Abstract
The collagen tripeptide fragments Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp were generated by hydrolyzing collagen from pig-skin, cattle-skin, fish-scales and chicken-feet, respectively, with Streptomyces collagenase. Collagenase treatment increased the concentration of tripeptides in the hydrolysates by 13–15% (w/w). Of the three peptides, Gly-Pro-Hyp was a true peptidic inhibitor of dipeptidylpeptidase-IV (DPP-IV), because DPP-IV could not hydrolyze the bond between Pro-Hyp. This tripeptide was a moderately competitive inhibitor (Ki = 4.5 mM) of DPP-IV, and its level in the collagen hydrolysates could be greatly increased (4–9% [w/w]) using Streptomyces collagenase.
Introduction
The Japanese Ministry of Health, Labor, and Welfare published a report on health and nourishment in 2007 according to which 22.1 million people are estimated to suffer from diabetes mellitus or are at risk to develop this condition. The incidence rate presented in this report was 1.2 times that reported in the previous decade, indicating an upward trend in the incidence of this disease.
The ubiquitously expressed enzyme dipeptidylpeptidase IV (EC 3.4.14.5, DPP-IV) modulates the biological activity of circulating peptide hormones by specifically cleaving the two N-terminal amino acids X-Pro and X-AlaCitation1, and also metabolizes insulinotropic hormone glucagon-like peptide-1 (GLP-1). Recently, DPP-IV inhibitors that protect active GLP-1 from being cleaved by DPP-IV have been used to control postprandial glycemia in type 2 diabetesCitation2,Citation3, and show efficacy in preventing and treating type 2 diabetes.
Collagens and their hydrolysates are interesting biomolecules with numerous commercial applications in the food, pharmaceutical, photographic film and cosmetic industries. In mature collagens, glycine and proline account for more than 20% of the total amino acids. The proline content in collagens is unusually high when compared to that in other natural proteins. The polypeptide chains of collagen molecules are composed of numerous repeats of a tripeptide unit, Gly-X-Y, where X and Y are generally proline and hydroxyproline. The Gly-Pro-Hyp tripeptide (10.5%) was most highly represented of the various tripeptide units; the other tripeptides include Gly-Pro-Ala, Gly-Ala-Hyp and Gly-Leu-Hyp (3.4–5.5%)Citation4. Gly-Pro-Ala is a known DPP-IV inhibitorCitation5. Therefore, we investigated whether the other collagen tripeptide fragments Gly-Pro-X and Gly-Ala-X could also act as DPP-IV inhibitors.
Streptomyces collagenase was cloned and characterized by Sakurai et al.Citation6 The enzyme belongs to Pz-peptidase family. Peptidases of this family can hydrolyze the synthetic substrate 4-phenylazobenzyloxycarbonyl-(Pz)-Pro-Leu-Gly-Pro-d-Arg, which contains the collagen specific sequence Gly-Pro-Y. Streptomyces collagenase also cleaves this substrate at the Leu-Gly bond, similar to other Pz-peptidases. Thus, Streptomyces collagenase is expected to produce collagen tripeptide fragments from the collagens used in this study, which were obtained from different sources.
Although diprotin A (Ile-Pro-Ile) and diprotin B (Val-Pro-Leu) are reported to act as DPP-IV inhibitorsCitation7, Rahfeld et al.Citation8 showed that the apparent competitive inhibition of DPP-IV by the diprotins is a kinetic artifact, derived from the substrate-like nature of tripeptides containing a penultimate proline residue. Based this finding, it is unclear whether the newly isolated peptide inhibitor of DPP-IV is hydrolyzed by the enzyme.
The present study focuses on the ability of collagen hydrolysates to inhibit human DPP-IV. In addition, we investigated the effects of the collagen tripeptide fragments Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp on DPP-IV activity.
Materials and methods
Materials
Collagen from pig-skin (product code CPAM-5, Jellice Co. Ltd., Miyagi, Japan), cattle-skin (product code AFC, Nippi Co. Ltd., Ibaraki, Japan) and fish-scales (product code FCP-AS-L, Nippi Co. Ltd.), chicken-feet (product code C-LAP, Nippon Meat Packers, Inc., Osaka, Japan) were used. All collagens were commercially available in this study. Synthetic peptides and Ala-Pro-p-nitroanilide (Ala-Pro-pNA) were obtained from Bachem AG (Bubendorf, Switzerland).
