Abstract
Prenatal diagnosis of truncus arteriosus with two-dimensional sonography requires expertise in fetal echocardiography. Indeed, truncus arteriosus shares with tetralogy of Fallot and pulmonary atresia with a ventricular septal defect (VSD) the sonographic finding of a single arterial trunk overriding a VSD. The diagnosis of truncus arteriosus can be confirmed when either the main pulmonary artery or its branches are visualized arising from the truncus itself. This requires sequential examination of multiple scanning planes and a process of mental reconstruction of their spatial relationships. The advantage of multiplanar imaging in three-dimensional and four-dimensional ultrasonography is that it allows for the simultaneous visualization of three orthogonal anatomic planes, which can be very important in diagnosing cardiac abnormalities. We report, first, a case of truncus arteriosus diagnosed in utero where the multiplanar display modality provided important insight into the differential diagnosis of this conotruncal anomaly, and then, review the diagnosis of truncus arteriosus on ultrasound.
Introduction
Truncus arteriosus is a congenital heart defect in which a single arterial trunk exits the heart by way of a single arterial valve, and gives rise directly to the coronary, systemic and one, or both, pulmonary arteries [Citation1]. This conotruncal anomaly is the result of an incomplete septation (persistence) of the distal portion of the cardiac outflow tract of the embryonic heart into the aorta and the pulmonary artery [Citation2]. Truncus arteriosus is a cyanotic congenital cardiac anomaly characterized by increased pulmonary blood flow [Citation3]. Early surgical repair in the neonatal period carries low morbidity and mortality [Citation4–12] and prevents the long-term sequelae of pulmonary over-circulation and heart failure [Citation12,Citation13].
We report the prenatal diagnosis of a case of truncus arteriosus using ultrasound, and describe the insights provided by novel three-dimensional (3D) imaging techniques in the differential diagnosis of this conotruncal anomaly. Moreover, we review the classification system of truncus arteriosus and summarize the chromosomal anomalies, genetic syndromes, and cardiac and non-cardiac defects that can be associated with this condition. Finally, we briefly discuss the clinical presentation at birth and major prognostic factors.
Case report
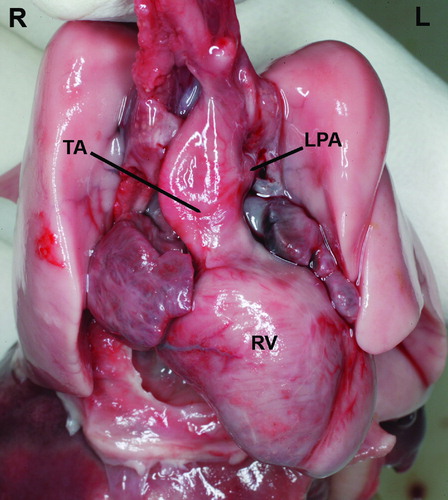
A 17-year-old G1P0 woman was referred to our unit at 23 weeks of gestation because a complex fetal cardiac anomaly was observed in a previous scan done elsewhere. Two-dimensional (2D) sonography of the outflow tracts identified a single, large vessel containing a single valve and overriding a ventricular septal defect (VSD) (). Multiplanar display of a volume dataset obtained with four-dimensional (4D) ultrasound demonstrated that the aortic arch and both pulmonary arteries arose from this large vessel (). Therefore, the prenatal diagnosis of truncus arteriosus type A2 in the Van Praagh classification [Citation14] was made. On parental request, a termination of pregnancy was performed, and a stillborn neonate weighing 490 g was delivered vaginally. The autopsy confirmed the sonographic findings of a single large vessel overriding a VSD, consistent with the prenatal diagnosis of truncus arteriosus type A2 in the Van Praagh classification (separate but adjacent origin of the pulmonary arteries from the arterial trunk) (). Additional findings included: a left superior vena cava, abnormal lung lobation, facial anomalies (low set and posteriorly rotated ears, hypertelorism, downward slanting of the palpebral fissures, long philtrum, midfacial hypoplasia with a small mandible), and limb anomalies (rocker-bottom feet, bilateral hypoplasia of the fifth digits, clinodactyly). The postnatal karyotype was normal (46 XY) and there was no 22q11.2 deletion found (DiGeorge/Velocardiofacial syndrome).
Figure 1. A single large vessel arises from the left and right ventricles. A thin vessel, subsequently confirmed to correspond to the left pulmonary artery, originates from the left side of this arterial trunk. RV, right ventricle; LV, left ventricle; CT, common arterial trunk; LPA, left pulmonary artery; SVC, superior vena cava; S, fetal spine.
