Abstract
Herein we introduce the derivation of a mathematical expression to evaluate the dissociation constant of a mixture of stereoisomers in equal amounts (KdMIX), when the corresponding dissociation constants (Kd) or medium response (MR50) of the pure stereoisomers are known; the final equation takes the form of the harmonic mean. In order to validate the equation, we carried out a bibliographic search of experimental data of enantiomeric molecules with biological activity, considering the Kd’s or MR50’s of the isolated enantiomers as well as that of the racemate. The comparisons between the experimental dissociation constants of the mixtures (KdEXP or MR50EXP) and the calculated values (KdMIX or MR50MIX) were consistent; the similarity between these values is supported through statistical analyses of group comparison and simple linear correlation. The equation we obtained, which corresponds to the harmonic mean, was used to predict the values of KdMIX (or MR50MIX) or Kd (or MR50) in systems when only two of the experimental values are known: either the dissociation constants of both enantiomers or the Kd (or MR50) of one of the enantiomers and dissociation constant of the racemate.
Introduction
A large fraction of drugs or substances with biological activity corresponds to organic molecules that can exist in two or more stereoisomeric forms. The synthesis of these compounds with a particular stereochemistry presents a challenge to chemists as well as to health specialists. Currently, a high fraction of the active principles of pharmaceutical drugs is commercialized as racematesCitation1–3. Nevertheless, in the last decade there has been a substantial increment in the distribution of enantiomerically pure substancesCitation1, as it has been shown that there is usually a pharmacokinetic and pharmacodynamic advantage of using only one enantiomer (eutomer) over the other (distomer)Citation4–6. On the other hand, the distomer may have a different kind of biological activity, even toxicityCitation5,Citation7, or it may have the same activity but different affinity to the receptor.
Herein we present the deduction of an equation intended to recognize the relationship between the dissociation constants of the individual components of a mixture of stereoisomers (Kd), particularly of a racemate, and the dissociation constant of the mixture as a whole (KdMIX). It is shown that the expression has the same form as that corresponding to the harmonic mean.
Methods
Bibliographic search
The revision focused on data reported in the last four decades on dissociation constants Kd, IC50 or ED50 with Hill coefficients equal 1 (nH = 1) of racemates as well as of the pure enantiomers. Particular emphasis was put on searching for data obtained on the same receptor and under the same methodology.
Calculation of KdMIX
This calculation was carried out using the equation proposed in this work (harmonic mean) starting from the Kd, IC50 or ED50 values reported for the pure enantiomers.
Validation and statistical analysis
The validation process was carried out through comparison of the Kd, IC50 or ED50 experimental values with the calculated KdMIX or MR50MIX values by means of the Mann--Whitney U test, with a statistically significant value of p < 0.05. The group comparison was performed through the normalized values which were determined by means of the following expressions:
where KdEXP or MR50EXP corresponds to the experimental dissociation constant or medium response of the racemate, KdMIX or MR50MIX to the value calculated through the proposed equation (harmonic mean), and KdEXPsmallest or MR50EXPsmallest to the experimental value of the pure enantiomer with the highest affinity for the receptor. To determine the sensitivity of the prediction of KdMIX (or MR50MIX), a correlation was carried out between pKdEXP (or pMR50EXP) and pKdMIX (or pMR50MIX) through a simple regression analysis validated by Student’s t test, where p < 0.05 is statistically significant. Finally, a covariance analysis between the slopes of both curves was carried out by Student’s t test, where p < 0.05 is statistically significant.
Results
The binding between a ligand L and a receptor R, leading to the formation of a ligand–receptor complex (R–L) can be described by the following equilibrium (8):
One way to evaluate the affinity between ligand and receptor is through the value of the dissociation constant Kd, which measures the tendency of the ligand–receptor complex to dissociate. The dissociation constant (Equation (1)) corresponds to the equilibrium constant for the inverse of the process described above:
where [R–L] is the concentration of the ligand–receptor complex; [R] and [L] are the concentrations of the free ligand and receptor, respectively. Low values of Kd indicate high affinity between ligand and receptor.
As an equilibrium constant, Kd is related to the Gibbs free energy of the ligand–receptor binding process shown before according to Equation (2). This energy is, in turn, determined by the intermolecular interactions that govern the complexation between both fragmentsCitation8.