Preparation of collagen hydrolysates using Streptomyces collagenase
Streptomyces collagenase (2000 proteolytic units/g) used was a gift from NAGASE ChemteX Co. Ltd. (Osaka, Japan). Collagen (2 g) was dispersed in 40 ml of distilled water, adjusted to pH 7.5 with 5 N NaOH and incubated at 45 °C for 17 h with shaking. The sample was hydrolyzed by incubation with a 1% (w/w) enzyme solution. The resultant hydrolysate was heated at 80 °C for 30 min and centrifuged at 2000 × g for 30 min to inactivate the protease. The supernatants were freeze-dried and stored at 4 °C until further analysis.
Gel filtration analysis
The molecular weight distributions of the collagen hydrolysates were determined by gel filtration. The peptides (10 mg/ml) were dissolved in 50 mM Tris–HCl (pH 7.5) containing 150 mM NaCl. A sample (200 μl) was injected into a Superdex peptide column (GE Healthcare Japan, Tokyo, Japan) with 50 mM Tris–HCl (pH 7.5) containing 150 mM NaCl at a flow rate of 0.7 ml/min. Elution was monitored at 215 nm using the ÄKTA FPLC apparatus (GE Healthcare). Aprotinin, Leu-Val and Gly were used as standards for calibration.
DPP-IV preparation
Pichia pastoris was used to extracellularly express recombinant human DPP-IV as described previouslyCitation9. The supernatant was concentrated by ammonium sulfate precipitation (70% saturation at 4 °C). The precipitate was centrifuged (20 000 × g for 30 min at 4 °C), dissolved in distilled water and dialyzed twice against distilled water. This dialysate was used as the enzyme preparation. DPP-IV activity was determined using 1.6 mM Ala-Pro-pNA in 0.1 M Tris–HCl (pH 7.5) at 37 °C. One unit of enzyme activity was defined as the amount of enzyme that can liberate 1 μmol p-nitroaniline per minute under the defined assay conditions. The assay was performed continuously at 405 nm (molecular extinction coefficient of pNA: εmM = 10.6).
DPP-IV inhibition assay
The inhibition assay was performed using a microplate reader (SH-8000Lab, Corona Electric Co. Ltd., Ibaraki, Japan) and 96-well microtiter plates. For the assay, dried samples of the hydrolysates were dissolved and diluted to different extents with distilled water. A sample (10 μl) was added to 10 μl of 2 mM Ala-Pro-pNA in 0.1 M Tris–HCl (pH 7.5) with 70 μl of distilled water in each well. Under the assay conditions, the samples were diluted to a final concentration between 0.31 and 5 mg/ml. Then, the mixture was pre-incubated at 37 °C for 5 min. The enzymatic reaction was initiated by adding 10 μl of the DPP-IV stock solution (0.3 U/ml). This mixture was incubated at 37 °C for 5 min. Absorbance at 405 nm was measured every 10 s, and the rate of the enzymatic reaction was estimated based on the increase in absorbance. The percentage of inhibition was determined relative to the velocity of the reaction without dipeptides. The half-maximal inhibitory concentration (IC50) values were calculated by plotting the logarithm of the sample concentration (mg/ml) against the inhibitory activity (%). Lineweaver--Burk and Dixon plots for synthetic peptides dissolved at a final concentration of 0.63–20 mM were used to determine the inhibition constant (Ki) and the type of inhibition, respectively.
Quantification of Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp in the collagen hydrolysates
Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp in the hydrolysates were quantified using an HPLC system with an evaporative light scattering detector (ELSD, Model 2424, Nihon Waters K. K., Tokyo, Japan). We modified the analytical method for the quantification of underivatized amino acidsCitation10 to quantify the tripeptides. The hydrolysates were dissolved at 5 mg/ml in distilled water. Each sample (20 μl) was injected into the system. The analysis was carried out using a Hypercarb column (2.1 mm × 100 mm, Thermo Fisher Scientific K. K., Yokohama, Japan). The elution was performed using 0.3% nonafluoropentanoic acid (Tokyo Chemical Industry Co. Ltd., Tokyo, Japan) in water for 1 min, followed by a linear gradient of 10% acetonitrile for 30 min. Next, the column was washed with 90% acetonitrile for 4 min and equilibrated with 0.3% nonafluoropentanoic acid in water for 5 min. The flow rate and column temperature were 1.0 ml/min and 40 °C, respectively. The conditions used for ESLD were as follows: gain, 100; nebulizer gas pressure, 40 psi; drift tube temperature, 50 °C; nebulizer, cooling. Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp in the hydrolysates were identified by comparing the retention times of synthetic peptides. To quantify the peptides, standard curves were plotted using synthetic peptides (2–10 µg).