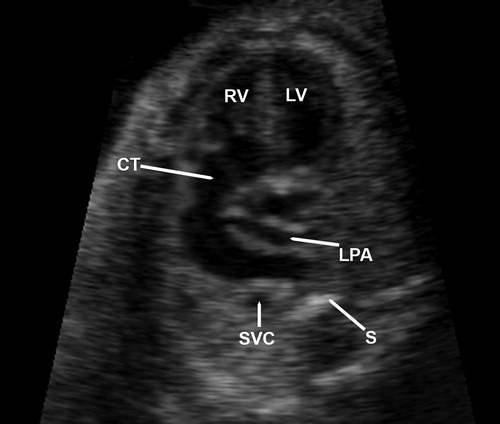
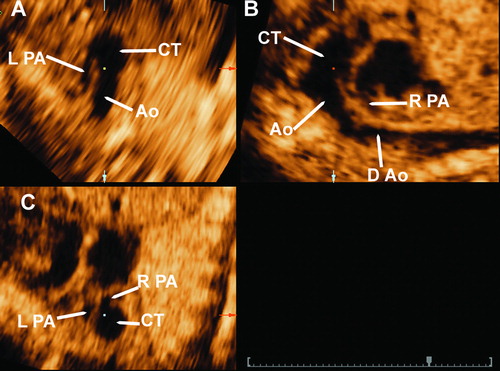
Figure 2. 3D multiplanar display imaging of the common arterial trunk. Panel A: in this transverse plane the left pulmonary artery is seen arising from the common arterial trunk. Panel B: in this sagittal plane the right pulmonary artery and the aortic arch are seen arising from the common arterial trunk. Panel C: coronal view, in which both left and right pulmonary arteries are demonstrated to originate from the common trunk. Ao, aorta; CT, common arterial trunk; RPA, right pulmonary artery; LPA, left pulmonary artery; D Ao, descending aorta.
Discussion
Truncus arteriosus is a conotruncal anomaly which accounts for 1% of all congenital heart diseases detected in prenatal life [Citation15]. Natural fetal wastage makes the intrauterine incidence even higher [Citation16]. Both sexes are equally affected [Citation17,Citation18], although a predominant distribution in females has been reported in one series [Citation19].
Truncus arteriosus belongs to the group of ectomesenchymal tissue migration abnormalities that are due to anomalous migration of neural crest cells through the branchial arch vessels during cardiogenesis [Citation20]. This heart defect occurs when there is incomplete septation (persistence) of the truncus arteriosus (the distal portion of the cardiac outflow tract of the embryonic heart tube) into the pulmonary artery and the aorta [Citation2]. The morphologic characteristics of a common arterial trunk are: (1) the common arterial trunk itself; (2) a common arterial orifice; and (3) a VSD in the outflow region (common outlet) [Citation21]. During the embryonic period, defective separation at the level of the aortic sac, the ventriculo-arterial junction, and the outlet, respectively, may account for these defects [Citation21]. The VSD is usually located in the infundibular region, immediately beneath the truncal valve. However, this defect can be absent (type B, common aorticopulmonary trunk) [Citation14,Citation22–24], or very small [Citation25]. The single valve at the origin of the truncus is often malformed, stenosed, or incompetent. In most cases, the ductus arteriosus is small, present as a ligamentous remnant or absent (it is uncommon to find a moderate or large ductus) [Citation18,Citation26].
Classification of truncus arteriosus
Collett and Edwards [Citation27] classified the truncus arteriosus anomaly according to the anatomic origin of the pulmonary arteries and to the spatial relationship between these vessels (). In 1965, Van Praagh and Van Praagh proposed another anatomical classification which also takes into account the presence or absence of a VSD in association with the truncus arteriosus () [Citation18]. The frequencies of the four anatomic types according to the classification proposed by Van Praagh and Van Praagh [Citation18] () were evaluated in a series of 100 cases [Citation17]. Type A1 was the most frequent (47–50%), whereas type A3 was the least frequent (2–8%) [Citation17].
Table I. Classification of Collett and Edwards.
Table II. Classification of Van Praagh and Van Praagh*.
