Abstract
The synthesis of some new pyrazino[1,2-a]benzimidazole derivatives and investigation of their anticancer activities were aimed in this work. Thus, 2-acetylbenzimidazole was reacted with appropriate α-bromoacetophenones and potassium carbonate in acetone to give 2-(2-acetyl-1H-benzimidazol-1-yl)-1-phenylethanone derivatives (3a–d). These diketone compounds were reacted with varied benzylamines in acetic acid to obtain 2-benzyl-1-methylidene-3-aryl-1,2-dihydropyrazino[1,2-a]benzimidazole derivatives (4a–t). The structures of the obtained compounds were elucidated by using IR, 1H-NMR, 13C-NMR, MS spectral data and elemental analyses results. Anticancer activities of the selected compounds were investigated in National Cancer Institute, Bethesda, MD. 3c and 4n showed remarkable anticancer activity comparing with standard drugs, melphalan and cisplatin.
Introduction
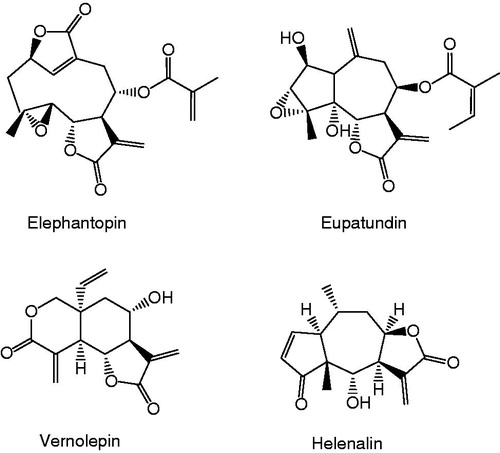
Natural and synthetic α-methylene-γ-lactone and quinone methide containing compounds are known with high anticancer activity by mechanism acting as DNA alkylating agentCitation1–5. For these compounds, it was found that one of the structural requirement for significant cytotoxicity was an O = C–C = CH2 system as part of an ester, a ketone or lactonCitation6. Natural compounds namely elephantopin, eupatundin, vernolepin and helenalin including α-methylene-γ-lactone moiety in their structure () are some of the compounds that have known with anticancer activities and also high cytoxicitiesCitation7–9. Toxic properties of these natural compounds leaded medicinal chemists to synthesize new derivatives including α,β-unsaturated carbonyl moiety as a pharmacophoric group (), which are thought to have high antitumor activity with low cytotoxicityCitation10.
Quinone methide moiety is also an important anticancer active group, which has α,β-unsaturated carbonyl structureCitation11–13. In studies, indole, benzimidazole and quinazoline rings have been used as precursors of quinone methides because of their excessive reactivityCitation14–17. Reversibly binding to nucleophilic sites on telomeric DNA via Michael addition reaction is thought to be responsible for the anticancer activity of both of α-methylene-γ-lactone and quinone methide chemical groupsCitation18–21.
In the light of these studies and as an extension of our previous works on anticancer active pyrazino[1,2-a]benzimidazole derivativesCitation22–24, we now report on the synthesis and the anticancer activity testing of some 2-benzyl-1-methylidene-3-phenyl-1,2-dihydropyrazino[1,2-a]benzimidazole derivatives.
Experimental section
Chemistry
All chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO) and Merck Chemicals (Merck KGaA, Darmstadt, Germany). Melting points were determined using an Electrothermal 9100 digital melting point apparatus (Electrothermal, Essex, UK) and were uncorrected. Spectroscopic data were recorded on the following instruments. IR: Shimadzu 8400 FTIR spectrophotometer (Shimadzu, Tokyo, Japan); 1H-NMR: Bruker DPX 500 NMR spectrometer (Bruker Bioscience, Billerica, MA), in DMSO-d6, TMS as internal standard; 13C-NMR: Bruker DPX 125 NMR spectrometer (Bruker Bioscience), in DMSO-d6MS: AB SCIEX-3200 Q-TRAP LC/MS/MS MASS spectrometer (Fisons Instruments Vertriebs GmbH, Mainz, Germany). Elemental analyses were performed on a Leco TruSpec Micro CHN/CHNS elemental analyzer (Leco, St. Joseph, MI).
2-(1-Hydroxyethyl)benzimidazole (1)
o-Phenylenediamine (100 mmol) and lactic acid (100 mmol) were refluxed in 100 mL 4 N HCl solution for 8 h. The solution was cooled, poured into ice water and neutralized with ammonia. The precipitate was filtered and crystallised from ethanol–water. Yield: 71% m.p. 178–180 °C (ref. 178.5–179.5 °C)Citation25.
2-Acetylbenzimidazole (2)
2-(1-Hydroxyethyl)benzimidazole (10 mmol) was dissolved in 100 mL acetic acid and heated to 90 °C. The solution of chromium trioxide (7.5 mmol) in 15 mL water was added dropwise to this mixture and the temperature was fixed at 90 °C. After the addition, the mixture was cooled and poured into water. The precipitate was filtered and extracted with chloroform. The solvent was evaporated at low pressure and the residue was recrystallized from toluene.
Yield: 76% m.p. 188–190 °C (ref. 188–189 °C)Citation26. IR(KBr) νmax (cm–1): 3288–2400 (–N–H), 1674 (–C = O), 1600–1420 (–C = C, –C = N). 1H-NMR (DMSO-d6) δ: (ppm) 2.71 (3H, s, –COCH3), 7.36–7.48 (2H, m, Ar-H), 7.86–8.14 (2H, m, Ar-H), 12.4 (1H, brs, N–H).
2-(2-Acetyl-1H-benzimidazol-1-yl)-1-arylethanone derivatives (3a–d)
A mixture of the appropriate 2-acetylbenzimidazole (5 mmol), 2-bromoacetophenone (5 mmol) and potassium carbonate (5 mmol) was stirred in acetone (50 mL) at room temperature. Stirring was continued at room temperature until the disappearance of the starting materials by TLC (4–6 h). The solvent was evaporated at low pressure, and the residue was washed with water and then ethanol. The raw product was recrystallized from ethanol.
2-(2-Acetyl-1H-benzimidazol-1-yl)-1-phenylethanone (3a)
Yield 75%. m.p. 167 °C (ref. 166–168 °C)Citation27. IR (KBr) νmax (cm−1): 3132–3061 (aromatic –C–H), 2937–2850 (aliphatic –C–H), 1686 and 1676 (–C = O), 1593–1446 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 2.71 (3H, s, –COCH3), 6.23 (2H, s, –CH2CO), 7.36–7.48 (2H, m, Ar-H), 7.60 (1H, m, Ar-H), 7.72–7.82 (2H, m, Ar-H), 7.92 (2H, d, J: 7.95 Hz) Ar-H), 8.13 (2H, d, J: 8.09 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δ 27.56, 52.13, 112.39, 123.03, 124.57, 126.42, 129.43, 130.36, 134.04, 135.21, 140.12, 143.36, 147.02, 193.82 and 194.10. For C17H14N2O2 calculated: 73.37% C, 5.07% H, 10.07% N; found: 73.56% C, 5.02% H, 10.21% N. MS: m/z 279 (M + 1).