It is well known that many biologically active substances are chiral and that they are usually obtained as racemates. In this case, we formally have two different substances competing for a given receptor. If we assume that each enantiomer is expected to have an independent affinity for the chiral receptor, we can expect the racemate to have a value of the dissociation constant, KdMIX, corresponding to some kind of average of the Kd values of both enantiomers. However, how do we obtain this average? It is expected that the Kd of the enantiomer with the higher affinity for the receptor contributes the most to the value of KdMIX; thus, the latter could be defined as the sum of the weighted contributions of the individual Kd values of the enantiomers. According to the definition of the weighted mean, , we can propose the expression shown in Equation (3)Citation9.
where Kd1 and Kd2 are the dissociation constants of each enantiomer, and w1 and w2 correspond to the weight in which each enantiomer contributes to the overall complexation of the mixture to the receptor. If we assume that the total number of receptors is constant, and that each enantiomer of the racemic mixture will bind to a fraction of this number, we can expect that w1 + w2 = 1, so that Equation (3) becomes Equation (4).
Once the expression for KdMIX has been defined, it is fundamental to describe the expressions corresponding to the weights (wi), which are defined in terms of the Kd’s of the pure enantiomers and of the relationship between them as shown in Equation (5):
It can be seen that each weight wi corresponds to the fraction of the binding of enantiomer j to the receptor, with respect to enantiomer i, in relation to the overall binding of both enantiomers to the receptor; simplification of Equation (5) leads to Equation (6).
This expression can be further simplified to Equation (7).
Generalization
We can now turn our attention to the case in which we have three or more molecules competing for binding to the same receptor, as would be the case of a mixture of stereoisomers of molecules with more than one stereogenic center. According to the above (Equation (4)), KdMIX would be described by Equation (8) for three stereoisomers present in equal amounts in a mixture:
In this case, the definition of the weights is the same as before, however, in order to obtain wi, now we have to consider the fraction of binding of isomers j and k with respect to isomer i. This can be done by obtaining first the fraction of binding of isomer j with respect to isomer k (or isomer k with respect to isomer j) as done in the two-component mixture (Equation (5)), and then referring this result to isomer i, as shown in Equation (9).
By substituting Equation (9) into Equation (8), and carrying out the corresponding algebra we obtain Equation (10), which describes the average binding of the mixture as a function of the individual binding constants of its components.
It can be shown that this expression can be further simplified to Equation (11).
Note that Equations (7) and (11) describe the average dissociation constants of two- and three-component mixtures, and that these equations are particular cases of a more general case, which is described by Equation (12) for an n-component mixture.
It can be readily seen that Equation (12) is the same as that of the harmonic mean (H)Citation9 of a set of n numbers xi (Equation (13)) so that KdMIX is the harmonic mean of the individual dissociation constants of the components of the mixture.
Discussion
The harmonic mean has been used for a long time to explain the total lethal effect on rats of mixtures of chemicals used extensively in industryCitation10. Similarly, the effect of a large variety of mixtures of toxic substances on laboratory animals has been studied and explained statistically through the use of the harmonic mean for mixtures of two or more componentsCitation11. The harmonic mean has already been used in other cases where dissociation constants are involved. For example, Raffa used the harmonic mean to relate the Kd of mutant receptors or ligands to the Kd of the wild-type species, something that could not be achieved by neither the arithmetic nor the geometric meansCitation12. In studies more related to ours, Chou and Talalay described in a broad and detailed study the average effect of mixtures of mutually exclusive enzymatic inhibitors, which follow a first-order kinetics, using the harmonic meanCitation13. Houghten et al. used the harmonic mean to predict the IC50 value of mixtures of components derived from combinatorial libraries for which the corresponding individual IC50 values as well as that of the mixture were knownCitation14. We should point out that in both instances the harmonic mean was applied directly, without the mathematical derivation that we introduced earlier.
With the goal of validating Equation (12) we carried out a search of experimental data reported in the literature, considering only those reports containing average responses for the pure enantiomers and for the racemates of substrates onto different receptors. For each case, we calculated KdMIX or MR50MIX from the corresponding experimental values of dissociation constants () or half maximal concentrations (MR50) () and compared them to the experimental average response of the racemates.
Table 1. Comparison between the calculated KdMIX with the Kd experimental values of racemates.
Table 2. Comparison between the calculated MR50MIX with the medium response (MR50) experimental values of racemates.
