Abstract
This study presents the synthesis, antiproliferative and antimicrobial evaluation of a new series of Mannich base derivatives containing 1,2,4-triazole system. New compounds were prepared by the reaction of 4,5-disubstituted 1,2,4-triazole-3-thiones with formaldehyde and various amines. The structures of the prepared compounds were confirmed by means of 1H NMR, 13C NMR and elemental analyses. Twelve compounds were evaluated for their in vitro antiproliferative activities against six chosen cancer cell lines. All synthesized compounds were screened for their in vitro antimicrobial activity by using the agar dilution technique. For 17 potentially active compounds, their antibacterial activity was confirmed on the basis of MIC (minimal inhibitory concentration) by broth microdilution method using the reference Gram-positive and Gram-negative bacterial strains.
Introduction
The search for novel antibacterial and antitumor agents devoid of side effects continues to be an active area of research in medicinal chemistry. Among all heterocyclic systems the therapeutic importance of 1,2,4-triazoles and their derivatives is well documented and they have been reported to possess various biological activities such as analgesicCitation1, antifungalCitation2,Citation3, antibacterialCitation4,Citation5, antiviralCitation6, antiphlogisticCitation7,Citation8, and antitubercularCitation9. Therefore, 4,5-substituted 1,2,4-triazoles seems to be suitable candidates for further chemical modifications and might be of interest as pharmacologically active compounds.
Our interest was directed to Mannich bases which in recent years have gained importance due to their application in pharmaceutical chemistry and comprehensive bioactivities like antibacterialCitation10–13, antifungalCitation14,Citation15, anticancerCitation16, analgesicCitation17 and anticonvulsantCitation18 properties. They are also used in polymer industry as paints and surface active reagentsCitation19.
Mannich reaction is a three-component condensation reaction involving an active hydrogen containing compound, formaldehyde and a secondary amine. The scope of Mannich reaction has been expanded in recent years to include a wide variety of amines, ammonia equivalents, imines and acetylated iminesCitation20. The aminomethylation of aromatic substrates by Mannich reaction is of considerable importance for the synthesis and modification of biological active compoundsCitation21.
Literature survey also reveals that piperazine or morpholine ring is significant for antimicrobial activityCitation22,Citation23. Mannich bases of 1,2,4-triazole containing N-methylpiperazine moiety are known as antimicrobial agentsCitation24. Some in vitro cytotoxic activitiy of Mannich bases on Maurine P388 lyphocytic leukemia have also been reportedCitation25,Citation26.
Prompted by these observations and in an attempt to continue our studies on the synthesis of biologically active nitrogen and sulfur containing heterocycles, we herein report the synthesis, spectral data and evaluation of biological activities of new Mannich bases containing 1,2,4-triazole moiety.
Experimental
Chemistry
General
All reagents were purchased from Sigma-Aldrich (Munich, Germany) and Merck Co. (Darmstadt, Germany) and used without further purification. Melting points were determined in Fisher-Johns blocks (Fisher Scientific, Schwerte, Germany) and presented without any corrections. The 1H NMR spectra were recorded on a Bruker Avance 300 apparatus (Bruker BioSpin GmbH, Rheinstetten/Karlsruhe, Germany) in DMSO-d6 with TMS as internal standard. The 13C NMR spectra were recorded on a Bruker Avance 300 apparatus. Chemical shifts are given in ppm (δ-scale). Multiplicities of NMR signals are represented as singlet (s), doublet (d), doublet of doublet (dd), triplet (t), quartet (q) and multiplet (m). The purity of obtained compounds was checked by TLC on aluminium oxide 60 F254 plates (Merck Co. Whitehouse Station, NJ), in a CHCl3/C2H5OH (10:1, v/v) solvent system. The spots were detected by exposure to a UV lamp at 254 nm. Elemental analyses of the obtained compounds were performed for C, H, N, S on AMZ 851 CHX analyser (PG, Gdańsk, Poland). The maximum percentage differences between calculated and found values for each element were within the error and amounted to ±0.4%.
Preparation of thiosemicarbazide derivatives
A mixture of appropriate hydrazide: cyclopropopanecarbohydrazide, cyclopentanecarbohydrazide, cyclohexanecarbohydrazide or nicotinic hydrazide (10 mmol) and 10 mmol appropriate isothiocyanate was heated in an oil bath at 50–90 °C for 8–12 h. The product was washed with diethyl ether to remove unreacted isothiocyanate. Then it was filtered off, dried and crystallized from ethanol.
N-(4-chlorophenyl)-2-(cyclopropylcarbonyl)hydrazinecarbothioamide (1a)
CAS Registry Number: 685113-56-8. Yield: 80%; m. p. 170 °C–172 °C (dec.). Temperature of reaction: 70 °C for 12 h. 1H NMR (DMSO-d6): δ (ppm) = 0.63–0.66 (m, 2H, CH2-cyclopropyl), 0.75–0.81 (m, 2H, CH2-cyclopropyl), 1.42–1.50 (m, 1H, CH-cyclopropyl), 7.32–7.58 (m, 4H, Ar-H), 9.17 (s, 1H, NH), 9.77 (s, 1H, NH), 10.11 (s, 1H, NH). 13C NMR: δ (ppm) = 10.76 (2 × 2 × CH2-cyclopropyl), 16.93 (CH-cyclopropyl), 121.95, 128.20, 129.37 (5Car), 139.93 (Car), 176.35 (C=O), 179.38 (C=S). Analysis for C11H12ClN3OS (269.75); Calculated: C, 49.98; H, 4.48; N, 15.58; S, 11.89; Found: C, 49.96; H, 4.45; N, 15.62; S, 11.86%.
2-(Cyclopentylcarbonyl)-N-(2,4-dichlorophenyl)hydrazinecarbothioamide (1b)
CAS Registry Number: 891381-19-4. Yield: 91%; m. p. 162 °C–164 °C (dec.). Temperature of reaction: 90 °C for 12 h. 1H NMR (DMSO-d6): δ (ppm) = 1.50–1.83 (m, 8H, CH2-cyclopentyl), 2.63–2.73 (m, 1H, CH-cyclopentyl), 7.39–7.72 (m, 3H, Ar-H), 9.37 (s, 1H, NH), 9.79 (s, 1H, NH), 9.94 (s, 1H, NH). Citation13C NMR: δ (ppm) = 26.12, 32.26 (4 × CH2-cyclopentyl), 46.12 (CH-cyclopentyl), 125.30, 128.73, 129.00, 129.96, 130.95, 134.63 (6Car), 176.59 (C=O), 180.86 (C=S). Analysis for C13H15Cl2N3OS (332.25); Calculated: C, 46.99; H, 4.55; N, 12.65; S, 9.65; Found: C, 46.92; H, 4.57; N, 12.61; S, 9.62%.
N-(4-chlorophenyl)-2-(cyclohexylcarbonyl)hydrazinecarbothioamide (1c)
CAS Registry Number: 891077-72-8. Yield: 90%; m. p. 172 °C–174 °C (dec.). Temperature of reaction: 90 °C for 12 h. 1H NMR (DMSO-d6): δ (ppm) = 1.04–2.28 (m, 10H, cyklohexyl), 2.63 (m, 1H, CH), 7.35–7.57 (m, 4H, Ar-H), 9.07, 9.63, 9.84 (3s, 3H, 3NH). Citation13C NMR: δ (ppm) = 25.15, 25.92, 27.93 (5 × CH2-cyclohexyl), 42.43 (CH-cyclohexyl), 121.95, 128.20, 129.37, 139.93 (6Car), 176.11 (C=O), 179.38 (C=S). Analysis for C14H18ClN3OS (311.83); Calculated: C, 53.92; H, 5.82; N, 13.48; S, 10.28; Found: C, 53.89; H, 5.88; N, 13.45; S, 10.25%.