Proper distinction between type A1 and A2 defects (Van Praagh classification) may be difficult even with angiographic and anatomical evaluations, if the defective aorticopulmonary septum is very short. Thus, it has been proposed that type A1 and A2 defects should be merged into one group [Citation17]. In addition, truncus type IV (based on Collett and Edwards classification) resembles, in its description, pulmonary atresia with a VSD with major aortopulmonary collaterals and, thus, it is probably a misnomer. More recently, members of the Society of Thoracic Surgeons – Congenital Heart Surgery Database Committee, and representatives from the European Association for Cardiothoracic Surgery, have proposed a unified truncus arteriosus nomenclature system for use in a surgical database, based on the revised classification proposed by Van Praagh [Citation14] in 1976 () [Citation28]. This new nomenclature has been proposed based on the observations that: (1) description of the origins of the left and right pulmonary branches from the common arterial trunk, as imaged by angiography and echocardiography, is frequently inconsistent with the appearance during surgery; (2) even when preoperative studies suggest the presence of a partially formed aorticopulmonary septum, the surgeon can rarely isolate and band a pulmonary artery segment; and (3) rare cases of truncus arteriosus without a VSD (in the classification of Van Praagh) are often associated with either conjoined aortic and pulmonary semilunar valves, or a large pulmonary valve and aortic atresia; thus, they may not represent true cases of truncus, but rather cases of a large aorticopulmonary window or of aortic atresia [Citation28].
Table III. Truncus arteriosus nomenclature.
Prenatal diagnosis of truncus arteriosus
Conotruncal anomalies, such as truncus arteriosus, can be prenatally diagnosed with accuracy in up to 80% of cases when multiple sonographic planes, Doppler color flow mapping, and pulsed Doppler interrogation are used [Citation29]. Experience in fetal echocardiography is a requirement for the prenatal assessment, as the four-chamber view, essential in cardiac prenatal screening, is unremarkable in up to 40–50% of the cases in which there is a major congenital heart defect [Citation30,Citation31]. Moreover, abnormalities in the four-chamber view are present in only 30% of cases of conotruncal anomalies [Citation32]. Prenatal diagnosis of truncus arteriosus with fetal echocardiography has been reported as early as the first trimester (using high resolution transvaginal ultrasound) [Citation33], at 16 weeks of gestation in a fetus with increased nucal translucency and bilateral echogenic foci [Citation34], and at 18 weeks of gestation in a fetus with intrauterine growth restriction [Citation35]. However, most cases are diagnosed between 20 and 25 weeks [Citation15,Citation16,Citation32,Citation36–40].
The role of novel sonographic imaging techniques in the differential diagnosis of truncus arteriosus
Visualization of a single arterial vessel overriding a VSD (rather than a distinct aorta and pulmonary artery) during examination of the outflow tracts, is a common finding of three cardiac defects: truncus arteriosus, tetralogy of Fallot, and pulmonary atresia with VSD [Citation41]. In tetralogy of Fallot and pulmonary atresia with VSD, a distinct pulmonary artery exiting from the right ventricle is anatomically present; however, the degree of pulmonary stenosis/atresia may be such that it is not recognizable by ultrasound, and only a single vessel (aorta) can be identified arising from the fetal heart. Thus, differentiating truncus arteriosus from these two other congenital heart defects relies on the sonographic demonstration that the main pulmonary artery, or at least one of its branches, arise from the single arterial trunk [Citation41]. In a recent observational study conducted at three referral centers, the prenatal echocardiographic diagnosis of truncus arteriosus was made in 24 cases, and confirmed by either autopsy or at postnatal echocardiography or surgery in 23 cases [Citation16]. The remaining neonate had a pulmonary atresia with VSD, which was erroneously diagnosed in utero as a truncus arteriosus [Citation16].
Because truncus arteriosus is a complex conotruncal anomaly and has multiple subtypes, the prenatal diagnosis requires sequential examination of multiple scanning planes and a process of mental reconstruction of their spatial relationships, which demands expertise and knowledge in fetal echocardiography. Such a process, however, can be facilitated by novel display modalities in 3D and 4D ultrasound, including multiplanar display, tomographic ultrasound imaging (TUI), and power Doppler rendering. The advantages of 3D and 4D over 2D sonography in obstetrics have been described [Citation42–46]. 2D sonography traditionally relies on standard anatomic planes for a thorough examination of the fetal heart. However, 3D and 4D sonography can facilitate the visualization of these planes, further simplify the examination of the fetal heart, and can potentially reduce operator dependency that is characteristic of 2D fetal echocardiography. By reslicing volume datasets of the fetal heart (which can only be obtained with 3D and 4D sonography), sonographic planes can be easily obtained [Citation47].