The experimental average responses of the racemates of the substrates summarized in are very similar to the calculated values, independently of the pharmacologic or biochemical relationship to the receptor: agonist, antagonist, substrate, inhibitor, etc. As expected and according to the definition of the harmonic meanCitation9, in all cases the calculated values of KdMIX are closer to that of the enantiomer with the smallest average response, which is that with the highest affinity to the receptor. It can also be seen that the calculated KdMIX values are not very much affected by the largest Kd values of the stereoisomer with the lowest affinity to the receptor.
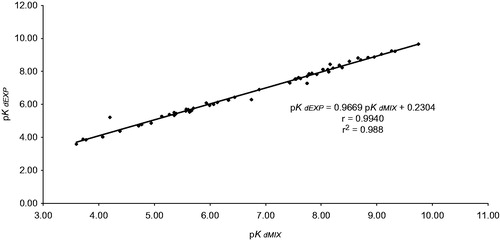
The statistical analysis of the correlation between pKdEXP and pKdMIX shows a linear relationship with a slope value very near 1.0; this means that prediction of Kd through the harmonic mean leads to values of practically the same magnitude as those of KdEXP ().
Figure 1. Correlation between pKdEXP and pKdMIX. Statistical simple linear regression analysis; b = 0.2304 ± 0.3008 at 95.0% confidence interval (p > 0.05); m = 0.9669 ± 0.0291 at 95.0% confidence interval (p < 0.001) and r = 0.9885 (p < 0.001) by Student’s t test.
Similarly, we analyzed those results where average effects were reported as IC50 or EC50 (both also known as MR50). We performed a correlation and a simple linear regression analysis between MR50EXP and MR50MIX () which do not show significant differences between theoretical and normalized experimental values.
Figure 2. Correlation between pMR50EXP and pMR50MIX. Statistical simple linear regression analysis; b = 0.3034 ± 0.5510 at 95.0% confidence interval (p > 0.2); m = 0.9311 ± 0.0869 at 95.0% confidence interval (p < 0.001) and r = 0.9782 (p < 0.001) by Student’s t test.
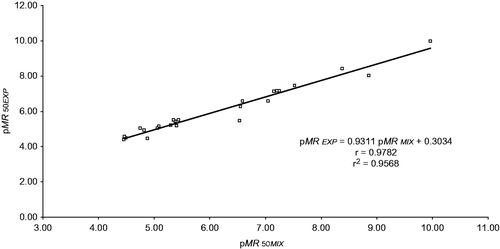
As before, pMR50EXP and pMR50MIX show a linear correlation with a slope very close to 1.0; again, prediction of MR50 through the harmonic mean leads to values of practically the same magnitude as those of MR50EXP. Thus the harmonic mean can predict satisfactorily Kd values as well as MR50 values.
The Kd and MR50 values shown in and were obtained through in vitro experiments. Both types of results are not necessarily equivalent between each otherCitation48; however, their values can be predicted independently through the harmonic mean. Therefore, we performed a covariance analysis between the straight lines in and , to show whether there is similarity between the slopes ().
Figure 3. ♦: Dissociation constants (pKd); □: Medium responses (pMR50). ANCOVA: the difference of slopes between both groups is not large enough to exclude the possibility that the difference is due to random sampling variability; there is no statistically significant difference (p > 0.5). One way ANOVA: the difference of slopes between both groups is not large enough to exclude the possibility that the difference is due to random sampling variability; there is no statistically significant difference (p > 0.25).
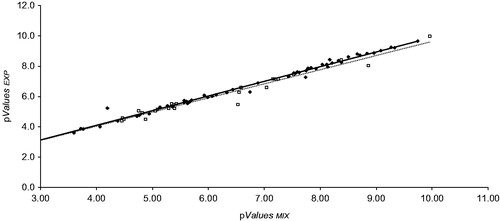
Based on analysis of covariance (ANCOVA) and one-way analysis of variance (ANOVA) for both sets data, we conclude that the harmonic mean predicts with the same magnitude and accuracy the KdMIX and MR50MIX values starting from experimental data, regardless of the type of variable (Kd or MR50), provided these variables describe the affinity of the components of the mixture to the same site of the receptor or enzyme.
In addition, in we have estimated KdMIX or MR50 for the racemic mixtures of a series of compounds using Equation (12), starting from the reported experimental Kd or MR50 values of the pure enantiomers.