N-phenyl-2-(pyridin-3-ylcarbonyl)hydrazinecarbothioamide (1d)
CAS Registry Number: 54584-49-5. Yield: 92%; m. p. 120 °C–122 °C (dec.). Temperature of reaction: 50 °C for 8 h. 1H NMR (DMSO-d6): δ (ppm) = 7.13–7.15 (m, 1H, Ar-H), 7.28–7.32 (m, 2H, Ar-H), 7.34–7.38 (m, 2H, Ar-H), 7.57–7.59 (m, 1H, Ar-H); 8.21–8.23 (m, 1H, Ar-H), 8.69–8.71 (m, 1H, Ar-H), 8.90–8.92 (m, 1H, Ar-H), 9.34, 9.71, 10.09 (3s, 3H, 3NH). Citation13C NMR: δ (ppm) = 121.54, 122.68, 124.47, 132.64, 137.58, 149.45, 150.91 (11Car), 168.05 (C=O), 179.39 (C=S). Analysis for C13H12N4OS (272.32); Calculated: C, 57.34; H, 4.44; N, 20.57; S, 11.77; Found: C, 57.41; H, 4.40; N, 20.61; S, 11.81%.
Preparation of 1,2,4-triazole derivatives
A mixture of appropriate thiosemicarbazide 1a–1d (10 mmol) and 20–40 ml of 2% aqueous solution of sodium hydroxide was refluxed for 2 h. Then, the solution was neutralized with diluted hydrochloric acid and the formed precipitate was filtered off and crystallized from ethanol.
4-(4-Chlorophenyl)-5-cyclopropyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (2a)
CAS Registry Number: 1038358-95-0. Yield: 37%; m. p. 172 °C–174 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 0.78–0.92 (m, 4H, CH2-cyclopropyl), 1.46–1.55 (m, 1H, CH-cyclopropyl), 7.52–7.54 (dd, 2H, Ar-H, J = 6 Hz), 7.65–7.67 (dd, 2H, Ar-H, J = 6 Hz), 13.65 (s, 1H, NH). Citation13C NMR: δ (ppm) = 6.67 (CH-cyclopropyl), 7.31 (2 × CH2-cyclopropyl), 129.93, 130.84, 133.20, 134.52 (6Car), 154.04 (C-5 triazole), 168.01 (C=S). Analysis for C11H10ClN3S (251.73); Calculated: C, 52.48; H, 4.00; N, 16.69; S, 12.74; Found: C, 52.47; H, 4.05; N, 16.57; S, 12.72%.
5-Cyclopentyl-4-(2,4-dichlorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2b)
Yield: 89%; m. p. 203 °C–205 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.44–1.78 (m, 8H, CH2-cyclopentyl), 2.63–2.73 (m, 1H, CH-cyclopentyl), 7.67–7.73 (m, 2H, Ar-H), 7.96–7.99 (m, 1H, Ar-H), 13.83 (s, 1H, NH). Citation13C NMR: δ (ppm) = 26.12, 34.79 (4 × CH2-cyclopentyl), 38.72 (CH-cyclopentyl), 129.46, 130.82, 131.69, 135.53, 136.44, 137.07 (6Car), 156.90 (C-5 triazole), 167.17 (C=S). Analysis for C13H13Cl2N3S (314.23); Calculated: C, 49.69; H, 4.17; N, 13.37; S, 10.20; Found: C, 49.72; H, 4.21; N, 13.42; S, 10.08%.
4-(4-Chlorophenyl)-5-cyclohexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (2c)
Yield: 93%; m. p. 212 °C–214 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.04–1.80 (m, 10H, cykloheksyl), 2.84 (m, 1H, CH), 7.52–7.55 (dd, 2H, Ar-H, J = 9 Hz), 7.69–7.72 (dd, 2H, Ar-H, J = 9 Hz), 11.04 (s, 1H, NH). Citation13C NMR: δ (ppm) = 25.15, 29.92, 29.47 (5 × CH2-cyclohexyl), 34.20 (CH-cyclohexyl), 129.74, 130.25, 133.45, 135.65 (6Car), 157.97 (C-5 triazole), 167.24 (C=S). Analysis for C14H16ClN3S (293.81); Calculated: C, 57.23; H, 5.49; N, 14.30; S, 10.91; Found: C, 57.29; H, 5.53; N, 14.27; S, 10.87%.
4-Phenyl-5-(pyridin-3-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2d)
CAS Registry Number: 57600-03-0. Yield: 89%; m. p. 222–223 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 7.16–7.18 (m, 1H, Ar-H), 7.34–7.38 (m, 2H, Ar-H), 7.46–7.50 (m, 2H, Ar-H), 7.57–7.59 (m, 1H, Ar-H), 8.28 (m, 1H, Ar-H), 8.69–8.71 (m, 1H, Ar-H), 8.72–8.74 (m, 1H, Ar-H), 10.98 (s, 1H, NH). Citation13C NMR: δ (ppm) = 126.04, 128.71, 129.36, 131.48, 134.49, 136.86, 146.07, 146.57 (11Car), 157.02 (C-5 triazole), 168.22 (C=S). Analysis for C13H10N4S (254.31); Calculated: C, 61.40; H, 3.96; N, 22.03; S, 12.61; Found: C, 61.51; H, 3.98; N, 21.98; S, 12.67%.
Preparation of Mannich base derivatives
To a solution of corresponding compounds 2a–2d (10 mmol) in 10 ml of 96% ethanol, appropriate amine (10 mmol) and formaldehyde (37%, 0.2 ml) were added. The mixture was stirred under reflux at room temperature for 1h. Next, distilled water was added and the precipitate formed was filtered off and crystallized from ethanol.
4-(4-Chlorophenyl)-5-cyclopropyl-2-(pyrrolidin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (3a)
Yield: 65%; m. p. 108 °C–110 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 0.88–0.95 (m, 4H, 2 × CH2-cyclopropyl), 1.53–1.62 (m, 1H, CH-cyclopropyl), 1.66–1.70 (m, 4H, 2 × CH2-pyrrolidine), 2.77–2.81 (m, 4H, 2 × CH2-pyrrolidine), 5.10 (s, 2H, CH2), 7.55–7.57 (dd, 2H, Ar-H, J = 6 Hz), 7.68–7.70 (dd, 2H, Ar-H, J = 6 Hz). Citation13C NMR: δ (ppm) = 6.50 (CH-cyclopropyl), 7.53 (2 × CH2-cyclopropyl), 29.31, 50.19 (4 × CH2-pyrrolidine), 65.21 (–CH2–), 129.37, 130.87, 133.64, 134.64 (6Car), 152.70 (C-5 triazole), 168.84 (C=S). Analysis for C16H19ClN4S (334.87); Calculated: C, 64.18; H, 6.40; N, 18.71; S, 10.71; Found: C, 64.15; H, 6.45; N, 18.67; S, 10.65%.
