13C NMR spectra also indicate the presence of the keto group at 189.3–189.8 ppm, while the carbonyl carbon of the ester group resonates in the range 164.1–165.9 ppm. The C-3″ carbon resonates in the range 154.2–161.2 ppm, while C-2″ in the range 99.8–102.2 ppm.
The quinolone ring in 20a–d was constructed through intramolecular cyclization of (19a–d) in which the chlorine atom at position 2 was replaced by the amino group via nucleophilic aromatic substitution (SNAr) assisted by the neighboring nitro group and fluorine atomCitation28,Citation29.
In this group of compounds, 1H NMR showed the disappearance of N–H broad peak at 11–12.7 ppm present in their acyclic precursors 19a–d; the spectrum showed a change in the doublet peak of CH to a singlet one. Both H-5 and H-2 in 20a–d are more deshielded than the corresponding protons in the 19a–d precursors. These changes constitute diagnostic criteria for ring closure (19a–d → 20a–d).
The Citation13C NMR spectra of compounds 20a–d reveal that most of the carbons are deshielded except for C-2, C-4 and C-8a.
In the Citation13C NMR spectrum, the carbon of the keto group [=O on C-4] for 20a–d resonates in the range 170.4–170.9 ppm, which is more shielded than the corresponding C-4 in compounds 19a–d (188–189 ppm); similarly, C-2 resonates around 152 ppm and C-8a resonates around 130 ppm and are more shielded than the corresponding carbons in 19a–d (154 ppm) and (148 ppm), respectively.
Having the essential quinolone compounds in hand, we could easily convert them to the diamino compounds 22a–d as shown in , in which the chlorine atom was replaced with the azide moiety via the standard nucleophilic aromatic substitution. The structure of compounds 21a–d is supported by spectral (NMR and MS) data which are in agreement with the literature data. In the 1H NMR spectra, there is not any difference in the chemical shift compared to the compounds 20a–d. In the Citation13C NMR spectra there is a small difference for C-7, which appears downfield in compounds 21a–d (∼128 ppm) compared to the corresponding C-7 in 20a–d due to the replacement of –Cl by the azide group.
Scheme 2. Synthesis of ethyl 7,8-diamino-1-(aryl/alkyl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylates (22a–d).
Reduction of the particular ethyl 7-azido-1-(alkyl/aryl)-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate (21a–d) with anhydrous stannous chloride in concentrated hydrochloric acid afforded the respective 7,8-diamino compounds (22a–d) in good yield (78–85%). The effective reduction of 21a–d to afford 22a–d was deduced from the presence of NH band at 3462–3295 cm−1 in the IR spectra. The IR spectra of 22a–d also show absorption bands (1672, 1632 cm−1), which indicate the presence of a conjugated keto group, while the carboxy group absorbs at 1718 cm−1. The structure of 22a–d is further supported by NMR spectral data. The 1H NMR spectra of 22a–d indicate the appearance of the amine protons signals at 5.05 (NH2 on C-8) and 5.43 (NH2 on C-7), while H-5 appears as a doublet at 7.19 ppm, and is thus shielded compared to its position in 21a–d. The Citation13C NMR spectra show the C-8 and C-5 resonating around 125.7 and 100.6 ppm, respectively, which are shielded compared to their positions in 21a–d (133.1 and 115.8 ppm, respectively).
Cyclocondensation of the synthesized diamine 22a–d using thionyl chloride produces the targeted bicyclic benzo[c][1,2,5]thiadiazoles system 23/24 (). The driving force of formation is due to: (i) strong electrophilicity of thionyl chloride, and (ii) stability of the tricyclic product due to aromaticity. It is worth mentioning that upon the workup procedure, where methanol was used to destroy the excess thionyl chloride, trans-esterification of the ethyl ester produced completely the methyl ester 23a, while a 1:1 ratio of methyl and ethyl esters were isolated for the product 23b/24b and 23d/24d. Compound 24c was isolated as the ethyl ester only.
Scheme 3. Synthesis of 9-(aryl/alkyl)-4-fluoro-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acids 25a--d.