2-(2-Acetyl-1H-benzimidazol-1-yl)-1-(4-methoxyphenyl)ethanone (3b)
Yield 65%. m.p. 141–142 °C (ref. 141–142 °C)Citation27 IR (KBr) νmax (cm–1): 3052–3016 (Aromatic –C–H), 2983–2851 (Aliphatic –C–H), 1689 and 1674 (–C = O), 1641–1463 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 2.71 (3H, s, –COCH3), 3.72 (3H, s, –COCH3), 6.24 (2H, s, –CH2CO), 7.15–7.24 (4H, m, Ar-H), 7.58 (2H, d, J: 8.15 Hz, Ar-H), 7.92 (2H, d, J: 8.14 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δ 27.68, 52.94, 56.06, 113.08, 123.44, 124.52, 127.29, 130.07, 132.14, 139.52, 140.63, 142.45, 147.53, 150.12, 193.69 and 194.55. For C18H16N2O3 calculated: 70.12% C, 5.23% H, 9.09% N; found: 70.16% C, 5. 25% H, 9.11% N. MS: m/z 309 (M + 1).
2-(2-Acetyl-1H-benzimidazol-1-yl)-1-(3-chlorophenyl)ethanone (3c)
Yield 72%. m.p. 151–153 °C. IR (KBr) νmax (cm–1): 3068–3020 (Aromatic –C–H), 2993–2931 (Aliphatic –C–H), 1695 and 1675 (–C = O), 1620–1452 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 2.71 (3H, s, –COCH3), 6.24 (2H, s, –CH2CO), 7.39–7.42 (1H, m, Ar-H), 7.45–7.49 (1H, m, Ar-H), 7.69 (1H, t, J: 7.88 Hz, Ar-H), 7.82 (1H, d, J: 8.24 Hz, Ar-H), 7.84–7.87 (1H, m, Ar-H), 7.91 (1H, d, J: 8.13 Hz, Ar-H), 8.06–8.09 (1H, m, Ar-H), 8.17 (1H, t, J: 1.82 Hz, J: 1.77 Hz, J: 1.81 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δ 27.98, 52.78, 112.66, 122.53, 124.90, 127.44, 128.02, 129.15, 132.28, 135.03, 135.17, 137.68, 138.19, 142.35, 147.21, 194.02 and 194.30. For C17H13ClN2O2 calculated: 65.29% C, 4.17% H, 8.96% N; found: 63.56% C, 4.02% H, 8.11% N. MS: m/z 312.9 (M + 1).
2-(2-Acetyl-1H-benzimidazol-1-yl)-1-(4-chlorophenyl)ethanone (3d)
Yield 71%. m.p. 161–162 °C (ref. 161–162 °C)Citation22. IR (KBr) νmax (cm–1): 3045–3025 (Aromatic –C–H), 2933–2833 (Aliphatic –C–H), 1695 and 1674 (–C = O), 1587–1452 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 2.73 (3H, s, –COCH3), 6.24 (2H, s, –CH2CO), 7.39–7.44 (2H, m, Ar-H), 7.70 (2H, d, J: 6.12 Hz, Ar-H), 7.96–7.97 (1H, m, Ar-H), 8.10 (2H, d, J: 8.15 Hz, Ar-H), 8.22 (1H, d, J: 7.75 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δ 27.99, 52.62, 112.68, 122.52, 124.87, 127.10, 130.37, 131.35, 134.54, 138.22, 140.29, 142.36, 147.24, 193.97 and 194.31. For C17H13ClN2O2 calculated: 65.29% C, 4.17% H, 8.96% N; found: 63.66% C, 4.05% H, 8.15% N. MS: m/z 313.1 (M + 1).
1-Methylidene-2-(4-substituted benzyl)-3-(4-substituted phenyl)pyrazino[1,2-a]benzimidazole (4a–t)
A mixture of the 3a–d (2 mmol) and an appropriate benzylamine (2 mmol) was refluxed for 10 h in acetic acid (50 mL). At the end of this time after cooling the mixture, ice water was poured into it and the aqueous mixture was neutralized with sodium carbonate. After removal of water, the obtained cohesive precipitate was crystallized from ethanol.
2-Benzyl-1-methylidene-3-phenyl-1,2-dihydropyrazino[1,2-a]benzimidazole (4a)
Yield 52%. m.p. 82–84 °C. IR (KBr) νmax (cm–1): 3057–3000 (Aromatic –C–H), 2926–2852 (Aliphatic –C–H), 1601–1471 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.64 (2H, d, J: 6.22 Hz, Ar-CH2–), 6.62 (1H, s, Ar-H), 7.13 (1H, t, J: 6.28 Hz, Ar-H), 7.23 (1H, t, J: 7.33 Hz, Ar-H), 7.32–7.38 (4H, m, Ar-H), 7.45–7.50 (5H, m, Ar-H), 7.68 (2H, d, J: 8.02 Hz, Ar-H), 7.83 (1H, d, J: 8.16 Hz, =CH2), 8.35 (1H, d, J: 8.14 Hz, =CH2), 8.64 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.58, 101.17, 112.33, 113.49, 120.12, 121.89, 126.13, 126.27, 127.75, 128.06, 128.4, 128.66, 129.63, 130.14, 131.40, 137.50, 139.04, 140.72, 143.24 and 144.64. For C24H19N3 calculated: 82.49% C, 5.48% H, 12.03% N; found: 82.44% C, 5.17% H, 12.28% N. MS: m/z 350 (M + 1).
2-(3-Methoxybenzyl)-1-methylidene-3-phenyl-1,2-dihydropyrazino[1,2-a]benzimidazole (4b)
Yield 50%. m.p. 105 °C. IR (KBr) νmax (cm–1): 3052–3024 (Aromatic –C–H), 2985–2886 (Aliphatic –C–H), 1573 – 1482 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.72 (3H, s, –CH3), 4.66 (2H, d, J: 6.02 Hz, Ar-CH2--), 6.56 (1H, s, Ar-H), 7.10 (1H, t, J: 6.33 Hz, Ar-H), 7.18 (1H, t, J: 7.22 Hz, Ar-H), 7.28–7.41 (4H, m, Ar-H), 7.45–7.53 (4H, m, Ar-H), 7.62 (1H, d, J: 7.82 Hz, Ar-H), 7.78–7.80 (1H, m, Ar-H), 7.83 (1H, d, J: 8.21 Hz, =CH2), 8.37 (1H, d, J: 8.0 Hz, =CH2), 8.67 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.82, 56.28, 102.52, 112.85, 113.68, 114.12, 115.23, 121.23, 121.87, 125.56, 126.47, 128.87, 129.79, 130.74, 131.45, 131.56, 132.74, 137.58, 139.10, 144.32 and 157.52. For C25H21N3O calculated: 79.13% C, 5.58% H, 11.07% N; found: 79.17% C, 5.61% H, 11.12% N. MS: m/z 380 (M + 1).
2-(3-Chlorobenzyl)-1-methylidene-3-phenyl-1,2-dihydropyrazino[1,2-a]benzimidazole (4c)
Yield 54%. m.p. 74–75 °C. IR (KBr) νmax (cm–1): 3055–3030 (Aromatic –C–H), 2956–2902 (Aliphatic –C–H), 1585–1479 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.65 (2H, d, J: 6.03 Hz, Ar-CH2--), 6.56 (1H, s, Ar-H), 7.25–7.55 (10H, m, Ar-H), 7.69 (2H, d, J: 8.07 Hz, Ar-H), 7.84 (1H, d, J: 8.21 Hz, =CH2), 8.36 (1H, d, J: 8.0 Hz, =CH2), 8.66 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.04, 102.52, 113.26, 114.41, 122.58, 127.23, 128.04, 129.19, 129.78, 130.03, 130.77, 131.48, 132.96, 138.46, 138.96, 139.56, 143.54 and 144.68. For C24H18ClN3 calculated: 75.09% C, 4.73% H, 10.95% N; found: 75.24% C, 4.77% H, 11.10% N. MS: m/z 383.6 (M + 1).