Multiplanar imaging in 3D and 4D ultrasonography is a display modality that allows for the simultaneous visualization of three orthogonal anatomic planes (transverse, sagittal and coronal). An imaging tool, referred to as the ‘reference dot’, can be used to identify and track anatomic structures in these planes. For example, placement of the reference dot in the sagittal view of the common trunk, at the origin of the right pulmonary artery and aortic arch (Panel B of ), allowed the visualization of the left pulmonary artery arising from the common trunk in the transverse and coronal planes (, panels A and B, respectively). Although the left pulmonary artery was visualized by 2D sonography, the confirmation of its nature, and also that of the right pulmonary artery and aortic arch, was accomplished by placing the reference dot on these vascular structures in the multiplanar display, and by rotating the volume dataset along the x- and y-axes (‘Spin’ technique) [Citation48]. It has also been demonstrated that multiplanar display may help in the evaluation of abnormal vascular connections [Citation49].
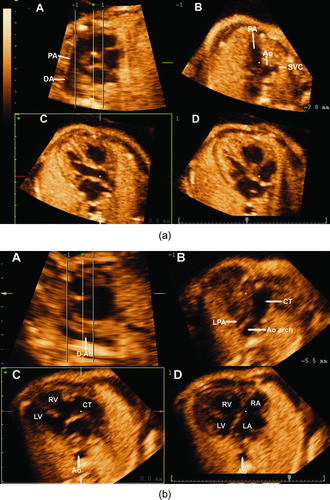
TUI is another new display modality available in 3D and 4D ultrasonography that allows simultaneous visualization of up to eight parallel anatomical planes. TUI has been used for the examination of the fetal heart [Citation47,Citation50–53] and other fetal organs [Citation54]. A recently described novel algorithm combining spatio-temporal image correlation and TUI allowed the simultaneous visualization of the four-chamber view, three-vessel and trachea view, and both outflow tracts [Citation47]. This algorithm allowed for visualization of the standard planes for fetal echocardiography in most fetuses with and without heart defects. The application of this algorithm to a 4D volume dataset from a normal fetus and that from the fetus with truncus arteriosus are displayed in and , respectively. In , a large VSD is observed in the four-chamber view (panel D), and a single vessel overriding the VSD is observed in panel C. The cross-section of the aortic root was not visualized in the short axis view ( panel A, and supplementary video clip 1).
Figure 4. (a) A novel algorithm using tomographic ultrasound imaging (TUI) allows for the simultaneous visualization of the short axis of the aorta in panel A, the three vessel and trachea view in panel B, the long axis of the left outflow tract in panel C and the four-chamber view in panel D. (b) The application of this algorithm to the present case of truncus arteriosus demonstrates a large ventricular septal defect (VSD) in the four-chamber view in panel D. Panel C displays a large vessel overriding the VSD. Of note, the root of the aorta is not visualized in panel A, which corresponds to the short axis of the heart. This indicates that the anatomic orientation of the common arterial trunk is similar to that of a normal pulmonary artery. Panel B displays the common trunk giving rise to the left pulmonary artery (LPA) and the aortic arch (Ao arch).
B-flow is a new display modality in 4D ultrasound that digitally enhances signals from weak blood reflectors from vessels and, at the same time, suppresses strong signals from the surrounding tissues. This display modality may have less signal drop out when the ultrasound beam is perpendicular to the vessel [Citation55]. The use of B-flow in the case presented herein demonstrated that this imaging modality provided important insight into the location of the left pulmonary artery and aortic arch ( and supplementary video clip 2).
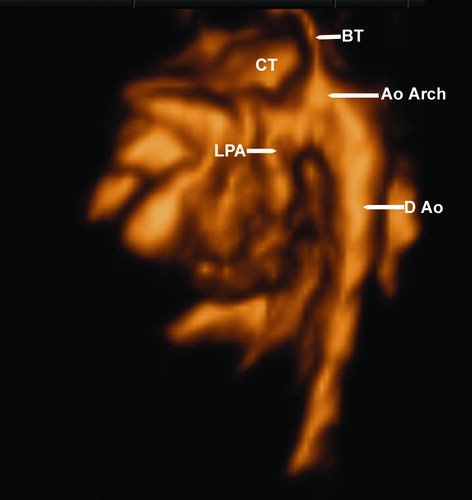
Figure 5. B-flow imaging displaying the common truncus (CT) and some of its branches, including the aortic arch (Ao Arch) and the left pulmonary artery (LPA); the image is seen from the left. D Ao, descending aorta, BT, brachiocephalic trunk.
Rendered images of 4D volume datasets obtained with power Doppler have been previously used to demonstrate the crisscrossing of the great arteries in a normal fetus, and the absence of this crisscrossing in a fetus with transposition of the great arteries [Citation56]. Supplementary video clip 3 displays the rendered images of a volume dataset obtained with power Doppler in the current case of truncus arteriosus. During diastole, the rendered Doppler signals are visualized entering both ventricles, and during systole, the power Doppler signal indicates that only one vessel arises from both ventricles. Thus, power Doppler rendering may help in the diagnosis of truncus arteriosus. Therefore, we found that the use of 3D/4D sonography provided important insight into the differential diagnosis of truncus arteriosus.