DPP-IV-catalyzed tripeptide hydrolysis
Peptides (2.0 mM, 90 μl) in 10 mM Tris–HCl (pH 7.5) buffer were pre-incubated for 5 min at 37 °C. DPP-IV (0.3 U/ml, 10 μl) was added to the substrate solution at 37 °C. The mixture was incubated at 37 °C for 1 or 17 h and then incubated at 99.9 °C for 5 min to denature DPP-IV, and the peptides were quantified using the HPLC system with ELSD and the Hypercarb column. For quick analysis, the gradient profile was modified as follows: the elution was performed with 0.3% nonafluoropentanoic acid in water for 1 min, followed by a linear gradient to 50% acetonitrile over 5 min. The washing and equilibration steps were the same as in the section titled “Quantification of Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp in the collagen hydrolysates”. The flow rate and the column temperature were 1.0 ml/min and 40 °C. Ten microliters of the reaction mixtures and synthetic peptide solutions were injected to quantify the decrease in the concentration of substrates after the incubation with DPP-IV.
Kinetic studies
The kinetic study was carried out using Gly-Ala-Hyp (1.8–20.0 mM, 40 μl) in 10 mM Tris–HCl (pH 7.5) buffer, after a 5-min pre-incubation at 37 °C. DPP-IV (1.5 U/ml, 10 μl) was added to the substrate solution at 37 °C. The mixture was incubated at 37 °C for 10 h followed by incubation at 99.9 °C for 5 min to denature DPP-IV. The resulting mixtures were diluted 10-fold with distilled water and used for HPLC analysis, as described in the earlier section.
When Gly-Pro-Ala and diprotin A were used as substrates (0.18–2.0 mM, 90 μl) after pre-incubation at 37 °C for 5 min in the same buffer, DPP-IV (6.0 or 0.75 U/ml, 10 μl) was added to the substrate solution at 37 °C. The mixture was incubated at 37 °C for 30 min and then heated to 99.9 °C for 5 min to denature DPP-IV. The resulting mixtures were used for the HPLC analysis.
Results
Inhibition of DPP-IV by the collagen hydrolysates
The activity and the protein concentration of the enzyme preparation were determined as 1.5 U/ml and 0.54 mg/ml, respectively, and the Km value for Ala-Pro-pNA was calculated to be 0.23 ± 0.02 mM.
The inhibitory activities of various collagen hydrolysates against DPP-IV are summarized in . Based on the IC50 values, the Streptomyces collagenase-treated pigskin collagen hydrolysate was over 3 times as effective in inhibiting DPP-IV compared to the untreated hydrosylate. The molecular weight distributions of the pigskin collagen with or without Streptomyces collagenase treatment are shown in . Treatment with Streptomyces collagenase produced small peptides as well as amino acids. The molecular distributions of the Streptomyces collagenase-treated collagens from other sources were similar to that of the collagenase-treated pigskin collagen (data not shown).
Figure 1. Effect of Streptomyces collagenase treatment on molecular weight distributions of pigskin collagen using gel filtration. The black and gray lines indicate with or without the Streptomyces collagenase treatment, respectively. Arrows indicate molecular weights at each peak. The molecular weight for each peak was estimated based on the elution volumes of the standards. The elution volumes of aprotinin, Leu-Val and Gly were 11.89, 16.91 and 19.42 ml, respectively. Inset, the standard curve is shown.
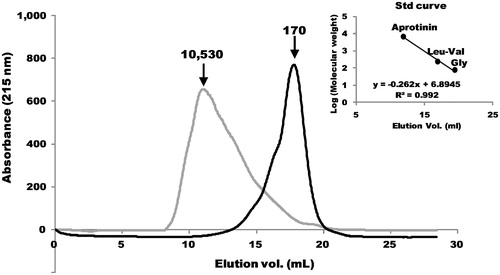
Table 1. Effect of collagenase treatment on DPP-IV inhibition and contents of Gly-X-Y tripeptides.