Table 3. Prediction of KdMIX or MR50MIX calculated from Kd or MR50 experimental values of pure enantiomers.
On the other hand, when the experimental values of Kd of only one of the enantiomers and of the racemic mixture of a compound are available, it is possible to predict the Kd or EC50 values of the other enantiomer. shows examples of this for a number of cases.
Table 4. Prediction of Kd or MR50 of a pure enantiomer from the experimental values of Kd or MR50 of the other pure enantiomer and Kd or MR50 of the racemate.
Conclusions
We have deduced the expression corresponding to the average dissociation constant of a mixture of isomers KdMIX, as a function of the weights of the Kd’s of individual components of the mixture. We come to the conclusion that KdMIX corresponds to the harmonic mean of the Kd’s of the components.
When the values of KdMIX obtained with the harmonic mean equation are compared with the experimental values reported in the literature for the Kd of racemic mixtures, a close match between both sets is observed. When only the Kd values of the pure enantiomers are known, it is possible to predict the value of the Kd of the racemic mixture. If only the Kd value of one of the enantiomers is known, along with the Kd value of the racemic mixture, it is possible to predict the Kd (or MR50) value of the other enantiomer.
Declaration of interest
The authors state no conflicts of interest.
DQZ, HAJV and JTF are fellows of the COFAA-IPN and EDI-IPN fellowship programs.
References
- Shimazawa R, Nagai N, Toyoshima S, Okuda H. Present state of new chiral drug development and review in Japan. J Health Sci 2008;54:23–9
- McConathy J, Owens MJ. Stereochemistry in drug action. Prim Care Companion J Clin Psychiatry 2003;5:70–3
- Nguyen LA, He H, Pham-Huy C. Chiral drugs. An overview. Int J Biomed Sci 2006;2:85–100
- Lien EJ. Chirality and drug targeting: Pros and Cons. J Drug Target 1995;2:527–32
- Hutt AJ, Valentová J. The chiral switch: the development of single enantiomer drugs from racemates. Acta Facult Pharm Univ Comenianae 2003;50:7–23
- Waldeck B. Three-dimensional pharmacology, a subject ranging from ignorance to overstatements. Pharmacol Toxicol 2003;93:203–10
- Smith SW. Chiral toxicology: it’s the same thing…only different. Toxicol Sci 2009;110:4–30
- Copeland RA. Enzymes: a practical introduction to structure, mechanism and data analysis. New York: John Wiley & Sons. Inc; 2000:76–8
- Marques de Cantú MJ. Probabilidad y estadística para ciencias químico-biológicas. México DF: McGraw-Hill Interamericana; 1991:192–3
- Smyth HF, Weil CS, Wets JS, Carpeter CP. An exploration of joint toxic action: twenty-seven industrial chemical intubated in rats in all possible pairs. Toxicol Appl Pharmacol 1969;14:340–7
- Hoel DG. Statistical aspects of chemical mixtures. In: Vouk VB, Butler GC, Upton AC, et al., eds. Methods for assessing the effects of mixtures of chemicals. Great Britain: John Wiley & Sons. Inc; 1987:369–77
- Raffa RB. Harmonic mean relationship between affinity for wild-type receptors and alanine-scan mutants. J Theor Biol 2002;218:207–14
- Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem 1981;115:207–16
- Santos RG, Giulianotti MA, Dooley CT, et al. The use and implications of the harmonic mean model on mixtures for basic research and drug discovery. ACS Comb Sci 2011;13:337–44
- Bellemann P, Schade A, Towart R. Dihydropiridine receptor in rat brain labeled with [3H]nimodipine. Proc Natl Acad Sci U S A 1983;80:2356–60
- Maan AC, Hosey MM. Analysis of the properties of binding of calcium-channel activators and inhibitors to dihydropiridine receptors in chick heart membranes. Circ Res 1987;61:379–88
- Wahlund G, Nerme V, Abrahamsson T, Sjöquist P-O. The β1- and β2-adrenoceptor affinity and β1-blocking potency of S- and R-metoprolol. Br J Pharmacol 1990;99:592–6