2-(4-Methoxybenzyl)-1-methylidene-3-phenyl-1,2-dihydropyrazino[1,2-a]benzimidazole (4d)
Yield 63%. m.p. 102–103 °C. IR (KBr) νmax (cm–1): 3063–3015 (Aromatic –C–H), 2956–2898 (Aliphatic –C–H), 1554–1469 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ 3.71 (ppm) (3H, s, –CH3), 4.56 (2H, d, J: 6.25 Hz, Ar-CH2–), 6.57 (1H, s, Ar-H), 6.90 (2H, d, J: 8.72 Hz, Ar-H), 7.02 (1H, t, J: 6.28 Hz, Ar-H), 742–7.34 (4H, m, Ar-H), 7.47–7.50 (3H, m, Ar-H), 7.71 (2H, d, J: 7.38 Hz, Ar-H), 7.83 (1H, d, J: 8.17 Hz, =CH2), 8.35 (1H, d, J: 8.16 Hz, =CH2), 8.64 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): 46.06, 56.25, 101.84, 113.48, 114.56, 122.87, 127.48, 128.64, 129.42, 129.89, 130.21, 130.79, 131.86, 132.74, 138.63, 138.97, 139.82, 143.43, 146.54 and 160.10. For C25H21N3O calculated: 79.13% C, 5.58% H, 11.07% N; found: 79.19% C, 11.03% H, 11.08% N. MS: m/z 380 (M + 1).
2-(4-Chlorobenzyl)-1-methylidene-3-phenyl-1,2-dihydropyrazino[1,2-a]benzimidazole (4e)
Yield 65%. m.p. 104–106 °C. IR (KBr) νmax (cm–1): 3057–3032 (Aromatic –C–H), 2893–2845 (Aliphatic –C–H), 1562–1481 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.64 (2H, d, J: 6.38 Hz, Ar-CH2--), 6.53 (1H, s, Ar-H), 7.22 (1H, t, J: 6.48 Hz, Ar-H), 7.35–7.40 (4H, m, Ar-H), 7.46–7.50 (5H, m, Ar-H), 7.69 (2H, d, J: 7.73 Hz, Ar-H), 7.83 (1H, d, J: 8.18 Hz, = CH2), 8.35 (1H, d, J: 8.10 Hz, = CH2), 8.66 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.74, 101.16, 112.47, 113.50, 121.92, 126.15, 127.78, 128.68, 129.56, 130.15, 130.27, 131.39, 132.53, 137.35, 138.98, 139.89, 143.20 and 144.64. For C24H18ClN3 calculated: 75.09% C, 4.73% H, 10.95% N; found: 74.95% C, 5.02% H, 10.68% N. MS: m/z 383.7 (M + 1).
2-Benzyl-1-methylidene-3-(4-methoxyphenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4f)
Yield 51%. m.p. 136 °C. IR (KBr) νmax (cm–1): 3063–3012 (Aromatic C–H), 2923–2876 (Aliphatic C–H), 1574–1486 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.81 (3H, s, –CH3), 4.64 (2H, d, J: 6.20 Hz, Ar-CH2–), 6.50 (1H, s, Ar-H), 7.03 (2H, d, J: 8.63 Hz, Ar-H), 7.09 (1H, t, J: 6.30 Hz, Ar-H), 7.23 (1H, t, J: 7.17 Hz, Ar-H), 7.32–7.37 (3H, m, Ar-H), 7.46–7.49 (3H, m, Ar-H), 7.61 (2H, d, J: 8.59 Hz, Ar-H), 7.82 (1H, d, J: 8.18 Hz, =CH2), 8.34 (1H, d, J: 7.11 Hz, =CH2), 8.57 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): 46.07, 55.66, 102.41, 113.26, 115.23, 122.63, 128.42, 128.76, 129.02, 129.89, 130.22, 130.65, 131.49, 132.46, 136.54, 137.86, 138.72, 139.12, 143.57, 146.86 and 157.46. For C25H21N3O calculated: 79.13% C, 5.58% H, 11.07% N; found: 79.18% C, 5.59% H, 11.10% N. MS: m/z 380 (M + 1).
2-(3-Methoxybenzyl)-1-methylidene-3-(4-methoxyphenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4g)
Yield 61%. m.p. 99 °C. IR (KBr) νmax (cm–1): 3046–3021 (aromatic –C–H), 2964–2859 (aliphatic –C–H), 1565–1446 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.71 (3H, s, –CH3), 3.81 (3H, s, –CH3), 4.60 (2H, d, J: 6.20 Hz, Ar-CH2--), 6.52 (1H, s, Ar-H), 6.80 (1H, d, J: 8.81 Hz, Ar-H), 7.03–7.10 (5H, m, Ar-H), 7.25 (1H, t, J: 7.80 Hz, Ar-H), 7.35 (1H, t, J: 7.40 Hz, Ar-H), 7.48 (1H, t, J: 7.64 Hz, Ar-H), 7.62 (2H, d, J: 8.46 Hz, Ar-H), 7.82 (1H, d, J: 8.20 Hz, =CH2), 8.33 (1H, d, J: 8.00 Hz, = CH2), 8.57 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.03, 55.69, 56.33, 102.40, 113.32, 113.46, 114.68, 121.69, 121.95, 123.44, 125.27, 126.75, 127.14, 127.76, 128.72, 130.61, 132.57, 135.24, 137.48, 141.54, 143.66, 145.58, 158.22 and 159.12. For C26H23N3O2 calculated: 76.26% C, 5.66% H, 10.26% N; found: 76.23% C, 5.69% H, 10.20% N. MS: m/z 410 (M + 1).
2-(3-Chlorobenzyl)-1-methylidene-3-(4-methoxyphenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4h)
Yield 61%. m.p. 91–92 °C. IR (KBr) νmax (cm–1): 3061–3028 (Aromatic –C–H), 2961–2877 (Aliphatic –C–H), 1563–1486 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.81 (3H, s, –CH3), 4.65 (2H, d, J: 6.32 Hz, Ar-CH2–), 6.56 (1H, s, Ar-H), 7.04 (2H, d, J: 8.55 Hz, Ar-H), 7.22 (1H, t, J: 6.37 Hz, Ar-H), 7.28–7.38 (3H, m, Ar-H), 7.44–7.50 (2H, m, Ar-H), 7.57 (1H, m, Ar-H), 7.62 (2H, d, J: 8.55 Hz, Ar-H), 7.83 (1H, d, J: 8.18 Hz, =CH2), 8.34 (1H, d, J: 8.15 Hz, =CH2), 8.58 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.54, 57.45, 101.54, 110.62, 113.49, 115.89, 120.24, 121.46, 125.48, 126.36, 128.42, 129.12, 130.34, 131.45, 131.86, 132.85, 137.69, 139.73, 143.14, 144.37 and 159.46. For C25H20ClN3O calculated: 72.55% C, 4.87% H, 10.15% N; found: 72.52% C, 4.79% H, 10.10% N. MS: m/z 413.6 (M + 1).