Quantification of tripeptides Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp in the collagen hydrolysates
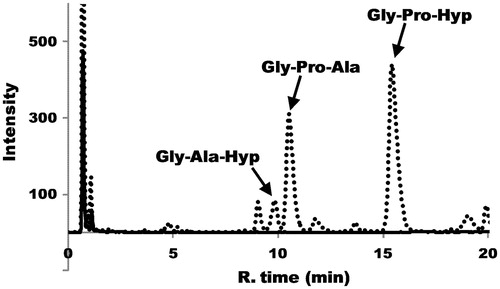
The three collagen tripeptides in the hydrolysate were quantified using HPLC. The pigskin collagen HPLC chromatograms with or without Streptomyces collagenase treatment are shown in . The composition of the three peptides in various collagen hydrolysates is summarized in .
Figure 2. The HPLC chromatograms of pigskin collagen with or without Streptomyces collagenase treatment. The dotted and black lines indicate with or without the Streptomyces collagenase treatment, respectively. Arrows indicate the peaks of collagen tripeptide fragments Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp, which were identified by comparison with the retention times of synthetic peptides.
Effect of the peptides on DPP-IV
The IC50 values of the collagen tripeptide fragments Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp and the known peptidic inhibitor diprotin A against DPP-IV were investigated. All four peptides showed inhibitory effects; however, the effects of collagen tripeptides were lower than that of diprotin A ().
Table 2. DPP-IV catalyzed hydrolysis of tripeptides.
Next, using HPLC with ELSD, we examined the ability of DPP-IV to hydrolyze the collagen tripeptides and diprotin A (). DPP-IV hydrolyzed Gly-Pro-Ala at a faster rate than diprotin A (). A comparison of the kinetic parameters also suggests that Gly-Pro-Ala is a better substrate for DPP-IV than diprotin A ().
Figure 3. DPP-IV-catalyzed hydrolysis of tripeptides. The time course of the hydrolysis reaction using HPLC is shown. The gray, hatched and black lines indicate the reaction times at 0, 1 and 17 h, respectively. All peaks indicated by arrows were identified by comparison with the retention times of synthetic peptides. (A) In the case of Gly-Ala-Hyp, the resulting Hyp was not retained and eluted at void volume with the buffer matrix under the experimental conditions. (B) Gly-Pro-Ala hydrolysis is shown at the reaction times of 0 and 1 h because Gly-Pro-Ala was completely hydrolyzed to Gly-Pro and Ala after 1 h. The resulting Ala was eluted at void volume with the buffer matrix under the experimental conditions. (C) Gly-Pro-Hyp could not be hydrolyzed by DPP-IV. (D) Hydrolysis of Ile-Pro-Ile (diprotin A).
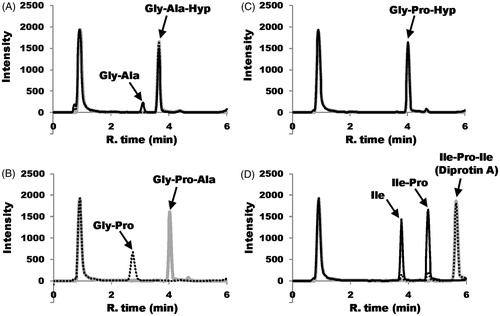
On the other hand, amongst Gly-Pro-Ala, Gly-Ala-Hyp and diprotin A, the rate of hydrolysis of Gly-Ala-Hyp was the lowest (). Thus, DPP-IV poorly hydrolyzed the bond between Ala-Hyp. Moreover, no hydrolysis of Gly-Pro-Hyp was observed ( and ).
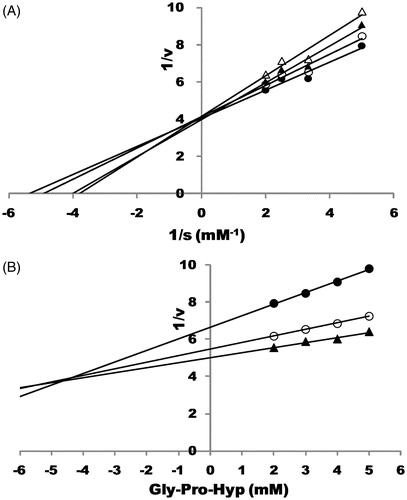
Finally, the mode of action of Gly-Pro-Hyp was found to be competitive, and its Ki value was estimated to be ca. 4.5 mM ().