Association with chromosomal anomalies and genetic syndromes
Chromosomal anomalies are detectable in 8.7% of cases of truncus arteriosus [Citation16]. Despite the reported sporadic association with trisomy 18 [Citation57], duplication of 8q [Citation58,Citation59], terminal deletion of 7q [Citation60], and mosaicism for ring chromosome 22 [Citation35], the most frequent chromosomal abnormality in fetuses with truncus arteriosus is microdeletion of 22q11, detectable in up to 40% of cases when a cytogenetic examination is performed [Citation16,Citation34,Citation61–64]. In fact, conotruncal heart defects are the most common cardiovascular malformations seen in the presence of 22q11 deletion [Citation65]. Moreover, monosomy 22q11 has been associated with distinct phenotypes affecting the derivatives of the third and fourth branchial pouches, such as: DiGeorge sequence, velocardiofacial syndrome, conotruncal anomaly face syndrome, facial dysmorphic features, CHARGE syndrome (coloboma of the eye, heart defects, atresia of the nasal choanae, retardation of growth and/or development, genital and/or urinary abnormalities, ear abnormalities and deafness), and cases of isolated complex cardiovascular malformations [Citation66,Citation67]. Therefore, a prompt examination for extracardiac manifestations on prenatal sonography is indicated in these cases.
Nonsyndromic conotruncal heart defects, including isolated truncus arteriosus anomaly, have been proposed to occur sporadically [Citation68], or as a result of a complex and multifactorial inheritance [Citation69], where the effects of one or more genes interact with environmental factors [Citation70,Citation71]. The familial occurrence of truncus arteriosus supports a potential genetic basis for this anomaly. Indeed, several individuals of a family can be affected by this congenital heart defect [Citation72,Citation73], and a higher frequency of truncus arteriosus has been reported among siblings [Citation74–76]. Furthermore, monozygotic [Citation77] and dizygotic [Citation78] twins concordant for truncus arteriosus, as well as a case of double truncus arteriosus in thoracopagus twins [Citation79], have been reported. A monogenic–oligogenic inheritance has been proposed for conotruncal anomalies such as truncus arteriosus [Citation69,Citation80,Citation81], and pedigree analysis of families with a history of first cousin marriages and truncus arteriosus have revealed compatibility with an autosomal recessive inheritance [Citation72]. Recurrence rates of congenital cardiac malformations in families of index cases of truncus arteriosus range from 6.6% in the absence of associated cardiac malformations, to 13.6% in the presence of complex truncus arteriosus [Citation82]. Finally, truncus arteriosus and other conotruncal defects have been experimentally induced in fetuses of rats exposed to teratogens [Citation83], and an association has been proposed between maternal exposure to alcohol, dyes, lacquers, paints, upper respiratory infections during the first trimester, and conotruncal anomalies in the developing fetus [Citation71].
Association with other cardiac and non-cardiac defects
The prevalence of associated heart and great vessel anomalies in fetuses with truncus arteriosus is 35% [Citation16]. The spectrum of aortic arch anomalies associated with persistent truncus arteriosus includes: interruption of the aortic arch (9–19% of cases), and left (58–66%) or right-sided (21–34%) aortic arch [Citation14,Citation17]. The combination of truncus arteriosus with interrupted aortic arch is limited to case reports and small series [Citation36,Citation84–91], but carries a high early mortality and high risk of re-interventions in survivors [Citation92]. The association of truncus arteriosus with double aortic arch is rare [Citation93–96]. A high incidence of coronary ostial and arterial abnormalities (44–49%) has also been described in the presence of truncus arteriosus [Citation14,Citation17]. Although in most cases these are not of functional importance [Citation14,Citation17], they can contribute to a high operative mortality rate, and may be a cause of late sudden death [Citation97]. Truncus arteriosus has been described in association with tricuspid atresia/absence of right atrioventricular connection [Citation98–103], atrioventricular septal defects [Citation104], atrioventricular canal [Citation105,Citation106], single ventricle [Citation76,Citation107–109], and pulmonary atresia [Citation110,Citation111].
DiGeorge syndrome (facial, palate and ear anomalies, hypocalcemia, and defective thymic-dependent cellular immunity) is present in 30% of patients with truncus arteriosus [Citation112]. Other anomalies such as holoprosencephaly [Citation40], splenic agenesis/asplenia syndrome [Citation113], polysplenia syndrome [Citation105], diaphragmatic hernia, and esophageal atresia with distal tracheoesophageal fistula [Citation114] have been reported with truncus arteriosus.