4-(4-Chlorophenyl)-5-cyclopropyl-2-(piperidin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (3b)
Yield: 93%; m. p. 132 °C–134 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 0.85–0.95 (m, 4H, 2 × CH2-cyclopropyl), 1.35–1.38 (m, 2H, CH2-piperidine), 1.49–1.54 (m, 4H, 2 × CH2-piperidine), 1.55–1.62 (m, 1H, CH-cyclopropyl), 2.66–2.70 (m, 4H, 2 × CH2-piperidine), 4.98 (s, 2H, CH2), 7.56–7.58 (dd, 2H, Ar-H, J = 6 Hz), 7.67–7.69 (dd, 2H, Ar-H, J = 6 Hz). Citation13C NMR: δ (ppm) = 10.50 (CH-cyclopropyl), 10.71 (2 × CH2-cyclopropyl), 23.42, 24.57, 53.90 (5 × CH2-piperidine), 63.51 (–CH2–), 129.72, 130.29, 133.71, 135.24 (6Car), 154.66 (C-5 triazole), 170.69 (C=S). Analysis for C17H21ClN4S (348.89); Calculated: C, 58.52; H, 6.07; N, 16.06; S, 9.19; Found: C, 58.53; H, 6.09; N, 16.11; S, 9.22%.
4-(4-Chlorophenyl)-5-cyclopropyl-2-(piperazin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (3c)
Yield: 15%; m. p. 222 °C–224 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 0.83–0.95 (m, 2H, CH2-cyclopropyl), 1.03–1.13 (m, 2H, CH2-cyclopropyl), 1.51–1.60 (m, 1H, CH-cyclopropyl), 2.68–2.82 (m, 8H, CH2-piperazine), 5.12 (s, 2H, CH2), 7.54–7.58 (dd, 2H, Ar-H, J = 12 Hz), 7.64–7.68 (dd, 2H, Ar-H, J = 12 Hz), 8.78 (s, 1H, NH). Citation13C NMR: δ (ppm) = 10.50 (CH-cyclopropyl), 10.71 (2 × CH2-cyclopropyl), 45.59, 53.21 (4 × CH2-piperazine), 63.51 (–CH2–), 129.72, 130.29, 133.71, 135.24 (6Car), 154.66 (C-5 triazole), 170.69 (C=S). Analysis for C16H20ClN5S (349.88); Calculated: C, 54.92; H, 5.76; N, 20.02; S, 9.16; Found: C, 54.95; H, 5.79; N, 20.05; S, 9.14%.
4-(4-Chlorophenyl)-5-cyclopropyl-2-{[4-(2-hydroxyethyl)piperazin-1-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (3d)
Yield: 73%; m. p. 154 °C–156 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 0.74–0.97 (m, 4H, 2 × CH2-cyclopropyl), 1.54–1.63 (m, 1H, CH-cyclopropyl), 2.32–2.40 (m, 4H, 2 × CH2-piperazine), 2.70–2.76 (m, 4H, 2 × CH2-piperazine), 2.89–2.91 (t, 2H, CH2), 3.46–3.48 (t, 2H, CH2), 4.99 (s, 2H, CH2), 7.34–7.71 (m, 4H, Ar-H), 8.89 (s, 1H, OH). Citation13C NMR: δ (ppm) = 6.48 (CH-cyclopropyl), 7.50 (2 × CH2-cyclopropyl), 52.43, 53.58 (4 × CH2-piperazine), 50.21 (–CH2–), 59.25 (–CH2–), 63.51 (–CH2–), 120.32, 125.98, 129.13, 129.94, 130.85, 139.06 (6Car), 154.66 (C-5 triazole), 170.69 (C=S). Analysis for C18H24ClN5OS (393.93); Calculated: C, 54.88; H, 6.14; N, 17.78; S, 8.14; Found: C, 54.92; H, 6.17; N, 17.80; S, 8.10%.
4-(4-Chlorophenyl)-5-cyclopropyl-2-[(4-phenylpiperazin-1-yl)methyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (3e)
CAS Registry Number: 1286639-67-5. Yield: 65%; m. p. 108 °C–110 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 0.85–0.98 (m, 4H, 2 × CH2-cyclopropyl), 1.54–1.63 (m, 1H, CH-cyclopropyl), 2.86–2.89 (t, 4H, 2 × CH2-piperazine), 3.12–3.15 (t, 4H, 2 × CH2-piperazine), 5.09 (s, 2H, CH2), 6.76–6.81 (m, 1H, Ar-H), 6.93–6.95 (m 2H, Ar-H), 7.19–7.24 (m 2H, Ar-H), 7.58–7.61 (dd, 2H, Ar-H, J = 9 Hz), 7.67–7.70 (dd, 2H, Ar-H, J = 9 Hz). Citation13C NMR: δ (ppm) = 10.50 (CH-cyclopropyl), 10.71 (2 × CH2-cyclopropyl), 50.36, 51.43 (4 × CH2-piperazine), 63.51 (–CH2–), 116.77, 120.31 (2Car), 129.35, 129.72, 130.29, 133.71, 135.24 (8Car), 150.96 (Car), 154.66 (C-5 triazole), 170.69 (C=S). Analysis for C22H24ClN5S (425.98); Calculated: C, 62.03; H, 5.68; N, 16.44; S, 7.53; Found: C, 62.08; H, 5.61; N, 16.41; S, 7.56%.
5-Cyclopentyl-4-(2,4-dichlorophenyl)-2-(pyrrolidin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (4a)
CAS Registry Number: 929980-59-6. Yield: 68%; m. p. 72 °C–74 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.49–1.59 (m, 2H, CH2-cyclopentyl), 1.69–1.71 (m, 4H, 2 × CH2-pyrrolidine), 1.72–1.79 (m, 6H, 3 × CH2-cyclopentyl), 2.68–2.75 (m, 1H, CH-cyclopentyl), 2.76–2.81 (m, 4H, 2 × CH2-pyrrolidine), 5.14 (s, 2H, CH2), 7.40–7.76 (m, 2H, Ar-H), 8.00–8.02 (m, 1H, Ar-H). Citation13C NMR: δ (ppm) = 24.01, 25.31, 25.38, 30.47 (4 × CH2-cyclopentyl), 30.94 (CH2-pyrrolidine), 35.70 (CH-cyclopentyl), 39.99, 40.27 (2 × CH2-pyrrolidine), 50.09 (CH2-pyrrolidine), 65.18 (–CH2–), 129.41, 130.60, 131.43, 133.19, 134.02, 136.27 (6Car), 154.40 (C-5 triazole), 167.12 (C=S). Analysis for C18H22Cl2N4S (397.36); Calculated: C, 54.41; H, 5.58; N, 14.10; S, 8.07; Found: C, 54.45; H, 5.59; N, 14.13; S, 8.04%.
5-Cyclopentyl-4-(2,4-dichlorophenyl)-2-(piperidin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (4b)
Yield: 92%; m. p. 88 °C–90 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.32–1.38 (m, 2H, CH2-cyclopentyl), 1.47–1.54 (m, 6H, 3 × CH2-piperidine), 1.63–1.79 (m, 6H, 3 × CH2-cyclopentyl), 2.63–2.70 (m, 4H, 2 × CH2-piperidine), 2.72–2.79 (m, 1H, CH-cyclopentyl), 5.05 (s, 2H, CH2), 7.67–7.76 (m, 2H, Ar-H), 7.99–8.01 (m, 1H, Ar-H). Citation13C NMR: δ (ppm) = 24.00, 25.29, 25.36, 25.99 (4 × CH2-cyclopentyl), 30.94 (CH2-piperidine), 35.68 (CH-cyclopentyl), 40.25, 51.61 (4 × CH2-piperidine), 69.99 (–CH2–), 129.40, 130.60, 131.43, 133.19, 134.04, 136.25 (6Car), 154.21 (C-5 triazole), 168.95 (C=S). Analysis for C19H24Cl2N4S (411.39); Calculated: C, 55.47; H, 5.88; N, 13.62; S, 7.79; Found: C, 55.49; H, 5.91; N, 13.60; S, 7.75%.