Figure 4. The mode of action of Gly-Pro-Hyp toward DPP-IV. (A) Lineweaver–Burk plot showing the effect of Gly-Pro-Hyp on the catalysis rate of Ala-Pro-pNA by DPP-IV. Ala-Pro-pNA was used at the final concentrations of 0.20, 0.30 and 0.50 mM; Gly-Pro-Hyp was added at the final concentrations of 0.00, 1.25, 2.50 and 5.00 mM. Symbols: •, in the absence of Gly-Pro-Hyp; ○, in the presence of Gly-Pro-Hyp (1.25 mM); ▴, in the presence of Gly-Pro-Hyp (2.50 mM); Δ, in the presence of Gly-Pro-Hyp (5.00 mM). (B) Dixon plot showing the effect of Gly-Pro-Hyp on the catalysis rate of Ala-Pro-pNA by DPP-IV. Ala-Pro-pNA was used at final concentrations of 0.20, 0.30 and 0.50 mM. Gly-Pro-Hyp was added at final concentrations of 0.00, 1.25, 2.50 and 5.00 mM. Symbols: •, Ala-Pro-pNA was used at a final concentration of 0.20 mM; ○, Ala-Pro-pNA was used at a final concentration of 0.30 mM; ▴, Ala-Pro-pNA was used at a final concentration of 0.30 mM.
Discussion
Gly-Pro-Ala was identified as a DPP-IV inhibitor by BauvoisCitation5. In this study, we confirmed that this tripeptide is a good substrate for DPP-IV ( and ). Li-Chan et al.Citation11 reported the inhibition of DPP-IV by Gly-Pro-Ala-Glu and Gly-Pro-Gly-Ala derived from the gelatin of Atlantic salmon skin. The hydrolysis of these peptides by DPP-IV was similar to that of Gly-Pro-Ala. Although Tulipano et al.Citation12 reported that the bioactive peptide Ile-Pro-Ala derived from whey proteins can be regarded as a moderately strong DPP-IV inhibitor, it may be hydrolyzed in a similar manner to diprotin A (Ile-Pro-Ile).
To our knowledge, this is the first identification of Gly-Pro-Hyp as a true peptidic inhibitor of DPP-IV. Importantly, DPP-IV cannot hydrolyze the bond between Pro-Hyp. Although the inhibitory effect is not strong, it is found in high amounts in the collagen hydrolysates obtained after digestion by Streptomyces collagenase ( and ). Gly-Pro-Hyp is known to be biologically active. For instance, the tripeptide exerts chemotactic effects on fibroblasts, peripheral blood neutrophils and monocytes in cell culture systemsCitation13–15. Based on the size, the tripeptides are expected to be suitable for trans-epithelial transportCitation16. Aito-Inoue et al.Citation17 investigated the transport of Gly-Pro-Hyp across the intestinal brush-border membrane (BBM) in a porcine BBM model. They concluded that Gly-Pro-Hyp could not cross the epithelial apical membrane in an intact form and that it was hydrolyzed to Gly and Pro-Hyp by intestinal proteases. We confirmed that Pro-Hyp was not inhibitory against DPP-IV (data not shown). On the other hand, in studies using radiolabeled Gly-[Citation14C]Pro-Hyp, Watanabe-Kamiyama et al.Citation18 reported that Gly-Pro-Hyp remained intact in the plasma when administered to Wistar rats. The two studies reported conflicting results regarding the fate of Gly-Pro-Hyp after oral administration. We have not studied the bioavailability of the collagen hydrolysates produced by Streptomyces collagenase in vivo; therefore, it is not possible to draw any conclusions on the physiological relevance of the collagen hydrolysate as a DPP-IV inhibitor.