- Walle UK, Pesola GR, Walle T. Stereoselective sulphate conjugation of salbutamol in humans: comparison of hepatic, intestinal and platelet activity. Br J Clin Pharmacol 1993;35:413–18
- Meng F, Xie G-X, Thompson RC, et al. Cloning and pharmacological characterization of a rat κ opioid receptor. Proc Natl Acad Sci U S A 1993;90:9954–8
- Chen Y, Mestek A, Liu J, Yu L. Molecular cloning of a rat κ opioid receptor reveals sequence similarities to the µ and δ opioid receptors. Biochem J 1993;295:625–8
- Guard S, Boyle SJ, Tang KW, et al. The interaction of the NK1 receptor antagonist CP-96,345 with L-type calcium channels and its functional consequences. Br J Pharmacol 1993;110:385–91
- Yang T, Prakash C, Roden DM, Snyders DJ. Mechanism of block of a human cardiac potassium channel by terfenadine racemate and enantiomers. Br J Pharmacol 1995;115:267–74
- Lejeune F, Newman-Tancredi A, Audinot V, Millan MJ. Interactions of (+)- and (–)-8- and 7-hydroxy-2-(di-n-propylamino)tetralin at human (h)D3, hD2 and h serotonin1A receptors and their modulation of the activity of serotoninergic and dopaminergic neurons in rats. J Pharmacol Exp Ther 1997;280:1241–9
- Hirota K, Okawa H, Appadu BL, et al. Stereoselective interaction of ketamine with recombinant µ, κ and δ opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology 1999;90:174–82
- Sun J, Meyers MJ, Fink BE, et al. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-α or estrogen receptor-β. Endocrinology 1999;140:800–4
- Rothman RB, Baumann MH, Savage JE, et al. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 2000;102:2836–41
- Gillard M, Van der Perren C, Moguilevsky N, et al. Binding characteristics of cetirizine and levocetirizine to human H1 histamine receptors: contribution of Lys191 and Thr194. Mol Pharmacol 2002;61:391–9
- Booth RG, Moniri NH, Bakker RA, et al. A Novel Phenylaminotetralin radioligand reveals a subpopulation of histamine H1 receptors. J Pharmacol Exp Ther 2002;302:328–36
- Sánchez C, Bøgesø KP, Ebert B, et al. Escitalopram versus citalopram: the surprising role of R-enantiomer. Psychopharmacology 2004;174:163–76
- Li X-Q, Weidolf L, Simonsson R, Andersson TB. Enantiomer/Enantiomer interactions between the S- and R-isomers of omeprazole in human cytochrome P450 enzymes: major role of CYP2C19 and CYP3A4. J Pharmacol Exp Ther 2005;315:777–87
- Velkov T, Chuang S, Wielens J, et al. The interaction of lipophilic drugs with intestinal fatty acid-binding protein. J Biol Chem 2005;280:17769–76
- Keskinbora K, Aydınlı I. Atipik opioid analjezik: Tramadol. Ağri 2006;18:5–19
- McLean TH, Chambers JJ, Parrish JC, et al. C-(4,5,6-Trimethoxyindan-1-yl)methamine: a mescaline analogue designed using a homology model of the 5-HT2A receptor. J Med Chem 2006;49:4269–74
- Bingham B, Jones PG, Uveges AJ, et al. Species-specific in vitro pharmacological effects of cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol 2007;151:1061–70
- Hao Z, Zhang Y, Pan L, et al. Comparison of enantiomers of SPFF, a novel β2-adrenoceptor agonist, in bronchodilating effect in Guinea Pigs. Biol Pharm Bull 2008;31:866–72
- Stresser DM, Mason AK, Perloff ES, et al. Differential time- and NADPH-dependent inhibition of CYP2C19 by enantiomers or fluoxetine. Drug Metab Dispos 2009;37:695–8
- Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology 2009;150:1817–25
- Maayani S, Weinstein H, Cohen S, Sokolovski M. Acetylcholine-like molecular arrangement in psychomimetic anticholinergic drugs. Proc Natl Acad Sci U S A 1973;70:3103–7
- Butcher SP, Collins JF, Roberts PJ. Characterization of the binding of DL-[3H]-2-amino-4-phosphonobutyrate to L-glutamate-sensitive sites on rat brain synaptic membranes. Br J Pharmacol 1983;80:355–64
- Schinazi RF, McMillan A, Cannon D, et al. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroximethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother 1992;36:2423–31