2-(4-Methoxybenzyl)-1-methylidene-3-(4-methoxyphenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4i)
Yield 55%. m.p. 148–149 °C. IR (KBr) νmax (cm–1): 3076–3012 (Aromatic –C–H), 2917–2837 (aliphatic –C–H), 1549–1453 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.71 (3H, s, –CH3), 3.81 (3H, s, –CH3), 4.55 (2H, d, J: 6.10 Hz, Ar-CH2--), 6.53 (1H, s, Ar-H), 6.90 (2H, d, J: 8.40 Hz, Ar-H), 6.98 (1H, t, J: 7.60 Hz, Ar-H), 7.05 (2H, d, J: 8.49 Hz, Ar-H), 7.35 (1H, t, J: 7.33 Hz, Ar-H), 7.40 (2H, d, J: 8.17 Hz, Ar-H), 7.47 (1H, t, J: 7.58 Hz, Ar-H), 7.64 (2H, d, J: 8.51 Hz, Ar-H), 7.81 (1H, d, J: 8.19 Hz, =CH2), 8.33 (1H, d, J: 8.18 Hz, =CH2), 8.56 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.01, 55.79, 56.04, 101.26, 111.44, 113.43, 114.94, 115.51, 120.06, 121.73, 126.02, 128.88, 129.71, 131.35, 132.45, 137.38, 143.14, 144.61, 159.62 and 160.29. For C26H23N3O2 calculated: 76.26% C, 5.66% H, 10.26% N; found: 76.25% C, 5.71% H, 10.22% N. MS: m/z 410 (M + 1).
2-(4-Chlorobenzyl)-1-methylidene-3-(4-methoxyphenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4j)
Yield 52%. m.p. 155 °C. IR (KBr) νmax (cm–1): 3063–3016 (aromatic –C–H), 2967–2837 (aliphatic –C–H), 1569–1416 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.80 (3H, s, –CH3), 4.63 (2H, d, J: 6.33 Hz, Ar-CH2–), 6.49 (1H, s, Ar-H), 7.04 (2H, d, J: 8.58 Hz, Ar-H), 7.17 (1H, t, J: 6.34 Hz, Ar-H), 7.34–7.40 (3H, m, Ar-H), 7.46–7.50 (3H, m, Ar-H), 7.61 (2H, d, J: 8.62 Hz, Ar-H), 7.82 (1H, d, J: 8.22 Hz, =CH2), 8.33 (1H, d, J: 8.14 Hz, =CH2), 8.57 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.77, 56.03, 101.31, 111.65, 113.46, 115.51, 120.07, 121.79, 125.97, 126.04, 128.89, 129.55, 130.26, 131.23, 131.33, 132.52, 137.23, 139.91, 143.07, 144.61 and 160.31. For C25H20ClN3O calculated: 72.55% C, 4.87% H, 10.15% N; found: 72.53% C, 4.82% H, 10.13% N. MS: m/z 413.7 (M + 1).
2-Benzyl-1-methylidene-3-(3-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4k)
Yield 55%. m.p. 85–86 °C. IR (KBr) νmax (cm–1): 3059–3035 (Aromatic C–H), 2887 (aliphatic C–H), 1589–1465 (C = C, C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.66 (2H, d, J: 6.28 Hz, Ar-CH2--), 6.56 (1H, s, Ar-H), 7.13 (1H, t, J: 6.34 Hz, Ar-H), 7.23 (1H, t, J: 7.49 Hz, Ar-H), 7.32–7.44 (4H, m, Ar-H), 7.46–7.52 (4H, m, Ar-H), 7.65 (1H, d, J: 7.74 Hz, Ar-H), 7.78–7.80 (1H, m, Ar-H), 7.84 (1H, d, J: 8.23 Hz, =CH2), 8.37 (1H, d, J: 8.15 Hz, =CH2), 8.74 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): 46.24, 102.48, 113.36, 115.54, 122.28, 128.01, 128.13, 128.51, 129.04, 130.04, 130.47, 131.31, 133.52, 136.45, 137.88, 138.84, 139.17, 143.23 and 146.45. For C24H18ClN3 calculated: 75.09% C, 4.73% H, 10.95% N; found: 75.24% C, 4.97% H, 10.90% N. MS: m/z 383.7 (M + 1).
2-(3-Methoxybenzyl)-1-methylidene-3-(3-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4l)
Yield 62%. m.p. 115 °C. IR (KBr) νmax (cm–1): 3067–3012 (Aromatic –C–H), 2950–2837 (aliphatic –C–H), 1588–1451 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.71 (3H, s, –CH3), 4.62 (2H, d, J: 6.23 Hz, Ar-CH2--), 6.52 (1H, s, Ar-H), 7.35–7.52 (9H, m, Ar-H), 7.69 (1H, d, J: 7.68 Hz, Ar-H), 7.82 (1H, s, Ar-H), 7.86 (1H, d, J: 8.16 Hz, =CH2), 8.37 (1H, d, J: 8.12 Hz, =CH2), 8.74 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.01, 56.04, 101.26, 111.44, 113.43, 114.95, 115.51, 120.06, 121.73, 126.02, 128.89, 129.72, 131.35, 132.46, 137.37, 143.14, 144.61 and 159.62. For C25H20ClN3O calculated: 72.55% C, 4.87% H, 10.15% N; found: 72.50% C, 4.83% H, 10.11% N. MS: m/z 413.6 (M + 1).
2-(3-Chlorobenzyl)-1-methylidene-3-(3-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4m)
Yield 72%. m.p. 105–106 °C. IR (KBr) νmax (cm–1): 3097–3020 (aromatic C–H), 2840 (aliphatic C–H), 1591–1471 (C = C, C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.65 (2H, d, J: 6.03 Hz, Ar-CH2--), 6.56 (1H, s, Ar-H), 7.28–7.58 (9H, m, Ar-H), 7.67 (1H, d, J: 7.76 Hz, Ar-H), 7.80 (1H, s, Ar-H), 7.84 (1H, d, J: 8.26 Hz, =CH2), 8.38 (1H, d, J: 8.12 Hz, =CH2), 8.76 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.76, 101.66, 113.18, 113.60, 120.17, 122.07, 124.69, 126.27, 126.36, 127.18, 127.45, 128.01, 128.33, 128.47, 131.45, 131.49, 131.94, 134.35, 135.02, 137.37, 141.15, 143.18, 143.62 and 144.65. For C24H17Cl2N3 calculated: 68.91% C, 4.10% H, 10.05% N; found: 69.22% C, 4.07% H, 10.20% N. MS: m/z 419 (M + 1).
2-(4-Methoxybenzyl)-1-methylidene-3-(3-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4n)
Yield 64%. m.p. 88–89 °C. IR (KBr) νmax (cm–1): 3069–3029 (Aromatic –C–H), 2837 (aliphatic –C–H), 1567–1459 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.72 (3H, s, –CH3), 4.62 (2H, d, J: 6.20 Hz, Ar-CH2--), 6.54 (1H, s, Ar-H), 7.27–7.48 (9H, m, Ar-H), 7.69 (2H, d, J: 8.12 Hz, Ar-H), 7.85 (1H, d, J: 8.11 Hz, =CH2), 8.35 (1H, d, J: 8.05 Hz, =CH2), 8.65 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.96, 55.79, 101.62, 112.87, 113.55, 114.93, 120.14, 121.98, 124.76, 126.21, 126.34, 126.43, 127.44, 128.43, 129.79, 131.45, 131.96, 132.09, 132.39, 135.02, 137.55, 141.26, 143.27, 144.64 and 159.65. For C25H20ClN3O calculated: 72.55% C, 4.87% H, 10.15% N; found: 72.51% C, 4.82% H, 10.14% N. MS: m/z 414.5 (M + 1).