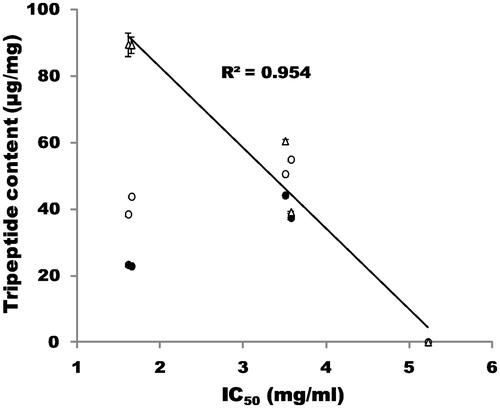
In this study, using Streptomyces collagenase, we successfully produced the collagen tripeptide fragments Gly-Ala-Hyp, Gly-Pro-Ala and Gly-Pro-Hyp from collagens obtained from various sources. Collagenase treatment led to an increase in the concentrations of the three tripeptides (13–15% [w/w]) in the hydrolysate. Our results are consistent with the findings of Fujita et al.Citation19, who used gelatin and immobilized Streptomyces collagenase and reported a yield of 4–14% for Gly-Pro-Hyp. The inhibitory effect of the tripeptides increased when Streptomyces collagenase was used as the enzyme source (). When the concentrations of the tripeptide fragments in collagen hydrolysates were plotted against IC50 values of DPP-IV inhibition by the hydrolysates, good correlations were observed between the concentration of Gly-Pro-Hyp and the IC50 values (). Gly-Pro-Hyp in the collagen hydrolysates is suggested to be mainly responsible for the DPP-IV inhibition in vitro. Thus, the capacity of collagens from pig-skin and cattle-skin to inhibit DPP-IV may be greater than that of collagens from fish-scales and chicken-feet because of the high Gly-Pro-Hyp content. The Japanese corporation UHA Mikakuto Co. Ltd. has secured the patent for the anti-diabetic effect of commercially available collagen peptides using a diabetic rat modelCitation20. It was shown that the collagen peptides inhibited DPP-IV activity, reduced blood glucose levels and enhanced effects on the levels of GLP-1 and insulin in blood. This patent did not address or identify the inhibitory effect of Gly-Pro-Hyp on DPP-VI. Moreover, the experiment was performed only in a rat model of diabetes. To date, no reports have addressed the anti-diabetic effects of Gly-Pro-Hyp in humans. Further, basic science and clinical studies are required to elucidate the inhibitory effect of collagen hydrolysates.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
The authors would like to thank Mr. Masashi Kusubata (Nippi Co. Ltd) for his helpful suggestions.
References
- Bjelke JD, Christensen J, Nielsen PF, et al. Dipeptidyl peptidases 8 and 9: specificity and molecular characterization compared with dipeptidyl peptidase IV. Biochem J 2006;396:391–9
- Richard EP. Overview of glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Medscape J Med 2008;10:171–83
- Richter B, Banderia-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag 2008;4:753–68
- Ramshow J, Shah N, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. J Struct Biol 1998;122:86–91
- Bauvois B. A collagen-binding glycoprotein on the surface of mouse fibroblasts is identified as dipeptidyl peptidase IV. Biochem J 1998;252:723–31
- Sakurai Y, Inoue H, Nishii W, et al. Purification and characterization of a major collagenase from Streptomyces parvulus. Biosci Biotech Biochem 2009;73:21–8
- Umezawa H, Aoyagi T, Ogawa K, et al. Diprotins A and B, inhibitors of dipeptidyl aminopeptidase IV, produced by bacteria. J Antibiot (Tokyo) 1984;37:422–5
- Rahfeld J, Scierhorn M, Hartrodt B, et al. Are diprotin A (Ile-Pro-Ile) and diprotin B (Val-Pro-Leu) inhibitors or substrates of dipeptidyl peptidase IV? Biochim Biophys Acta 1991;1076:314–16
- Hatanaka T, Inoue Y, Arima J, et al. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem 2012;134:797–802
- Chaimbault P, Petritis K, Elfakir C, Dreux M. Ion-pair chromatography on porous graphitic carbon stationary phase for the analysis of twenty underivatized protein amino acids. J Chromatogr A 2000;870:245–54
- Li-Chan E, Hunag SL, Jao CL, et al. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl peptidase IV inhibitors. J Agric Chem 2012;60:973–8
- Tulipano G, Sibilla V, Caroli AM, Cocchi D. Whey proteins as source of dipeptidyl peptidase IV (DPP-4) inhibitors. Peptides 2011;32:835–8
- Postlethwaite AE, King A. Collagen and collagen like peptide-induced chemotaxis of human peripheral blood neutrophils. J Exp Med 1976;143:1299–307
- Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II and III collagens and collagen-derived peptides. Proc Natl Acad Sci USA 1978;75:871–5
- Laskin DL, Kimura T, Sakakibara S, et al. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J Leukoc Biol 1986;39:255–66
- Satake M, Enjoh M, Nakamura Y, et al. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biotech Biotec Biochem 2002;66:378–84
- Aito-Inoue M, Lackeyram D, Fan M, et al. Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. J Pep Sci 2007;13:468–74
- Watanabe-Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agric Chem 2010;58:835–41
- Fujita A, Kawakita H, Saito K, Sugita K. Production of tripeptide from gelatin using collagenase-immobilized porous hollow-fiber membrane. Biotechnol Prog 2003;19:1365–7
- UHA Mikakuto Co., Ltd. Dipeptidyl peptidase-IV inhibitor. Japanese patent of JP 5176964 (WO/2008/066070); 2007