5-Cyclopentyl-4-(2,4-dichlorophenyl)-2-(piperazin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (4c)
Yield: 82%; m. p. 244 °C–246 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.23–1.29 (m, 2H, CH2-cyclopentyl), 1.48–1.56 (m, 6H, 3 × CH2-cyclopentyl), 2.05–2.13 (m, 1H, CH-cyclopentyl), 2.70–2.78 (m, 8H, 4 × CH2-piperazine), 5.09 (s, 2H, CH2), 7.66–7.75 (m, 2H, Ar-H), 7.78–8.06 (m, 1H, Ar-H), 9.17 (s, 1H, NH). Citation13C NMR: δ (ppm) = 26.12, 34.79 (4 × CH2-cyclopentyl), 39.66 (CH-cyclopentyl), 45.59, 53.21 (4 × CH2-piperazine), 63.51 (–CH2–), 129.56, 131.01, 131.62, 135.43, 135.98, 136.22 (6Car), 154.02 (C-5 triazole), 169.21 (C=S). Analysis for C18H23Cl2N5S (412.38); Calculated: C, 52.43; H, 5.62; N, 16.98; S, 7.78; Found: C, 52.46; H, 5.63; N, 16.96; S, 7.74%.
5-Cyclopentyl-4-(2,4-dichlorophenyl)-2-{[4-(2-hydroxyethyl)piperazin-1-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (4d)
Yield: 61%; m. p. 92 °C–94 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.47–1.57 (m, 2H, CH2-cyclopentyl), 1.60–1.80 (m, 6H, 3 × CH2-cyclopentyl), 2.33–2.37 (t, 2H, CH2), 2.69–2.74 (m, 4H, 2 × CH2-piperazine), 2.77–2.81 (m, 1H, CH-cyclopentyl), 3.44–3.50 (m, 4H, 2 × CH2-piperazine), 3.97 (s, 1H, OH), 4.38–4.42 (t, 2H, CH2), 5.06 (s, 2H, CH2), 7.40–7.77 (m, 2H, Ar-H), 8.00–8.01 (m, 1H, Ar-H). Citation13C NMR: δ (ppm) = 26.12, 34.79 (4 × CH2-cyclopentyl), 39.66 (CH-cyclopentyl), 52.43, 53.58 (4 × CH2-piperazine), 58.02 (–CH2–), 59.25 (–CH2–), 63.51 (–CH2–), 129.56, 131.01, 131.62, 135.43, 135.98, 136.22 (6Car), 154.02 (C5-triazole), 169.21 (C=S). Analysis for C20H27Cl2N5OS (456.43); Calculated: C, 52.63; H, 5.96; N, 15.34; S, 7.03; Found: C, 52.65; H, 5.98; N, 15.37; S, 7.01%.
5-Cyclopentyl-4-(2,4-dichlorophenyl)-2-[(4-phenylpiperazin-1-yl)methyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (4e)
Yield: 87%; m. p. 67 °C–69 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.49–1.56 (m, 2H, CH2-cyclopentyl), 1.63–.179 (m, 6H, 3 × CH2-cyclopentyl), 2.59–2.62 (t, 4H, 2 × CH2-piperazine), 2.84–2.94 (m, 1H, CH-cyclopentyl), 4.13--4.16 (t, 4H, 2 × CH2-piperazine), 5.16 (s, 2H, CH2), 6.78–6.81 (m, 1H, Ar-H), 6.94–6.96 (m, 2H, Ar-H), 7.18–7.25 (m, 2H, Ar-H), 7.66–7.78 (m, 2H, Ar-H), 8.00–8.02 (m, 1H, Ar-H). Citation13C NMR: δ (ppm) = 26.12, 34.79 (4 × CH2-cyclopentyl), 39.66 (CH-cyclopentyl), 50.36, 51.43 (4 × CH2-piperazine), 63.51 (–CH2–), 116.77, 120.31 (3Car), 129.35, 129.56, 131.01, 131.62, 135.43, 135.98, 136.22 (8Car), 150.96 (Car), 154.02 (C-5 triazole), 169.21 (C=S). Analysis for C24H27Cl2N5S (488.47); Calculated: C, 59.01; H, 5.57; N, 14.34; S, 6.56; Found: C, 59.06; H, 5.61; N, 14.37; S, 6.61%.
4-(4-Chlorophenyl)-5-cyclohexyl-2-(pyrrolidin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (5a)
Yield: 73%; m. p. 91 °C–93 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.16–1.62 (m, 10H, 5 × CH2-cyclohexyl), 1.70–1.92 (m, 4H, 2 × CH2-pyrrolidine), 2.38–2.46 (m, 1H, CH-cyclohexyl), 2.69–2.78 (m, 4H, 2 × CH2-pyrrolidine), 5.16 (s, 2H, CH2), 7.48–7.50 (dd, 2H, Ar-H, J = 6 Hz), 7.66–7.68 (dd, 2H, Ar-H, J = 6 Hz). Citation13C NMR: δ (ppm) = 24.92 (2 × CH2-pyrrolidine), 25.15, 25.92, 29.47 (5 × CH2-cyclohexyl), 34.92 (CH-cyclohexyl), 53.65 (2 × CH2-pyrrolidine), 62.10 (–CH2–), 129.72, 130.29, 133.71, 135.24 (6Car), 157.28 (C-5 triazole), 170.69 (C=S). Analysis for C20H27ClN4S (376.94); Calculated: C, 60.54; H, 6.68; N, 14.86; S, 8.51; Found: C, 60.58; H, 6.71; N, 14.82; S, 8.55%.
4-(4-Chlorophenyl)-5-cyclohexyl-2-(piperidin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (5b)
Yield: 78%; m. p. 87 °C–89 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.11–1.56 (m, 10H, 5 × CH2-cyclohexyl), 1.59–1.64 (m, 4H, 2 × CH2-piperidine), 2.06–2.18 (m 4H, 2 × CH2-piperidine), 2.39–2.44 (m, 1H, CH-cyclohexyl), 2.56–2.62 (m, 2H, CH2-piperidine), 5.35 (s, 2H, CH2), 7.37–7.40 (dd, 2H, Ar-H, J = 9 Hz), 7.44–7.47 (dd, 2H, Ar-H, J = 9 Hz). Citation13C NMR: δ (ppm) = 24.57, 25.10 (4 × CH2-piperidine), 25.15, 26.92, 29,47 (5 × CH2-cycohexyl), 34.92 (CH-cyclohexyl), 53.90 (CH2-piperidine), 63.51 (–CH2–), 129.72, 130,29, 133.71, 135.24 (6Car), 157.28 (C-5 triazole), 170.69 (C=S). Analysis for C20H27ClN4S (390.97); Calculated: C, 61.44; H, 6.96; N, 14.33; S, 8.20; Found: C, 61.38; H, 6.99; N, 14.30; S, 8.17%.