2-(4-Chlorobenzyl)-1-methylidene-3-(3-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4o)
Yield 60%. m.p. 106–108 °C. IR (KBr) νmax (cm–1): 3052–3016 (Aromatic –C–H), 2963–2841 (Aliphatic –C–H), 1590–1433 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.65 (2H, d, J: 6.39 Hz, Ar-CH2--), 6.55 (1H, s, Ar-H), 7.21 (1H, t, J: 6.41 Hz, Ar-H), 7.38–7.44 (4H, m, Ar-H), 7.47–7.52 (4H, m, Ar-H), 7.67 (1H, d, J: 7.74 Hz, Ar-H), 7.80 (1H, s, Ar-H), 7.84 (1H, d, J: 8.18 Hz, =CH2), 8.36 (1H, d, J: 8.17 Hz, =CH2), 8.75 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.52, 101.25, 112.68, 113.96, 121.36, 123.16, 124.75, 126.37, 126.89, 127.63, 128.29, 128.86, 128.94, 130.23, 131.53, 131.84, 133.28, 135.69, 138.21, 140.25, 142.69, 143.58 and 144.85. For C24H17Cl2N3 calculated: 68.91% C, 4.10% H, 10.05% N; found: 69.10% C, 4.27% H, 10.10% N. MS: m/z 419 (M + 1).
2-Benzyl-1-methylidene-3-(4-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4p)
Yield 54%. m.p. 174–176 °C. IR (KBr) νmax (cm–1): 3051–3012 (Aromatic –C–H), 2973–2850 (aliphatic –C–H), 1564–1469 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.64 (2H, d, J: 6.30 Hz, Ar-CH2--), 6.54 (1H, s, Ar-H), 7.14 (1H, t, J: 6.40 Hz, Ar-H), 7.23 (1H, t, J: 7.33 Hz, Ar-H), 7.33–7.39 (3H, m, Ar-H), 7.46–7.50 (3H, m, Ar-H), 7.53 (2H, d, J: 9.63 Hz, Ar-H), 7.73 (2H, d, J: 8.58 Hz, Ar-H), 7.83 (1H, d, J: 8.16 Hz, =CH2), 8.33 (1H, d, J: 8.18 Hz, =CH2), 8.70 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.69, 102.12, 113.54, 113.97, 122.89, 123.46, 124.15, 126.79, 126.87, 127.19, 128.47, 128.84, 129.04, 130.47, 131.24, 131.87, 133.47, 135.59, 138.76, 140.33, 142.72, 143.14 and 144.46. For C24H18ClN3 calculated: 75.09% C, 4.73% H, 10.95% N; found: 75.20% C, 4.57% H, 10.78% N. MS: m/z 383.7 (M + 1).
2-(3-Methoxybenzyl)-1-methylidene-3-(4-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4q)
Yield 59%. m.p. 118 °C. IR (KBr) νmax (cm–1): 3058–3029 (aromatic –C–H), 2964–2863 (aliphatic –C–H), 1586–1451 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.72 (3H, s, –CH3), 4.64 (2H, d, J: 6.72 Hz, Ar-CH2--), 6.52 (1H, s, Ar-H), 7.17 (2H, d, J: 8.45 Hz, Ar-H), 7.26 (1H, t, J: 6.75 Hz, Ar-H), 7.25–7.34 (3H, m, Ar-H), 7.48–7.54 (2H, m, Ar-H), 7.59 (1H, m, Ar-H), 7.64 (2H, d, J: 8.45 Hz, Ar-H), 7.88 (1H, d, J: 8.13 Hz, =CH2), 8.37 (1H, d, J: 8.19 Hz, =CH2), 8.71 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.93, 56.08, 101.22, 113.12, 113.85, 114.76, 121.25, 121.89, 123.65, 125.47, 126.89, 127.12, 127.82, 128.13, 129.65, 130.79, 132.02, 132.53, 135.22, 137.14, 141.13, 143.56, 144.48 and 158.74. For C25H20ClN3O calculated: 72.55% C, 4.87% H, 10.15% N; found: 72.49% C, 4.83% H, 10.17% N. MS: m/z 413.6 (M + 1).
2-(3-Chlorobenzyl)-1-methylidene-3-(4-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4r)
Yield 62%. m.p. 191–193 °C. IR (KBr) νmax (cm–1): 3056–3021 (aromatic –C–H), 2937–2864 (aliphatic C–H), 1557–1450 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.66 (2H, d, J: 6.42 Hz, Ar-CH2--), 6.53 (1H, s, Ar-H), 7.24 (1H, t, J: 6.51 Hz, Ar-H), 7.36–7.40 (3H, m, Ar-H), 7.48–7.55 (3H, m, Ar-H), 7.54 (2H, d, J: 8.51 Hz, Ar-H), 7.74 (2H, d, J: 8.63 Hz, Ar-H), 7.84 (1H, d, J: 8.19 Hz, =CH2), 8.35 (1H, d, J: 8.18 Hz, =CH2), 8.70 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 46.22, 101.54, 112.75, 113.41, 121.24, 122.52, 124.46, 126.78, 127.13, 128.28, 128.76, 129.38, 131.89, 132.67, 132.83, 134.52, 135.29, 137.58, 141.29, 143.69, 143.92 and 144.46. For C24H17Cl2N3 calculated: 68.91% C, 4.10% H, 10.05% N; found: 68.66% C, 4.35% H, 10.20% N. MS: m/z 419 (M + 1).
2-(4-Methoxybenzyl)-1-methylidene-3-(4-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4s)
Yield 63%. m.p. 146 °C. IR (KBr) νmax (cm–1): 3059–3012 (Aromatic –C–H), 2954–2897 (aliphatic –C–H), 1565–1413 (–C = C, –C = N). 1H-NMR (DMSO-d6): δ (ppm) 3.71 (3H, s, –CH3), 4.55 (2H, d, J: 8.21 Hz, Ar-CH2--), 6.56 (1H, s, Ar-H), 6.89 (2H, d, J: 8.58 Hz, Ar-H), 7.03 (1H, t, J: 8.24 Hz, Ar-H), 7.39–7.41 (3H, m, Ar-H), 7.48 (1H, t, J: 7.4 Hz, Ar-H), 7.54 (2H, d, J: 8.46 Hz, Ar-H), 7.75 (2H, d, J: 8.48 Hz, Ar-H), 7.85 (1H, d, J: 8.17 Hz, =CH2), 8.34 (1H, d, J: 8.14 Hz, =CH2), 8.68 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.87, 55.85, 101.54, 113.64, 113.89, 114.77, 120.58, 121.54, 125.63, 126.57, 127.56, 128.94, 129.96, 131.62, 132.05, 132.86, 133.29, 135.54, 137.73, 141.69, 143.12, 144.44 and 158.98. For C25H20ClN3O calculated: 72.55% C, 4.87% H, 10.15% N; found: 72.48% C, 4.85% H, 10.12% N. MS: m/z 413.6 (M + 1).