Clinical presentation in the neonatal period
Truncus arteriosus, like transposition of the great arteries and total anomalous pulmonary venous return, is considered a cyanotic congenital cardiac anomaly characterized by increased pulmonary blood flow [Citation3]. Pulmonary blood flow, in contrast, is reduced in tetralogy of Fallot and tricuspid atresia, the other two major congenital cyanotic cardiopathies [Citation115]. In neonates affected by truncus arteriosus, cyanosis is not a constant feature. It can be minimal, intermittent, or even absent, because the large pulmonary flow itself may guarantee the resting arterial oxygen saturation to be close to normal [Citation116]. However, if the cardiac defect is unrecognized or left untreated, the increasing amount of mixed blood perfusing the pulmonary circulation leads to an increase in pulmonary vascular resistance and to cardiac heart failure, of which cyanosis is a constant feature [Citation116,Citation117]. Widened pulse pressure, bounding pulses [Citation117], as well as auscultatory murmurs [Citation116] are additional clinical features. Failure to thrive, respiratory infection or distress, and congestive heart failure [Citation116,Citation118] may manifest later according to the severity of the condition, and particularly, if the diagnosis and appropriate treatment are delayed. However, some cases are only diagnosed during adulthood [Citation119–122].
Prognostic factors
Predictors of poor neonatal outcome in the presence of truncus arteriosus include: severe truncal valve regurgitation, truncal valve stenosis, concomitant interrupted aortic arch, coronary abnormalities, associated anomalies, prematurity, birthweight <2.5–3.0 kg, as well as surgical variables (timing and type of surgical repair) [Citation6,Citation15,Citation118,Citation123–125]. Truncal valve regurgitation is the result of the presence of a single semilunar valve, which is frequently dysmorphic, dysfunctional or both [Citation126]. Persistency of moderate–severe insufficiency after surgical treatment reduces short- and long-term survival [Citation126]. Neonates with truncal valve stenosis (defined as a Doppler velocity ≥2 m/s across the valve on the postnatal echocardiogram) have a higher risk of early sudden death and can be potentially identified by prenatal Doppler velocimetry of the truncal valve [Citation15]. Prenatal assessment of these risk factors may assist in a more individualized parental counseling.
Acknowledgement
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Crupi G, Macartney FJ, Anderson RH. Persistent truncus arteriosus. A study of 66 autopsy cases with special reference to definition and morphogenesis. Am J Cardiol 1977;40:569–578.
- Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK. Septation and separation within the outflow tract of the developing heart. J Anat 2003;202:327–342.
- Grifka RG. Cyanotic congenital heart disease with increased pulmonary blood flow. Pediatr Clin North Am 1999;46:405–425.
- Appelbaum A, Bargeron LM Jr., Pacifico AD, Kirklin JW. Surgical treatment of truncus arteriosus, with emphasis on infants and small children. J Thorac Cardiovasc Surg 1976;71:436–440.
- Bove EL, Lupinetti FM, Pridjian AK, Beekman RH III, Callow LB, Snider AR, Rosenthal A. Results of a policy of primary repair of truncus arteriosus in the neonate. J Thorac Cardiovasc Surg 1993;105:1057–1065.
- Di Donato RM, Fyfe DA, Puga FJ, Danielson GK, Ritter DG, Edwards WD, McGoon DC. Fifteen-year experience with surgical repair of truncus arteriosus. J Thorac Cardiovasc Surg 1985;89:414–422.
- Ebert PA, Turley K, Stanger P, Hoffman JI, Heymann MA, Rudolph AM. Surgical treatment of truncus arteriosus in the first 6 months of life. Ann Surg 1984;200:451–456.
- Imamura M, Drummond-Webb JJ, Sarris GE, Mee RB. Improving early and intermediate results of truncus arteriosus repair: a new technique of truncal valve repair. Ann Thorac Surg 1999;67:1142–1146.
- Lacour-Gayet F, Serraf A, Komiya T, Sousa-Uva M, Bruniaux J, Touchot A, Roux D, Neuville P, Planche C. Truncus arteriosus repair: influence of techniques of right ventricular outflow tract reconstruction. J Thorac Cardiovasc Surg 1996;111:849–856.
- Pearl JM, Laks H, Drinkwater DC Jr., Milgalter E, Orrin AC, Giacobetti F, George B, Williams R. Repair of truncus arteriosus in infancy. Ann Thorac Surg 1991;52:780–786.