4-(4-Chlorophenyl)-5-cyclohexyl-2-(piperazin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (5c)
Yield: 92%; m. p. 182 °C–184 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.05–1.75 (m, 10H, 5 × CH2-cyclohexyl), 2.38–2.45 (m, 4H, 2 × CH2-piperazine), 2.68–2.74 (m, 4H, 2 × CH2-piperazine), 2.77–2.83 (m, 1H, CH-cyclohexyl), 5.03 (s, 2H, CH2), 7.52–7.54 (dd, 2H, Ar-H, J = 6 Hz), 7.67–7.69 (dd, 2H, Ar-H, J = 6 Hz), 8.71 (s, 1H, NH). Citation13C NMR: δ (ppm) = 25.45, 25.62, 30.41 (5 × CH2-cyclohexyl), 34.52 (CH-cyclohexyl), 40.26; 40.54 (4 × CH2-piperazine), 50.32 (–CH2–), 129.72, 130.15, 133.02, 135.24 (6Car), 157.28 (C-5 triazole), 170.69 (C=S). Analysis for C19H26ClN5S (391.96); Calculated: C, 58.22; H, 6.69; N, 17.87; S, 8.18; Found: C, 58.29; H, 6.72; N, 17.85; S, 8.15%.
4-(4-Chlorophenyl)-5-cyclohexyl-2-{[4-(2-hydroxyethyl)piperazin-1-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (5d)
Yield: 82%; m.p. 153 °C–155 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.22–1.48 (m, 10H, 5 × CH2-cyclohexyl), 2.48–2.58 (m, 4H, 2 × CH2-piperazine), 2.67–2.82 (m, 4H, 2 × CH2-piperazine), 2.84–2.89 (m, 1H, CH-cyclohexyl), 2.95 (t, 2H, CH2), 3.54 (t, 2H, CH2), 3.77 (s, 1H, OH), 4.78 (s, 2H, CH2), 7.24–7.48 (m, 4H, Ar-H). Citation13C NMR: δ (ppm) = 25.15, 25.92, 29.47 (5 × CH2-cyclohexyl), 34.92 (CH-cyclohexyl), 52.43, 53.58 (4 × CH2-piperazine), 58.02 (–CH2–), 59.25 (–CH2–), 63.51 (–CH2–), 129.72; 130.29, 133.71, 135.24 (6Car), 157.28 (C-5 triazole), 170.69 (C=S). Analysis for C21H30ClN5OS (436.01); Calculated: C, 57.85; H, 6.94; N, 16.06; S, 7.35; Found: C, 57.87; H, 6.97; N, 16.11; S, 7.32%.
4-(4-Chlorophenyl)-5-cyclohexyl-2-[(4-phenylpiperazin-1-yl)methyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (5e)
Yield: 79%; m. p. 68 °C–70 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.13–1.76 (m, 10H, 5 × CH2-cyclohexyl), 2.39–2.51 (m, 1H, CH-cyclohexyl), 2.68–2.72 (m, 4H, 2 × CH2-cyclohexyl), 3.13–3.17 (m, 4H, 2 × CH2-cyclohexyl), 5.14 (s, 2H, CH2), 6.76–6.81 (m, 2H, Ar-H), 6.93–6.96 (m, 2H, Ar-H), 7.19–7.25 (m, 3H, Ar-H), 7.53–7.56 (m, 1H, Ar-H), 7.66–7.70 (m, 1H, Ar-H), 8.72 (s, 1H, CH). Citation13C NMR: δ (ppm) = 25.15, 25.92, 29.47 (5 × CH2-cyclohexyl), 34.92 (CH-cyclohexyl), 50.36, 51.43 (4 × CH2-piperazine), 63.51 (–CH2–), 116, 120.31 (3Car), 129.35, 129.72, 130.29, 130.29, 133.71, 135.24 (8Car), 150.96 (Car), 157.28 (C-5 triazole), 170.69 (C=S). Analysis for C25H30ClN5S (468.06); Calculated: C, 64.15; H, 6.46; N, 14.96; S, 6.85; Found: C, 64.19; H, 6.48; N, 14.99; S, 6.88%.
4-Phenyl-2-(pyrrolidin-1-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (6a)
CAS Registry Number: 142529-43-9. Yield: 23%; m. p. 134 °C–136 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.67–1.76 (m, 4H, 2 × CH2-pyrrolidine), 2.88–2.92 (t, 4H, 2 × CH2- pyrrolidine), 5.29 (s, 2H, CH2), 5.29 (s, 2H, CH2), 7.44–7.54 (m, 6H, Ar-H), 7.69–7.73 (m, 1H, Ar-H), 8.52–8.63 (m, 2H, Ar-H). Citation13C NMR: δ (ppm) = 23.96, 50.26 (4 × CH2-pyrrolidine), 65.79 (–CH2–), 122.45, 124.00, 129.28, 129.95, 130.21, 135.15, 136.51, 147.56, 149.24 (9Car), 150.28, 151.66 (2Car), 154.80 (C-5 triazole), 170.04 (C=S). Analysis for C18H19N5S (337.44); Calculated: C, 64.07; H, 5.68; N, 20.75; S, 9.50; Found: C, 64.11; H, 5.64; N, 20.81; S, 9.45%.
4-phenyl-2-(piperidin-1-ylmethyl)-5-(pyridin-3-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (6b)
CAS Registry Number: 142529-46-2. Yield: 89%; m. p. 150 °C–152 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.30–1.54 (m, 6H, 3 × CH2-piperidine), 2.68–2.79 (m, 4H, 2 × CH2-piperidine), 5.12 (s, 2H, CH2), 7.30–8.63 (m, 9H, Ar-H). Citation13C NMR: δ (ppm) = 23.42, 24.57, 53.90 (5 × CH2-piperidine), 63.51 (–CH2–), 126.15, 128.65, 128.83, 129.36, 132.06, 134.57, 136.59 (9Car), 146.42, 149.92 (2Car), 154.70 (C-5 triazole), 168.26 (C=S). Analysis for C19H21N5S (351.47); Calculated: C, 64.93; H, 6.02; N, 19.93; S, 9.12; Found: C, 64.98; H, 6.07; N, 19.88; S, 9.08%.
4-Phenyl-2-(piperazin-1-ylmethyl)-5-(pyridin-3-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (6c)
Yield: 78%; m. p. 242 °C–244 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 1.04–1.14 (m, 2H, CH2-piperazine), 1.25–1.31 (m, 2H, CH2-piperazine), 3.20–3.30 (m, 4H, 2 × CH2-piperazine), 5.24 (s, 2H, CH2), 7.46–8.56 (m, 9H, Ar-H), 9.23 (s, 1H, NH). Citation13C NMR: δ (ppm) = 45.59, 53.21 (4 × CH2-piperazine), 63.51 (–CH2–), 126.15, 128.65, 128.83, 129.36, 132.06, 136.59 (9Car), 146.42, 146.92 (2Car), 154.70 (C-5 triazole), 168.26 (C=S). Analysis for C18H20N6S (352.46); Calculated: C, 61.34; H, 5.72; N, 23.84; S, 9.10; Found: C, 61.27; H, 5.78; N, 23.80; S, 9.06%.
2-{[4-(2-Hydroxyethyl)piperazin-1-yl]methyl}-4-phenyl-5-(pyridin-3-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (6d)
Yield: 70%; m. p. 168 °C–170 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 2.89–2.95 (m, 4H, 2 × CH2-piperazine), 3.21 (t, 2H, CH2), 3.36–3.43 (m, 4H, 2 × CH2-piperazine), 3.55 (t, 2H, CH2), 4.46 (s, 2H, CH2), 7.17–7.60 (m, 5H, Ar-H), 8.27–8.77 (m, 4H, Ar-H), 8.87 (s, 1H, OH). Citation13C NMR: δ (ppm) = 52.43, 53.58 (4 × CH2-piperazine), 58.02 (–CH2–), 59.25 (–CH2–), 63.51 (–CH2–), 126.15, 128.65, 128.83, 129.36, 132.06, 134.57, 136.59 (9Car), 146.42, 146.92 (2Car), 154.70 (C-5 triazole), 168.26 (C=S). Analysis for C20H24N6OS (396.50); Calculated: C, 60.58; H, 6.10; N, 21.20; S, 8.09; Found: C, 60.62; H, 6.05; N, 21.24; S, 8.01%.