2-(4-Chlorobenzyl)-1-methylidene-3-(4-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole (4t)
Yield 68%. m.p. 213–215 °C. IR (KBr) νmax (cm–1): 3041 (aromatic C–H), 2897 (aliphatic C–H), 1587–1460 (C = C, C = N). 1H-NMR (DMSO-d6): δ (ppm) 4.64 (2H, d, J: 6.42 Hz, Ar-CH2--), 6.53 (1H, s, Ar-H), 7.24 (1H, t, J: 6.51 Hz, Ar-H), 7.36–7.40 (3H, m, Ar-H), 7.47–7.51 (3H, m, Ar-H), 7.54 (2H, d, J: 8.76 Hz, Ar-H), 7.74 (2H, d, J: 8.63 Hz, Ar-H), 7.84 (1H, d, J: 8.19 Hz, =CH2), 8.35 (1H, d, J: 8.18 Hz, =CH2), 8.70 (1H, s, pyrazinobenzimidazole C4–H). Citation13C NMR (125 MHz, DMSO-d6): δ 45.69, 101.46, 112.89, 113.45, 120.46, 122.68, 124.69, 127.21, 127.78, 128.57, 128.89, 129.01, 131.63, 131.79, 132.21, 134.46, 135.34, 137.42, 141.63, 143.52, 143.88 and 144.17. For C24H17Cl2N3 calculated: 68.91% C, 4.10% H, 10.05% N; found: 68.75% C, 3.92% H, 9.90% N. MS: m/z 419 (M + 1).
Anticancer activity tests
The cytotoxic and/or growth inhibitory effects of the compounds were evaluated in vitro against approximately 60 human tumor cell lines derived from nine neoplastic diseases namely leukemia (L, four or six cell lines), non-small cell lung cancer (NSCLC, nine cell lines), colon cancer (CC, seven cell lines), central nervous system cancer (CNSC, six cell lines), melanoma (M, eight or nine cell lines), ovarian cancer (OC, six or seven cell lines), renal cancer (RC, eight cell lines), prostate cancer (PC, two cell lines) and breast cancer (BC, six or eight cell lines). The evaluation of anticancer activity was performed at the National Cancer Institute (NCI) of Bethesda, MD, USA, following the in vitro screening program at 10-fold dilutions of five concentrations ranging from 10–4 to 10–8 M. The percentage growth was evaluated spectrophotometrically versus controls not treated with test agents. A 48 h continuous drug exposure protocol was followed and a sulforhodamine B protein assay was used to estimate cell viability of growth. Three dose response parameters (GI50, TGI and LC50) were calculated for each experimental agentCitation28–31.
Results and discussions
Chemistry
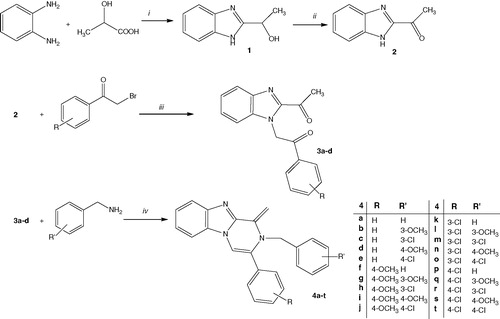
In this study, the syntheses of the new pyrazinobenzimidazoles (4a–t), have been carried out as shown in . First, o-phenylenediamine and lactic acid were reacted with 4 N HCl solution according to Phillips method to give 2-(1-hydroxyethyl)benzimidazole. The obtained compound (1) was oxidized with chromium trioxide to give 2-acetyl benzimidazole (2) compound, which was then reacted with appropriate α-bromoacetophenones in the presence of potassium carbonate. IR, 1H-NMR and Citation13C-NMR spectra were measured for the compounds 2 and 3a–d. In the spectrum of the compound 2, bands were observed at about 3288–2400 cm–1 and 1674 cm–1 belonging to –N–H and –C = O bonds, respectively. Proton of the benzimidazole nitrogen was observed at 12.4 ppm as broad singlet peak as expected. In the spectrum of the compounds 3a–d, carbonyl bands were observed 1695–1674 cm–1 and methylene protons were observed at about 6.23–6.24 ppm. At the final step, 2-(2-acetyl-1H-benzimidazol-1-yl)-1-(subtituted phenyl)ethanone derivatives (3a–d) and varied benzylamines were refluxed in acetic acid to afford corresponding 2-(4-substituted benzyl)-1-methylidene-3-(4-substituted phenyl)pyrazino[1,2-a]benzimidazole (4a–t) derivatives. The resulting products were yielded in the range of 50–72%. In the IR spectra of the final compounds (4a–t), all bands were observed in expected areas and in addition disappearance of stretching bands about 1695–1674 cm–1 belonging to ketone carbonyl was an evidence for ring closure analyzed by IR spectroscopy. In the 1H-NMR spectra of the final compounds, doublet peak was observed at 4.64–4.66 ppm belonging to –N–CH2 protons and the resonating of these protons as doublet was thought to be due to magnetic anisotropy. The peaks seen at 7.83–8.38 ppm were assigned for methylene protons in the first position of pyrazinobenzimidazole ring system. The signal belonging to the C4–H proton of the pyrazinobenzimidazole condensed ring system was observed much further downfield at about 8.64–8.75 ppm according to the other aromatic protons. The other characteristic aromatic protons were observed at expected areas, about 6.52–7.80 ppm. In the Citation13C NMR spectra of the compounds 3a–d, the characteristic peaks were seen at about 193.82–194.55 ppm and 27.56–27.99 ppm belonging to –C = O and –CH3 groups carbon. Peaks at about 45.54–46.82 ppm and 101.16–102.52 ppm belonging to –CH2– and =CH– carbon atoms were also seen in spectra of the final compounds (4a–t). The mass spectra of the compounds showed [M + 1] peaks, in agreement with their molecular weight. All compounds gave satisfactory elemental analysis results in correlation with the calculations. During the laboratory work, another similar compound 2-(2-acetyl-1H-benzimidazol-1-yl)-1-(3-methoxyphenyl)ethanone was synthesized but 2-(substituted benzyl)-1-methylidene-3-(3-methoxyphenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole derivatives which are planned to synthesize from this diketone compound could not be obtained purely. So, the indicated compounds did not include to this study.
Scheme 1. The synthetic protocol of the compounds 3a–d and 4a–t.
The synthetic protocol of the compounds (4a–t). Reagents: (i) 4N HCl, reflux, 8 h, 71%; (ii) CrO3, CH3COOH, 90 °C during the addition of chromium trioxide solution addition then cooled, 76%; (iii) K2CO3, acetone, rt, 4–6 h, 65–75%; (iv) CH3COOH, reflux, 10 h, 50–72%.
Anticancer activity evaluation
All final compounds (4a–t) and new diketone compound 3c were submitted to NCI for testing their anticancer activity according to in vitro drug screening protocol of the institute. The screening is a two-stage process, beginning with the evaluation of all compounds against the 60 cell lines at a single dose. 3c (NSC 748533), 4d (NSC 748537), 4e (NSC 748536), 4f (NSC 748535), 4i (NSC 748540), 4j (NSC 748539), 4n (NSC 748541), 4p (NSC 748534) and 4s (NSC 748538) were selected by NCI for the anticancer tests for single dose testing. In vitro single dose anticancer assay was performed in full NCI 60 cell panel representing L, NSCLC, CC, CNSC, M, OC, RC, PC and BC. In accordance with the protocol of the NCI, test results were determined as growth percent values at 10–5 M concentration of the tested compounds. Results for each compound were reported as a mean graph of the percent growth of the treated cells when compared to the untreated control cells. The obtained growth percent values of the selected nine compounds were depicted in .