![Scheme 3. Synthesis of 9-(aryl/alkyl)-4-fluoro-6-oxo-6,9-dihydro[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acids 25a--d.](/cms/asset/68ee4203-c4fe-4450-9be2-457d87342f7f/ienz_a_855925_f0005_b.jpg)
The following changes constitute diagnostic criteria for ring closure: the 1H NMR spectra of compounds (23/24) indicate the disappearance of the amine protons signals at 3.75–4.80 (NH2 on C-8) and 5.29–5.61 (NH2 on C-7), while H-5 appears as a doublet at 8.09–8.32 ppm, and is thus deshielded compared to its position in (22a–d). The Citation13C-NMR spectra show the C-3a and C-9b around 148.4 and 148.9 ppm, respectively, which are deshielded compared to their positions in 22a–d.
The hydrolysis of ethyl(methyl) 9-(aryl/alkyl)-4-fluoro-6-oxo-6,9-dihydro[1,2,5]thiadiazolo- [3,4-h]quinoline-7-carboxylate (23/24a–d) gave the corresponding acid products (25a–d) in ∼90 % yield by using 12% aqueous HCl under reflux conditions.
The structures of 25a–d are supported by spectral (1H NMR and MS) data. The 1H NMR spectra show the disappearance of ethyl or the methyl protons signals (in previous esters 23/24b–d) from the spectra and the appearance of a broad signal at 14.9 (exchangeable CO2H proton). Citation13C-NMR spectra show the disappearance of ethyl or the methyl carbons signals (in previous esters 23/24b–d) from the spectra.
Antimicrobial activity
The in vitro antimicrobial properties of the acids 25a–d, their esters 23a and 24b–d and of the starting compounds 19/22a–d were detected against Gram positive and Gram negative bacteria, yeasts and mould.
summarizes the results obtained when compounds 23a, 24b–d and 25a–d were assayed against B. subtilis and S. aureus Gram positive bacteria and E. coli and H. influenzae Gram negative ones. The antibacterial activity of desfluorinated N-ethylquinolone acid 14 and of the 6-fluoro-N–Cyclopropyl analogous 16 together with its ethyl ester is also reported for an useful comparison.
Table 1. In vitro antibacterial activity of thiadiazoloquinolones (minimum inhibitory concentration in μg/mL).
The thiadiazoloquinolone acids 25a–d are the most active compounds. N-ethyl derivative 25a and N-4-fluorophenyl derivative 25c had strong inhibitory effect on the growth of both Gram positive and Gram negative tested strains (MIC 0.15–3 μg/mL). Significant inhibition was also exhibited by phenyl derivative 25b with MIC values in the 1.5–50 μg/mL range. A lower activity was observed for 4-methoxyphenyl derivative 25d against B. subtilis and S. aureus (MIC 100–200 μg/mL). Gram positive B. subtilis was the most sensitive microorganism. Among thiadiazoloquinolone esters, N-ethyl derivative 23a and N-4-fluorophenyl derivative 24c showed good antimicrobial properties against B. subtilis at 12 μg/mL, whereas phenyl derivative 24b had some activity against the same strain at the concentration of 100 μg/mL. Compound 24c was the only ester that inhibited the growth of H. influenzae and S. aureus (MIC 200–400 μg/mL, respectively).
All the newly synthesized compounds exhibited antibacterial activity lower than that of ciprofloxacin used as reference substance. Furthermore, no inhibition was exerted against S. cerevisiae, C. tropicalis and A. niger fungal strains up to the concentration of 400 μg/mL (data not shown; MICs of miconazole 12 μg/mL, 6 μg/mL and 3 μg/mL, respectively).
Structure--activity relationship shows that, among the newly synthesized thiadiazoloquinolone acids and among their corresponding esters, the N-4-fluorophenyl derivatives 24c and 25c exhibited the highest activity, followed, in the order, by N-ethyl- (23a and 25a), N-phenyl- (24–25b) and N-4-methoxyphenyl- (24–25d) quinolones. However, these new thiadiazoloquinolones exhibited MIC values higher than those of the N–Cyclopropyl acid 16 and its ethyl ester, confirming the current knowledge that the replacement of the cyclopropyl at the N-1 position of the quinolone ring with ethyl or substituted and unsubstituted phenyl moiety plays a negative role on the antibacterial properties. Likewise, the introduction of fluorine at position C-6, in compound 25a, enhanced, by a factor four or two, the activity of 14 against S. aureus and E. coli, respectively.