4-Phenyl-5-(pyridin-3-yl)-2-[(4-phenylpiperazin-1-yl)methyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (6e)
CAS Registry Number: 915144-29-5. Yield: 95%; m. p. 154 °C–156 °C (dec.). 1H NMR (DMSO-d6): δ (ppm) = 2.62–2.65 (t, 2H, CH2-piperazine), 3.00–3.04 (t, 2H, CH2-piperazine), 3.15–3.19 (t, 2H, CH2-piperazine), 3.46–3.48 (t, 2H, CH2-piperazine), 5.32 (s, 2H, CH2), 6.79–6.84 (m, 2H, Ar-H), 6.96–7.07 (m, 4H, Ar-H), 7.22–7.27 (m, 4H, Ar-H), 7.42–7.63 (m, 2H, Ar-H), 7.73–7.77 (m, 1H, Ar-H), 8.57–8.65 (m, 1H, Ar-H). Citation13C NMR: δ (ppm) = 50.36, 51.43 (4 × CH2-piperazine), 63.51 (–CH2–), 116.77 (2Car), 120.31 (Car), 126.15, 128.65, 128.83, 129.35, 129.36, 132.06, 134.57, 136.59 (11Car), 146.42, 146.92 (2Car), 150.96 (Car), 154.70 (C-5 triazole), 168.26 (C=S). Analysis for C24H24N6S (428.55); Calculated: C, 67.26; H, 5.64; N, 19.61; S, 7.48; Found: C, 67.34; H, 5.61; N, 19.64; S, 7.44%.
Antiproliferative activity
Materials and methods
The experiment was conducted on reference cancer cell lines: A549 (Adenocarcinomic human alveolar basal epithelial cells), HeLa (Human cervical cancer cells), TOV-112D (Human ovarian cancer cell line), T47D (Human ductal breast epithelial tumor cell line) and L929 (Murine aneuploid fibrosarcoma cell line) and GMK (Green monkey kidney cell line) as a normal cell lines. The control group was the chosen cell line untreated with compounds. Cell lines were obtained from European Collection of Cell Cultures (ECAACC). Cell cultures were grown at 37 °C in a humidified atmosphere consisting 5% CO2 in air. Cultures were maintained at density of 2–5 × 106 cell/ml in exponential growth serum free conditions containing RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS, Sigma), 100 U/ml of penicillin (Sigma), 100 μg/ml streptomycin (Sigma), and routinely passaged twice a week. Cell viability was assessed by the ability to exclude trypan blue dye (0.5% v/v, Sigma). Next cultures were incubated in the presence of tested compounds (3a, 3b, 3c, 3e, 4a, 4b, 4d, 5c, 5e, 6a, 6b, 6e) at the concentrations of 50, 100 and 150 μM during 24, 48 and 72 h. The investigated 12 compounds were dissolved in dimethylsulfoxide (DMSO, Sigma) and then diluted in cell culture media. The antiproliferative activity of novel compounds was assessed by 5-bromo-2′-deoxyuridine test (BrdU, Sigma) on Elx808iu ELISA reader. In these method, 5-bromo-2′-deoxyuridine, a thymidine analog, replaces [3H] thymidine. BrdU is incorporated into newly synthesized DNA strands of actively proliferating cells. Following partial denaturation of double stranded DNA, BrdU is detected immunochemically allowing the assessment of the population of cells, which are synthesizing DNA. All results were done in triplicates.
Microbiology
Materials and methods
All the derivatives were screened for their in vitro antibacterial activity using agar plate method (with 1000 µg/ml concentration of compounds) and next the compounds with potential antibacterial activity were tested using microdilution technique to estimate minimal inhibitory concentration (MIC), as was described earlier Citation27. Eleven reference strains of aerobic bacteria from American Type Culture Collection were used. There were representing Gram-positive bacteria Staphylococcus aureus ATCC 6538, S. aureus ATCC 25923, S. aureus ATCC 43300, S. epidermidis ATCC 12228, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 10876, Micrococcus luteus ATCC 10240 and representing Gram-negative bacteria Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Proteus mirabilis ATCC 12453 and Pseudomonas aeruginosa ATCC 27853. All the bacterial cultures were adjusted to 0.5 McFarland standards 150 × 106 CFU/ml (CFUs – colony forming units) in sterile 0.85% NaCl. All stock solutions of the tested compounds were prepared in DMSO (dimethyl sulfoxide). The medium with DMSO at the final concentration and without the tested compounds served as negative control – no microbial growth inhibition was observed. Cefuroxime (second generation of cephalosporins) was used as a positive control.
Using Mueller–Hinton agar plate method with 1000 µg/ml concentration of each derivative, 10 μl of each bacterial suspension was put onto the prepared solid media and antibacterial activity was detected after incubation (37 °C for 18 h) on the basis of the microbial growth inhibition. MIC of compounds, which inhibited the growth of bacteria on agar medium, was tested through the Mueller–Hinton broth microdilution method as recommended by Clinical Laboratory Standards InstituteCitation28. MIC is usually defined as the lowest concentration of the compound at which there was no visible growth of microorganisms. The 96-well microplates and Mueller–Hinton broth with a series of two-fold dilution of the tested compound in the range of final concentrations from 7.82 to 1000 μg/ml were used. After incubation (at 35 °C for 18 h), spectrophotometric measurements of optical density (OD600) of the bacterial cultures with the tested compounds were performed in order to determine MIC. OD600 of bacterial cultures in the medium without the tested compounds and the blank wells with two-fold dilution of each of the tested compounds added to broth without bacterial suspension were used as controls. In our study, the bioactivity of tested compounds against bacteria was defined as mild (MIC > 500 µg/ml), moderate (MIC in the range >125–500 µg/ml), good (MIC in the range ≥31.25–125 µg/ml), according to O’Donnell et al.Citation29.
Results and discussion
Chemistry
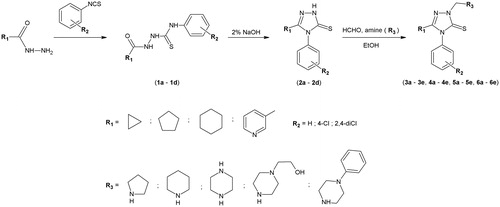
The hydrazides of cyclopropionic acid, cyclopentanoic acid, cyclohexanoic acid and nicotinic acid were initial substrates for the synthesis of new Mannich base derivatives 3a–3e, 4a–4e, 5a–5e, 6a–6e. In the first step, thiosemicarbazide derivatives 1a–1d were obtain by the reaction of corresponding carboxylic acid hydrazide with approptiate isothiocyanate. The substracts were heated in an oil bath, temperatures were selected experimentally (t = 50–90 °C). Subsequently, the reaction of thiosemicarbazide derivatives 1a–1d with 2% aqueous solution of sodium hydroxide lead to the formation of 4,5-disubstituted 1,2,4-triazole-3-thione derivatives 2a–2d. The treatment of 1,2,4-triazole derivatives with formaldehyde and corresponding secondary amine in ethanol afforded new Mannich base derivatives 3a–3e, 4a–4e, 5a–5e, 6a–6e in moderate to good yields.