Table 1. Sixty human tumor cell lines’ anticancer screening data at single dose assay as percent cell growth promotion of selected compounds.
As can be seen from the , according to the mean values 3c including 3-chloro phenyl moiety and 4n derived from 3c including 3-chloro phenyl and 4-methoxy benzyl moieties possessed significant low growth percentages among the other tested compounds, which were found 24.53% and 22.62% (mean values), respectively. Among all of the cell lines, L cells were the most susceptible cells against all tested compounds. Compounds 3c and 4n had the lowest growth percentages, which were −7.27% and −0.37% (mean values) against L cells. Among the L cells, HL-60 (TB), SR and RPMI-8236 cell lines were defined as the most susceptible cell lines against the compounds 3c and 4n. NCI-H522 was found to be the most sensitive cell line through NSCLC cells with the −15.98% and −10.93% growth percentages against 3c and 4n compounds. The pointed two compounds induced growth percentage below 13% on HCT-116 and KM12 cell lines through CC cells. Against CNSC cell lines, 3c and 4n caused 8.27% and 10.72% growth percentages, and among these cell lines and among CNSC cancer cell lines and also all cell lines SF-539 was found to be one of most sensitive cell line with a growth value of −31.29%. The active two compounds were brought about −32.33 to 90.58% growth percentage against M cell lines. The highest activity was seen against MDA-MB-43 cell line, which was a M cancer cell line by compound 4n with a value of −32.33%. Compounds 3c and 4n caused −31.30, −28.13% growth percentage against OVCAR-3 and 4p caused −8.06% growth percentage against OVCAR-4, which were OC cell lines. Tested compounds showed the lowest activity to the RC cell lines with the growth percentages between 2 and 155.99% among all tested cancer cell lines. As well as other types of cancer, compounds 3c and 4n was found to be the most active compounds with the respective values of 5.27 and 15.29% against PC. BT-549 and T-47D cell lines were found to be the most sensitive cells through BC.
Compounds 3a, 3b and 3d were synthesized, and 3a and 3d were investigated for their anticancer activities in our previous studyCitation22. In addition similar diketone compounds and ring closure products obtained from these diketone compounds were studied and anticancer activities of diketone compounds were found to be higher than final compounds. This situation supports the finding that intermediate diketone compound 3b had significant activity compared with final products in this study. But increasingly, final compound 4n synthesized from 3c also showed as higher activity as 3c, in contrast to other studiesCitation22–24. Other final products were found to be inactive with growth percentages greater than 80%. Trying to explain the situation of the substituent effect is needed but anticancer test assay was not performed for all compounds due to NCI protocol. However, according to obtained test results, 4-methoxybenzyl moiety on the second position pyrazinobenzimidazole condensed ring system has increased activity a little bit with respect to chloro substitution. Another explanation is needed for the lack of the activity of the final compounds except 4n when evaluated older studies. According to earlier studies, 2-phenyl-attached pyrazinobenzimidazole compounds showed good anticancer activity, but in this study, the new compounds reported to have a benzyl group attached at the same position but the activity shown is less than the former compounds. This situation can be related with disconnecting of methylene group (=CH–) at the first position of the pyrazinobenzimidazole, which is considered to be the active part of the molecule and binds to biological receptor. Because of the molecular structure of benzyl residue (conformations); alkylation reaction of the methylene group with the nucleophilic sites of DNA can be avoided and so activity is lacked.
In the second stage of the anticancer screening tests, five-dose assay was performed to the selected compounds against same 60 cell line at five different concentrations. The compounds that reduced the growth of the cell lines to 32% or less (negative number indicate kills) were considered in vitro activeCitation32. Compounds 3c and 4n satisfied pre-determined threshold growth inhibition criteria and further selected for NCI full panel five-dose assay at 10-fold dilutions of five different concentrations (0.01, 0.1, 1, 10 and 100 µM) Dose response curves obtained from the NCI’s in vitro disease-oriented human tumor cells line of compounds 3c and 4n on nine cancer disease at five concentrations were shown in and . The results of tested compounds were also given by three response parameters (GI50, TGI and LC50) for each cell line from log concentration of % growth inhibition curves on nine cancer diseases (). The GI50 value (growth inhibitory activity) corresponds to the concentration of the compound causing 50% decrease in net cell growth, the TGI value (cytostatic activity) is the concentration of the compound resulting in total growth inhibition and LC50 value (cytotoxic activity) is the concentration of the compound causing net 50% loss of initial cells at the end of the incubation period of 48 h. A mean graph midpoint (MG-MID) was calculated for each subpanels of cancer types for the tested compounds and standard drugs cisplatin and melphalan, which are two of the commonly used chemotherapeutic agents by giving log10GI50, log10TGI and log10LC50 (). The test method states that the compounds having log10GI50 values greater than −4 were considered as inactive. It can be seen that both of the compounds log10GI50 values are smaller than −4. The title compounds under investigation (3c and 4n) exhibited remarkable anticancer activity against all the tested cell lines representing nine different subpanels with log10GI50 values between −7.61 and −5.18 for 3c and −6.70 and −4.81 for 4n. MDA-MB-43 (M cell line) and HL-60 (TB) (L cell line) were found to be the most sensitive cell lines against compounds 3c and 4n with log10GI50 values of −7.61 and −6.70, respectively. According to subpanel average log10GI50 values, L cancer was found to be the most sensitive cancer type against both of the tested compounds 3c and 4n. Compound 3c possessed a value of log10GI50 −6.64, which was smaller than standard drugs values (log10GI50-melphalan: −5.48, log10GI50-cisplatin: −6.39) against L. Furthermore, according to (all cancer types) subpanel average log10GI50 values, both of the two compounds possessed smaller values than melphalan. Therefore, we may conclude that both of our compounds under investigation provide a notable activity level compared with standard drugs.
Figure 3. Dose response curves (% growth verses sample concentration at NCI fixed protocol, µM) obtained from the NCI’s in vitro disease-oriented human tumor cells line of compound (3c) on nine cancer diseases.
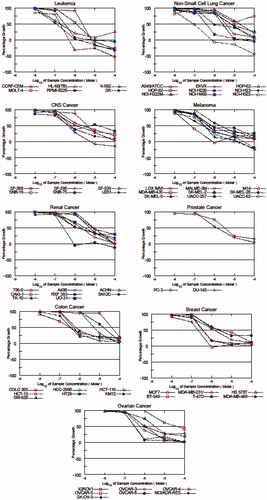
Figure 4. Dose response curves (% growth verses sample concentration at NCI fixed protocol, µM) obtained from the NCI’s in vitro disease-oriented human tumor cells line of compound (4n) on nine cancer diseases.
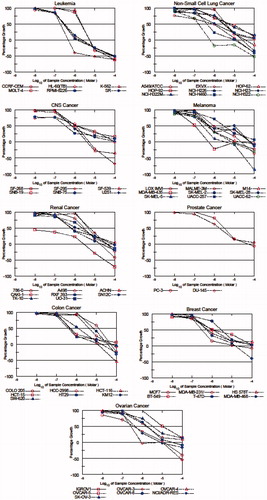
Table 2. NCI DTP in vitro testing results of compounds 3c and 4n at five-dose assay.
Table 3. Antiproliferative activities of the compounds (log10GI50).