- Rajasinghe HA, McElhinney DB, Reddy VM, Mora BN, Hanley FL. Long-term follow-up of truncus arteriosus repaired in infancy: a twenty-year experience. J Thorac Cardiovasc Surg 1997;113:869–878.
- Rodefeld MD, Hanley FL. Neonatal truncus arteriosus repair: surgical techniques and clinical management. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5:212–217.
- Shrivastava S. Timing of surgery/catheter intervention in common congenital cardiac defects. Indian J Pediatr 2000;67:S2–S6.
- Van Praagh R. Editorial: classification of truncus arteriosus communis (TAC). Am Heart J 1976;92:129–132.
- Duke C, Sharland GK, Jones AM, Simpson JM. Echocardiographic features and outcome of truncus arteriosus diagnosed during fetal life. Am J Cardiol 2001;88:1379–1384.
- Volpe P, Paladini D, Marasini M, Buonadonna AL, Russo MG, Caruso G, Marzullo A, Vassallo M, Martinelli P, Gentile M. Common arterial trunk in the fetus: characteristics, associations, and outcome in a multicentre series of 23 cases. Heart 2003;89:1437–1441.
- Calder L, Van PR, Van PS, Sears WP, Corwin R, Levy A, Keith JD, Paul MH. Truncus arteriosus communis. Clinical, angiocardiographic, and pathologic findings in 100 patients. Am Heart J 1976;92:23–38.
- Van Praagh R, Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol 1965;16:406–425.
- Samanek M. Boy:girl ratio in children born with different forms of cardiac malformation: a population-based study. Pediatr Cardiol 1994;15:53–57.
- Clark EB. Pathogenetic mechanisms of congenital cardiovascular malformations revisited. Semin Perinatol 1996;20:465–472.
- Bartelings MM, Gittenberger-de Groot AC. Morphogenetic considerations on congenital malformations of the outflow tract. Part 1: common arterial trunk and tetralogy of Fallot. Int J Cardiol 1991;32:213–230.
- Carr I, Bharati S, Kusnoor VS, Lev M. Truncus arteriosus communis with intact ventricular septum. Br Heart J 1979;42:97–102.
- Murdison KA, McLean DA, Carpenter B, Duncan WJ. Truncus arteriosus communis associated with mitral valve and left ventricular hypoplasia without ventricular septal defect: unique combination. Pediatr Cardiol 1996;17: 322–326.
- Van Praagh R. Truncus arteriosus: what is it really and how should it be classified? Eur J Cardiothorac Surg 1987;1: 65–70.
- Ozkutlu S, Ayabakan C, Alehan D. Truncus arteriosus with a very small ventricular septal defect diagnosed by echocardiography. Pediatr Cardiol 2002;23:244–245.
- Mello DM, McElhinney DB, Parry AJ, Silverman NH, Hanley FL. Truncus arteriosus with patent ductus arteriosus and normal aortic arch. Ann Thorac Surg 1997;64:1808–1810.
- Collett RW, Edwards JE. Persistent truncus arteriosus; a classification according to anatomic types. Surg Clin North Am 1949;29:1245–1270.
- Jacobs ML. Congenital heart surgery nomenclature and database project: truncus arteriosus. Ann Thorac Surg 2000;69:S50–S55.
- Tometzki AJ, Suda K, Kohl T, Kovalchin JP, Silverman NH. Accuracy of prenatal echocardiographic diagnosis and prognosis of fetuses with conotruncal anomalies. J Am Coll Cardiol 1999;33:1696–1701.
- Benacerraf BR. Sonographic detection of fetal anomalies of the aortic and pulmonary arteries: value of four-chamber view vs. direct images. AJR Am J Roentgenol 1994;163:1483–1489.
- Allan L, Benacerraf B, Copel JA, Carvalho JS, Chaoui R, Eik-Nes SH, Tegnander E, Gembruch U, Huhta JC, Pilu G, et al Isolated major congenital heart disease. Ultrasound Obstet Gynecol 2001;17:370–379.
- Paladini D, Rustico M, Todros T, Palmieri S, Gaglioti P, Benettoni A, Russo MG, Chiappa E, D'Ottavio G. Conotruncal anomalies in prenatal life. Ultrasound Obstet Gynecol 1996;8:241–246.
- Achiron R, Rotstein Z, Lipitz S, Mashiach S, Hegesh J. First- trimester diagnosis of fetal congenital heart disease by transvaginal ultrasonography. Obstet Gynecol 1994;84: 69–72.
- Machlitt A, Tennstedt C, Korner H, Bommer C, Chaoui R. Prenatal diagnosis of 22q11 microdeletion in an early second-trimester fetus with conotruncal anomaly presenting with increased nuchal translucency and bilateral intracardiac echogenic foci. Ultrasound Obstet Gynecol 2002;19:510–513.