Compounds 1a, 1b, 1c, 2a, 3e, 4a, 6a, 6b are registered in CAS (Chemical Abstracts Service) database but without references and methods of synthesis. Compounds 1dCitation30, 2dCitation31, 6eCitation32 were synthesized earlier.
All the newly obtained compounds are air stable solids and soluble in DMSO at ambient temperature. The purity of the synthesized compounds was checked by elemental analyses and thin layer chromatography. The structures of prepared derivatives were determined on the basis of 1H NMR and 13C NMR spectra and all of the synthesized compounds have satisfactory analyses for their proposed structures. 1H NMR spectral results for all compounds together with hydrogen assignments and 13C NMR spectra results are presented in Experimental Section.
1H NMR spectra of the thiosemicarbazide derivatives 1a–d show three proton signals typical for the NH group in the δ 9.07–10.11 ppm range, whereas in the 1H NMR spectra of the 1,2,4-triazole compounds 2a–d the singlet peak due to the proton of NH group appeared in the region of δ 11.04–13.65 ppm, which confirmed the successful formation of the desired products. All the 1H NMR spectra of Mannich base derivatives derivatives 3a–e, 4a–e, 5a–e, 6a–e confirmed adequate N-substitution of 1,2,4-triazole-3-thiones. The singlet signal for CH2 group was observed in the δ 4.46-5.35 ppm range (compounds 3a–e, 4a–e, 5a–e, 6a–e), for NH group (compounds 3c, 4c, 5c, 6c) δ 8.71–9.23 ppm, and for OH group in the region of 3.77–3.97 and 8.87–8.89 ppm (compounds 3d, 4d, 5d, 6d). All other aliphatic and aromatic protons for obtained compounds were observed at expected regions.
In the Citation13C NMR spectra of thiosemicarbazide derivatives 1a–d the carbon of C=S group had a typical signal at about 176 ppm and for C=O group at about 168–180 ppm. Similarly, in the 1,2,4-triazole derivatives 2a–d, the presence of the C=S group was also confirmed by a signal at about 167–168 ppm and for the C-5 in 1,2,3-triazole system in the range of 154–157 ppm. The 2a–d derivatives may occur in thione or thiol form. However, in our case the signal of C=S group in compounds 2a–d proved that they were obtained in thione form. The thione-thiol tautomerism was not observed. It is consistent with the studies recently conducted in our department which were focused on the cyclization mechanism of thiosemicarbazide derivativesCitation33,Citation34. In the Mannich base derivatives 3a–e, 4a–e, 5a–e, 6a–e the CH2 group was observed at about 50–69 ppm. All other aliphatic and aromatic signals were observed at expected regions.
The synthetic pathway leading to the new Mannich base derivatives 3a–e, 4a–e, 5a–e, 6a–e was carried out according to the steps shown in . The substituents of all obtained compounds 1–6 are presented in .
Table 1. Substituents of compounds 1–6.
Antiproliferative activity
The purpose of this study was to evaluate in vitro antiproliferative activities against chosen cell lines of the 12 compound based on of Mannich bases containing 1,2,4-triazole system. Evaluation of cell cycle progression is essential for investigations in many scientific fields. Measurement of [3H] thymidine incorporation as cells enter S phase has been a traditional method for detection of cell proliferation. Precentage of cells viability, before the foundation of the culture, was about 87–96%. The amount of BrdU was determined according to OD. The higher the OD, the higher the BrdU concentration in the sample. Results were presented as the percentage of cell viability in comparison to control. The influence of various concentrations of new synthesized compounds on the proliferative activity of chosen cell cultures is summarized in .
Table 2. Effects of tested compounds (3a, 3b, 3c, 3e, 4a, 4b, 4d, 5c, 5e, 6a, 6b, 6e) on the viability of chosen cell lines (%).
The investigated compounds proved to be low toxic for checked cell lines, since applied in concentrations of 50, 100 and 150 μM caused only slight viability decreases in examined cell cultures. Only 1 of the 12 investigated compounds was found to be little biologically active in vitro. We have noticed, that compound 6b evoked slight dose-dependent viability decreases in T47D, HeLa and A549 cell lines (15–20% growth inhibition was observed comparison to control). In case of HeLa cell line, except compound 6b, it was noticed that compound 6a, in the concentration of 100 and 150 μM also caused small decreases (10 and 20%, respectively) in cell viability versus control.
We have noted, that T47D cell line was the most susceptible cancer cell line, among the others, on compounds’ influencing. The slight cytotoxic activity against T47D cell line in the set of checked compounds (except compound 6b) were found also for compounds number 5e and 6a. In the concentration of 50, 100 and 150 μM, these compounds showed 15–25% growth inhibition against breast tumor cell line after 24, 48 and 72 h of incubation. The other compounds that affected on this cell line, but to a much lesser extent, were compound number 4b, 4d, 5c, 6a and 6e.
No visual changes in cell viability against TOV-112D cell line, L929 and GMK cell line during tested compound treatment, were not observed. Our study revealed that some of our synthesized compounds possess minor antiproliferative activity.
Microbiology
On the basis of the preliminary results obtained by agar plate method, it was shown that some of the newly synthesized compounds listed in have shown a potential activity against reference strains mainly of Gram-positive bacteria. On the basis of MIC estimation some compounds was found to possess good, moderate or mild bioactivity against Gram-positive bacterial species with MIC ranging from 31.25 to 500 µg/ml (). The highest activity possessed compounds 1a, 1b, 1c, 2a, 2c and 5c (MIC = 31.25–250 µg/ml). M. luteus ATCC 102740 was the most sensitive to the selected derivatives (MIC = 31.25–250 µg/ml). In our experiments, MIC of cefuroxime was 0.24–1.95 µg/ml for Staphylococcus species and 0.49–31.25 µg/ml for the other Gram-positive bacteria.
Table 3. The influence of synthesized compounds 1–6 on the growth of Gram-positive and Gram-negative bacteria of on the basis of MIC (µg/ml).
Among the tested compounds, only seven (3a, 3b, 4a, 4b, 6a, 6b and 6e) had mild or moderate inhibitory effect on the Gram-negative bacteria growth with MIC values ranging from 250 to ≥1000 µg/ml.
In general, our obtained thiosemicarbazide derivatives showed better activity as compared to cyclic compounds, what is consistent with the literature findingsCitation35. According to the literature, the Mannich base derivatives antimicrobial activity is strongly dependent on the kind of amine substituent in N2 positionCitation36, what can also be observed in our case study. Our results have shown that the tested derivatives exhibited good or moderate activity against both pathogenic (e.g. S. aureus) and opportunistic (e.g. S. epidermidis, M. luteus, B. subtilis or B. cereus) Gram-positive bacteria and may be of value for searching new derivatives showing better antimicrobial activity.
Conclusions
In the current study, we synthesized and characterized by 1H NMR, 13C NMR and elemental analyses a new series of Mannich base derivatives containing 1,2,4-triazole moiety.
Twelve of obtained compounds were analyzed for their in vitro antiproliferative acitivity against six cancer cell lines. Our study revealed that synthesized compounds 5e, 6a, 6e possess minor antiproliferative activity and some further studies in this direction need to be done.