- Koizumi T, Saita Y, Miyake A, et al. Antagonistic effect of YM022, an antiulcer agent in rats, on human cholecystokinin (CCK)B/Gastrin receptor. Jpn J Pharmacol 1996;71:307–13
- Morrissey I, Hoshino K, Sato K, et al. Mechanism of differential activities of ofloxacin enantiomers. Antimicrob Agents Chemother 1996;40:1775–84
- Neupert W, Brugger R, Euchenhofer C, et al. Effects of ibuprofen enantiomers and its coenzyme A thioesters on human prostaglandin endoperoxide synthases. Br J Pharmacol 1997;122:487–92
- Triggle DJ, Mitchell JM, Filler R. The pharmacology of physostigmine. CNS Drug Rev 1998;4:87–136
- Kinney GG, Sur C, Burno M, et al. The glycine transporter type 1 inhibitor N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine potentiates NMDA receptor-mediated responses in vivo and produces and antipsychotic profile in rodent behavior. J Neurosci 2003;23:7586–91
- Callahan RJ, Au JD, Paul M, et al. Functional inhibition by methadone of N-methyl-D-aspartate receptors expressed in Xenopus oocytes: stereospecific and subunit effects. Anesth Analg 2004;98:653–9
- Wang L, Wang Y, Zheng J, et al. Pharmacological profiles of an anticholinergic agent, phencynonate hydrochloride, and its optical isomers. Acta Pharmacol Sin 2005;26:527–32
- Copeland RA. Enzymes: a practical introduction to structure, mechanism and data analysis. New York: John Wiley & Sons, Inc; 2000:282–7
- U’Prichard DC, Bylund DB, Snyder SH. (+/−)-[3H]epinephrine and (–)-[3H]dihydroalprenolol binding to β1- and β2-noradrenergic receptor in brain, heart, and lung membranes. J Biol Chem 1978;253:5090–102
- Tam SW, Cook L. σ opiates and certain antipsychotic drugs mutually inhibit (+)-[3H]SKF 10,047 and [3H]haloperidol binding in guinea pig brain membranes. Proc Natl Acad Sci U S A 1984;81:5618–21
- Panara MR, Greco A, Santini G, et al. Effects of the novel anti-inflammatory compounds, N-[2-(cyclohexyloxy)-4-nitrophenyl] methanesulphonamide (NS-398) and 5-methanesulphonamido-6-(2,4-difluorothiophenyl)-1-indanone (L-745,337), on the cyclo-oxygenase activity of human blood prostaglanin endoperoxide synthases. Br J Pharmacol 1995;116:2429–34
- Damaj MI, Glassco W, Marks MJ, et al. Pharmacological investigation of (+)- and (–)-cis-2,3,3a,4,5,9b-hexahydro-1-methyl-1H-pyrrolo-[3,2-h] isoquinoline, a bridged-nicotine analog. J Pharmacol Exp Ther 1997;282:1425–34
- Morse KL, Behan J, Laz TM, et al. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther 2001;296:1058–66
- Griffin MT, Figueroa KW, Liller S, Ehlert FJ. Estimation of agonist activity at G protein-coupled receptors: analysis of M2 muscarinic receptor signaling through Gi/o, Gs and G15. J Pharmacol Exp Ther 2007;21:1193–207
- Yamamoto I, Deniau GP, Gavande N, et al. Agonist responses of (R)- and (S)-3-fluoro-γ-aminobutyric acids suggest an enantiomeric fold for GABA binding to GABAC receptors. Chem Commun 2011;47:7956–8
- Shannon M, Battaglia G, Glennon RA, Titeler M. 5-HT1 and 5-HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA). Eur J Pharmacol 1984;102:23–9
- Chidlow G, Melena J, Osborne NN. Betaxolol, a β1-adrenoceptor antagonist, reduces Na+ influx into cortical synaptosomes by direct interaction with Na+ channels: comparison with other β-adrenoceptor antagonists. Br J Pharmacol 2000;130:759–66
- Andersen J, Taboureau O, Hansen KB, et al. Location of the antidepressant binding site in the serotonin transporter. Importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J Biol Chem 2009;284:10276–84
- Sinnegger-Brauns MJ, Huber IG, Koschak A, et al. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol 2009;75:407–14
- Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate receptor ion channels: structure, regulation and function. Pharmacol Rev 2010;62:405–96