As concerns the intermediates 19/22a–d, only B. subtilis, the most sensitive microorganism, was inhibited by 22c at 100 μg/mL and by 7-chloro-8-nitroquinolines 20a and 20c at 200 μg/mL. A low antibacterial activity appeared when compounds 19 cyclize to quinolone structure 20. This inhibition disappeared in the derivatives 21, probably owing to the loss of chlorine substituent. Replacing the azido fragment with the amino group for substances 22 led to an increase in antibacterial properties of 4-fluorophenyl derivative 22c against B. subtilis. The subsequent cyclization of the two amino groups at 7 and 8 positions on the fused thiadiazole ring (compounds 23a and 24b-d) enhanced the activity. Compound 24c showed a MIC value of 12 μg/mL towards B. subtilis and broadened the range of its inhibition against S. aureus and H. influenzae. This change in the structure resulted also in increased activity of 23a and 24b towards B. subtilis (MIC 12 and 100 μg/mL, respectively).
As expected, the sizeable increase of activity was observed in the target compounds 25a--d, whose activity is discussed above, by replacing the C-3 ester group with the carboxylate that is considered critical and not yet improved by any change.
Conclusion
The aim of this study is to investigate whether the thiadiazolo fused ring, as a bridge between the critical 7 and 8 positions of the fluoroquinolone scaffold, offers new insight into the structural–activity relationship of the class of quinolones antibacterials. In this study, 26 new compounds were successfully synthesized and fully characterized. The synthesized thiadiazolofluoroquinolones show broad spectrum of activity. The fluoroquinolone 25c was the most active compound in this study. The activities of this class of compounds were expected as a consequence of the modifications at the positions 7 and 8 of the fluoroquinolone ring, and are in agreement with the analogous previous generations of fluoroquinolones. This modification is expected to increase the intracellular targets on the type II topoisomerase and to impair the action of efflux pump, thus lowering the development of quinolone resistance in Gram positive pathogens. Extensive mechanism of action for this class of compounds will be investigated.
Declaration of interest
The authors report no conflicts of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgements
We wish to thank the Deanship of Scientific Research (The University of Jordan, Amman, Jordan) for financial support.
References
- Wagman AS, Wentland MP. Quinolone antibacterial agents. In: Taylor JB, Triggle DJ, eds. Comprehensive medicinal chemistry II. Oxford: Elsevier Ltd; 2007:567–96
- Bryskier A. Fluoroquinolones. In: Bryskier A, ed. Antimicrobial agents: antibacterials and antifungals. Washington, DC: ASM Press; 2005:668–788
- Gootz TD, Brighty KE. Chemistry and mechanism of action of the quinolone antibacterials. In: Andriole VT, ed. The quinolones. San Diego (CA): Academic Press; 1998:29--80
- Emami S, Shafiee A, Foroumadi A. Structural features of new quinolones and relationship to antibacterial activity against Gram-positive bacteria. Mini Rev Med Chem 2006;6:375–86
- Li Q, Mitscher LA, Shen LL. The 2-pyridone antibacterial agents: bacterial topoisomerase inhibitors. Med Res Rev 2000;20:231–93
- Kathiravan MK, Khilare MM, Nikoomanesh K, et al. Topoisomerase as target for antibacterial and anticancer drug discovery. J Enzyme Inhib Med Chem 2013;28:419–35
- Mitscher LA. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem Rev 2005;105:559–92
- Chu DTW, Fernandes PB. Structure--activity relationships of the fluoroquinolones. Antimicrob Agents Chemother 1989;33:131–5