Conclusions
Twenty 2-benzyl-1-methylidene-3-aryl-1,2-dihydropyrazino[1,2-a]benzimidazole derivatives (4a–t) were synthesized from four different diketone compounds (3a–d). The obtained final compounds (4a–t) and diketone compounds (3a–d) were offered to American NCI. Anticancer activities of the selected compounds were evaluated in vitro against approximately 60 human cell lines derived from nine neoplastic diseases, and the results were given as growth percentage values. Compound 3c namely 2-(2-acetyl-1H-benzimidazol-1-yl)-1-(3chlorophenyl)ethanone and compound 4n derived from 3c namely 2-(4-methoxybenzyl)-1-methylidene-3-(3-chlorophenyl)-1,2-dihydropyrazino[1,2-a]benzimidazole were found to be as the most active compounds with the lowest growth percentages 24.53 and 22.62%, respectively. In addition, log10GI50 value (log10 of molar sample concentration resulting in 50% growth inhibition) of the (MG-MID) compound 3c was found to be −6.32, which was smaller than anticancer drug cisplatin (log10GI50: −6.20) and log10GI50 value of the compound 4n was found to be −5.87, which was smaller than anticancer drug melphalan (log10GI50: −5.09). In connection with the result of this work and earlier works, we plan to synthesize novel compounds including diketone substructure on the first and/or second position of benzimidazole ring in further anticancer drug design studies.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
The authors thank NCI (USA) and Anadolu University BIBAM (Türkiye) for anticancer test results and NMR spectra, respectively.
References
- Li X, Weng L, Gao X, et al. Antiproliferative and apoptotic sesquiterpene lactones from Carpesium faberi. Bioorg Med Chem Lett 2011;21:366–72
- Siriwana D, Naruse T, Tamura H. Effect of epoxides and α-methylene-γ-lactone skeleton of sesquiterpenes from yacon (Smallanthus sonchifolius) leaves on caspase-dependent apoptosis and NF-κB inhibition in human cercival cancer cells. Fitoterapia 2011;82:1093–101
- Ma G, Chong L, Li Z, et al. Anticancer activities of sesquiterpene lactones from Cyathocline purpurea in vitro. Cancer Chemother Pharmacol 2009;64:143–52
- Basarić N, Cindro N, Bobinac D, et al. Sterically congested quinone methides in photodehydration reactions of 4-hydroxybiphenyl derivatives and investigation of their antiproliferative activity. Photochem Photobiol Sci 2011;10:1910–25
- Zhang Y, Tu Y, Gao X, et al. Strong inhibition of celastrol towards UDP-glucuronosyl transferase (UGT) 1A6 and 2B7 indicating potential risk of UGT-based herb-drug interaction. Molecules 2012;17:6832–9
- Lee KH, Ibuka T, Kim SH, et al. Antitumor agents. 16. Steroidal .alpha.-methylene-.gamma.-lactones. J Med Chem 1975;18:812–17
- Hernandez R, Velazquez SM, Suarez E. Synthesis of (+)-8-deoxyvernolepin. J Org Chem 1994;59:6395–403
- Howie GA, Manni PE, Cassady JM. Synthesis of alkyl-substituted α,β-unsaturated γ-lactones as potential antitumor agents. J Med Chem 1974;17:840–3
- Kupchan SM, Eakin MA, Thomas AM. Tumor inhibitores. 69. Structure–cytotoxicity relationships among the sesquiterpene lactones. J Med Chem 1971;14:1147–52
- Rosowsky A, Papathanasopoulos N, Lazarus H, et al. Cysteine scavengers. 2. Synthetic α-methylenebutyrolactones as potential tumor inhibitors. J Med Chem 1974;17:672–6
- Lin AJ, Cosby LA, Shansky CW, Sartorelli AC. Potential bioreductive alkylating agents. 3. Synthesis and antineoplastic activity of acetoxymethyl and corresponding ethyl carbamate derivatives of benzoquinones. J Med Chem 1974;17:558–61
- Lin AJ, Pardini RS, Lillis BJ, Sartorelli AC. Potential bioreductive alkylating agents. 4. Inhibition of coenzyme Q enzyme systems by lipoidal benzoquinone and naphthoquinone derivatives. J Med Chem 1974;17:668–72
- Moore HW. Bioactivation as a model for drug design bioreductive alkylation. Science 1977;197:527–32
- Lee CH, Skibo EB. Active-site-directed reductive alkylation of xanthine oxidase by imidazo[4,5-g]quaninazoline-4,9-diones functionalized with a leaving group. Biochem 1987;26:7355–62
- Lemus RH, Lee CH, Skibo EB. Studies of extended quinone methides. Synthesis and physical studies of purine-like monofunctional and bifunctional imidazo[4,5-g]quinazoline reductive alkylating agents. J Org Chem 1989;54:3611–18
- Lemus RH, Skibo EB. Studies of extended quinone methides. Design of reductive alkylating agents based on the quinazoline ring system. J Org Chem 1988;53:6099–105
- Skibo EB. Studies of extended quinone methides. The hydrolysis mechanism of 1-methyl-2-(bromomethyl)-4,7-dihydroxybenzimidazole. J Org Chem 1986;51:522–7
- Pratt WB, Ruddon RW, Ensminger WD. (1994). The anticancer drugs. Part 2. New York: Oxford University Press
- Rajski SC, Williams RM. DNA cross-linking agents as antitumor drugs. Chem Rev 1998;98:2723–95
- Doria F, Nadai M, Folini M, et al. Hybrid ligand–alkylating agents targeting telomeric G-quadruplex structures. Org Biomol Chem 2012;10:2798–806
- Avendaño C, Menendez JC. Medicinal chemistry of anticancer drugs. Hungary: Elsevier; 2008. Chapter 6
- Demirayak S, Abu Mohsen U. Anticancer and anti-HIV activities of some pyrido/pyrazino-benzimidazole derivatives. Acta Pharm Turc 1998;40:9–12
- Demirayak S, Mohsen UA, Karaburun AC. Synthesis and anticancer and anti-HIV testing of some pyrazino[1,2-a]benzimidazole derivatives. Eur J Med Chem 2002;37:255–60
- Demirayak S, Kayagil I, Yurttas L. Microwave supported synthesis of some novel 1,3-diarylpyrazino[1,2-a]benzimidazole derivatives and investigation of their anticancer activities. Eur J Med Chem 2011;46:411–16
- Siegart WR, Day AR. Metabolit analogs. VII. Preparation of some benzimidazolyl analogs of ethyl pteroylglutamate. J Am Chem Soc 1957;79:4391–4
- Cheeseman GW. 2-Acetylbenzimidazole. J Chem Soc 1964;4645–6
- Demirayak S, Güven K. Synthesis of some pyrido- and pyrazino-benzimidazole derivatives and their antifungal activity. Pharmazie 1995;50:527–9
- Boyd MR. Status of the NCI preclinical antitumor drug discovery screen. Princip Prac Oncol 1989;3:1–12
- Monks A, Scudiero D, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a divers panel of cultured human tumor cell. J Natl Cancer Inst 1991;83:757–66
- Boyd MR, Paull KD. Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev Res 1995;34:91–109
- Husain A, Rashid M, Shaharyar M, et al. Benzimidazole clubbed with triazolo-thiadiazoles and triazolo-thiadiazines: new anticancer agents. Eur J Med Chem 2013;62:785–98
- Kode N, Chen L, Murthy D, et al. New bis-N9-(methylphenylmethyl)purine derivatives: synthesis and antitumor activity. Eur J Med Chem 2007;42:327–33