All the newly prepared compounds were also screened for their in vitro antimicrobial activity by agar dilution technique against 11 reference strains of Gram-positive and Gram-negative bacteria. Our antimicrobial study revealed that compounds 1a, 1b, 1c, 2a, 2c and 5c had good or moderate activity against the reference Gram-positive bacteria and the compounds 3a, 3b, 4a, 4b, 6a, 6b and 6e had mild or moderate inhibitory effect on the Gram-negative bacteria growth. Obtained compounds may be regarded as precursor compounds for searching new derivatives showing better antimicrobial activity against pathogenic or opportunistic bacteria. Nowadays due to several serious problems such as growing drug resistance of bacteria or undesirable side effects of drugs it is very important to find new substances with antimicrobial activity.
Declaration of interest
The project was partially supported by the Research Grant for Young Scientists (MNmb25). The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Turan-Zitouni G, Kaplanciki ZA, Erol K, Kiliç FS. Synthesis and analgesic activity of some triazoles and triazolothiadiazines. Farmaco 1999;54:218–23
- Omar K, Geronikaki A, Zoumpoulakis P, et al. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem 2012;18:426–32
- Colin X, Sauleau A, Coulon J. 1,2,4-Triazolo mercapto and aminonitriles as potent antifungal agents. Bioorg Med Chem Lett 2003;13:2601–5
- Byrak H, Demirbas A, Demirbas N, Karaoglu SA. Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur J Med Chem 2009;44:4362–6
- Padmavathi V, Sudhakar GR, Padmaja A, et al. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur J Med Chem 2009;44:2106–12
- Al-Soud YA, Al-Dweri MN, Al-Masoudi NA. Synthesis, antitumor and antiviral properties of some 1,2,4-triazole derivatives. Farmaco 2004;59:775–83
- Salgm-Cökşen U, Gökhan-Kelekçi N, Göktaş Ö, et al. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg Med Chem 2007;15:5738–51
- Palaska E, Şahin G, Kelicen P, et al. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco 2002;57:101–7
- Shiradkar MR, Murahari KK, Gangadasu HR, et al. Synthesis of new S-derivatives of clubbed triazolyl thiazole as anti-Mycobacterium tuberculosis agents. Bioorg Med Chem 2007;15:3997–4008
- Pandeya SN, Sriram D, Nath G, DeClercq E. Synthesis, antibaceterial and anti-HIV activities of Schiff and Mannich bases derived from isation derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur J Pharm Sci 1999;9:25–31
- Almajan GL, Barbuceanu S-F, Almajan E-R, et al. Synthesis, characeteriaztion and antibaceterial acitivity of some triazole Mannich bases carrying diphenylsulfone moieties. Eur J Med Chem 2009;44:3083–9
- Idhayadhulla A, Kumar RS, Nasser AJA, et al. Synthesis of some Mannich base derivatives and their antimicrobial activity study. Arab J Chem 2011. doi: 10.1016/j.arabjc.2010.12.025
- Koparir M, Orek C, Parlak AE, et al. Synthesi and biological activities of some novel aminomethyl derivatives of 4-substituted-5-(2-thienyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Eur J Med Chem 2013;63:340–6
- Shivarama H, Sooryanarayana R, Shirdhara K, Akberali PM. Studies on arylfuran derivatives Part XI. Synthesis, characterization and biological studies on some Mannich bases carrying 2,4-dichlorophenylfurfural moiety. Farmaco 2000;55:338–44
- Karthikeyan MS, Prasad DJ, Poojary B, et al. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg Med Chem 2006;14:7482–9
- Shivarama H, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich base derived form 1,2,4-triazoles. Eur J Med Chem 2003;38:759–67
- Sujith KV, Rao JN, Shetty P, Kalluraya B. Regioselective reaction: synthesis and pharmacological study of Mannich bases containing ibuprofen moiety. Eur J Med Chem 2009;44:3697–702
- Obniska J, Rzepka S, Kamiński K. Synthesis and anticonvulsant activity of new N-Mannich bases derived form 3-(2-fluorophenyl)- and 3-(2-bromophenyl)-pyrrolidine-2,5-diones. Part II. Bioorg Med Chem 2012;20:4872–80
- Tramontini M, Agioline L, Ghedeni N. Mannich bases in polymer chemistry. Polymer 1988;29:771–88
- Jia XD, Chen XN, De Huo C, et al. Cross double Mannich reaction catalyzed by I2: synthesis of highly substituted 4-piperidones. Chin Chem Lett 2012;23:309–12
- Tramontini M, Angiolini L. Further advances in the chemistry of Mannich bases. Tetrahedron 1990;46:1791–837
- Wyrzykiewicz E, Wendzonka M, Kedzia B. Synthesis and antimicrobial activity of new (E)-4-[piperidino (4′-methylpiperidino-, morpholino-)N-alkoxy]stilbenes. Eur J Med Chem 2006;41:519–25
- Foroumadi AR, Ghodsi S, Emami S, et al. Synthesis and antibacterial activity of new fluoroquinolones containing a substituted N-(phenethyl) piperazine moiety. Bioorg Med Chem Lett 2006;16:3499–503
- Holla BS, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 2003;38:759–67
- Dimmock JR, Chamankkah M, Allen TM, Halleran S. Mannich bases of 2-arylmethylenecyclohexanone with cytotoxic activity. Pharmazie 1995;50:221–2
- Dimmock JR, Kumar P. Anticancer and cytotoxic properties of Mannich bases. Curr Med Chem 1997;4:1–22
- Popiołek Ł, Kosikowska U, Dobosz M, Malm A. Synthesis and in vitro antimicrobial activity of new 4-phenyl-5-methyl-4H-1,2,4-triazole-3-thione derivatives. J Enzym Inhib Med Chem 2013;28:479–88
- CLSI. Performance standards for antimicrobial susceptibility testing; Eighteenth International Supplement. CLSI document M7-MIC. Wayne, PA: Clinical Laboratory Standards Institute; 2008
- O’Donnell F, Smyth TJP, Ramachandran VN, Smyth WF. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int J Antimicrob Agents 2010;35:30–8
- Makovik Yu V, Knish EG, Panasenko OI. Synthesis, transformations, antimicrobial and antifungal activities of 5-(pyridin-3-yl)-4-R-1,2,4-triazole-3-thiones. Medichna Khimiya 2007;9:95–8
- Popiołek Ł, Kosikowska U, Dobosz M, Malm A. Synthesis and antimicrobial properties of new thiosemicarbazide, 1,2,4-triazole, and 1,3,4-thiadiazole derivatives of sulfanylacetic acid. Phosphorus Sulfur 2012;187:468–81
- Pitucha M, Wujec M, Dobosz M, et al. Synthesis and biological action of 1-aminomethyl derivatives of 3-R-4-phenyl-Δ2-1,2,4-triazoline-5-thione. Acta Pol Pharm 2005;62:443–9
- Siwek A, Paneth P. Computational studies of the cyclization of thiosemicarbazides. J Phys Org Chem 2007;20:463–8
- Siwek A, Wujec M, Wawrzycka-Gorczyca I, et al. Thiol-thione tautomeric forms recognition on the example of 4-[3-(2-Methyl-furan-3-yl)-5-thioxo-1,2,4-triazolin-4-yl]acetic acid. Heteroatom Chem 2008;19:337–44
- Plech T, Wujec M, Siwek A, et al. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur J Med Chem 2011;46:241–8
- Plech T, Wujec M, Majewska M, et al. Microbiologically active Mannich bases derived from 1,2,4-triazoles. The effect of C-5 substituent on antibacterial activity. Med Chem Res 2013;22:2531–7