- Chu DTW, Fernandes PB, Claiborne AK, et al. Synthesis and structure--activity relationships of novel aryl flouroquinolone antibacterial agents. J Med Chem 1985;28:1558–64
- Odagiri T, Inagaki H, Sugimoto Y, et al. Design, synthesis, and biological evaluations of novel 7-[7-amino-7-methyl-5-azaspiro[2.4]heptan-5-yl]-8-methoxyquinolines with potent antibacterial activity against respiratory pathogens. J Med Chem 2013;56:1974–83
- Koga H, Itoh A, Murayama SS, Irikura T. Structure--activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinline-3-carboxylic acids. J Med Chem 1980;23:1358–63
- Felmingham D, O’Hare MD, Robbins MJ, et al. Comparative in vitro studies with 4-quinolone antimicrobials. Drugs Exp Clin Res 1985;11:317–29
- Petersen U, Bartel S, Bremm K-D, et al. The synthesis and biological properties of 6-fluoroquinolonecarboxylic acids. Bull Soc Chim Belg 1996;105:683–99
- Mor N, Vanderkolk J, Heifets L. Inhibitory and bactericidal activities of levofloxacin against Mycobacterium tuberculosis in vitro and in human macrophages. Antimicrob Agents Chemother 1994;38:1161–4
- Balfour JAB, Lamb HM. Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections. Drugs 2000;59:115–39
- Chu DTW, Fernandes PB, Maleczka RE Jr, et al. Synthesis and structure--activity relationship of 1-aryl-6,8-difluoroquinolne antibacterial agents. J Med Chem 1987;30:504–9
- Al-Tarawneh SA, Zahra JA, Kamal MR, et al. Synthesis and biological evaluation of tetracyclic fluoroquinolones as antibacterial and anticancer agents. Bioorg Med Chem 2010;18:5873–84
- Al-Tarawneh SA, El-Abadelah MM, Zahra JA, et al. Synthesis and biological evaluation of tetracyclic thienopyridones as antibacterial and antitumor agents. Bioorg Med Chem 2011;19:2541–8
- Al-Qawasmeh RA, Zahra JA, Zani F, et al. Synthesis and antibacterial activity of 9-cyclopropyl-4-fluoro-6-oxo-6,9-dihydro-[1,2,5]thiadiazolo[3,4-h]quinoline-7-carboxylic acid and its ethyl ester. Arkivoc 2009;xii:322–36
- Hutchinson DR. Modified release tizanidine. J Int Med Res 1989;17:565–73
- Kamen L, Henney HR, Runyan JD. A practical overview of tizanidine use for spasticity secondary to multiple sclerosis, stroke, and spinal cord injury. Curr Med Res Opin 2008;24:425–39
- Paisley S, Beard S, Hunn A, Wight J. Review clinical effectiveness of oral treatments for spasticity in multiple sclerosis: a systematic review. Multiple Sclerosis 2002;8:319–29
- Sharma KS, Kumari S, Singh RP. Condensed heterocycles; XI. Synthesis of 1,2,5-thia(selena)diazolo[3,4-b]quinolines and 1,2,5-thia(selena)diazolo[3,4-h]quinolines. Synthesis 1981;4:316–18
- Mataka S, Takahashi K, Ikezaki Y, et al. Sulfur nitride in organic chemistry. Part 19. Selective formation of benzo- and benzobis 1,2,5-thiadiazole skeleton in the reaction of tetrasulfur tetranitride with naphthalenols and related compounds. Bull Chem Soc Jpn 1991;64:68–73
- Klamann D, Koser W, Weyerstahl P, Fligge M. Pseudonitrosites, nitroximes, and furoxans. Chem Ber 1965;98:1831–6
- Pesin VG, Zolotova-Zolotukhina LV. Behavior of 4- and 5-aminobenzo-2,1,3-thiadiazoles under Hertz-Skraup reaction conditions. Khim Geterotsikl Soedin 1965:314–15; Chem Abstr 1965;63:39083
- National Committee for Clinical Laboratory Standards (NCCLS) (Wayne, PA): (a) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standards M7-A7 and 17th Informational Supplement M100-S17, 2007, 27. (b) Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard M27-A2, 2002, 22. (c) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, approved standard M38-A2, 2002, 22
- Pulla RM, Venkaiah CN. An improved process for the preparation of quinolone derivatives 2001; World Patent: WO 0185 692
- Grohe K, Heitzer H. Cycloaracylation of enamines. II. Synthesis of 1-amino-4-quinolone-3-carboxylic acids. Liebigs Ann Chem 1987;10:871–9