- Chen CP, Chern SR, Chang TY, Lee CC, Chen LF, Tzen CY, Wang W, Lin CJ, Yang BP, Yang LS. Prenatal diagnosis of mosaic ring chromosome 22 associated with cardiovascular abnormalities and intrauterine growth restriction. Prenat Diagn 2003;23:40–43.
- Marasini M, Cordone M, Zampatti C, Pongiglione G, Bertolini A, Ribaldone D. Prenatal ultrasonic detection of truncus arteriosus with interrupted aortic arch and truncal valve regurgitation. Eur Heart J 1987;8:921–924.
- Allan LD, Sharland GK, Milburn A, Lockhart SM, GrovesAM, Anderson RH, Cook AC, Fagg NL. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol 1994;23: 1452–1458.
- Muhler MR, Rake A, Schwabe M, Chaoui R, Heling KS, Planke C, Lembcke A, Fischer T, Kivelitz D. Truncus arteriosus communis in a midtrimester fetus: comparison of prenatal ultrasound and MRI with postmortem MRI and autopsy. Eur Radiol 2004;14:2120–2124.
- Costa P, Carrico A, Monterroso J, Matias A, Areias JC. Prenatal diagnosis of truncus arteriosus. Rev Port Cardiol 2005;24:135–136.
- Tongsong T, Khunamornpong S, Wanapirak C, Sirichotiyakul S. Prenatal sonographic diagnosis of truncus arteriosus associated with holoprosencephaly. J Clin Ultrasound 2005;33:193–196.
- Sharland GK. Common arterial trunk. Allan L. Hornberger L. Sharland GK. editors. Textbook of fetal cardiology. London: Greenwich Medical Media Limited; 2000. p 288–303.
- Pretorius DH, Nelson TR. Three-dimensional ultrasound. Ultrasound Obstet Gynecol 1995;5:219–221.
- Merz E, Bahlmann F, Weber G. Volume scanning in the evaluation of fetal malformations: a new dimension in prenatal diagnosis. Ultrasound Obstet Gynecol 1995;5:222–227.
- Benacerraf BR, Benson CB, Abuhamad AZ, Copel JA, Abramowicz JS, Devore GR, Doubilet PM, Lee W, Lev- Toaff AS, Merz E, et al Three- and 4-dimensional ultrasound in obstetrics and gynecology: proceedings of the American Institute of Ultrasound in Medicine Consensus Conference. J Ultrasound Med 2005;24:1587–1597.
- Benacerraf BR, Shipp TD, Bromley B. How sonographic tomography will change the face of obstetric sonography: a pilot study. J Ultrasound Med 2005;24:371–378.
- Goncalves LF, Nien JK, Espinoza J, Kusanovic JP, Lee W, Swope B, Soto E, Treadwell MC, Romero R. What does 2-dimensional imaging add to 3- and 4-dimensional obstetric ultrasonography? J Ultrasound Med 2006;25:691–699.
- Espinoza J, Kusanovic JP, Goncalves LF, Nien JK, Hassan S, Lee W, Romero R. A novel algorithm for comprehensive fetal echocardiography using 4-dimensional ultrasonography and tomographic imaging. J Ultrasound Med 2006;25:947–956.
- Devore GR, Polanco B, Sklansky MS, Platt LD. The ‘spin’ technique: a new method for examination of the fetal outflow tracts using three-dimensional ultrasound. Ultrasound Obstet Gynecol 2004;24:72–82.
- Espinoza J, Hassan SS, Gotsch F, Kusanovic JP, Lee W, Erez O, Goncalves LF, Schoen ML, Romero R. A systematic approach to the use of the multiplanar display in evaluation of abnormal vascular connections to the fetal heart using 4-dimensional ultrasonography. J Ultrasound Med 2007;26:1461–1467.
- Devore GR. Three-dimensional and four-dimensional fetal echocardiography: a new frontier. Curr Opin Pediatr 2005;17:592–604.
- Espinoza J, Romero R, Kusanovic JP, Gotsch F, Lee W, Goncalves LF, Hassan SS. Standardized views of the fetal heart using four-dimensional sonographic and tomographic imaging. Ultrasound Obstet Gynecol 2008;31:233–242.
- Goncalves LF, Espinoza J, Romero R, Kusanovic JP, SwopeB, Nien JK, Erez O, Soto E, Treadwell MC. Four-dimensional ultrasonography of the fetal heart using a novel Tomographic Ultrasound Imaging display. J Perinat Med 2006;34:39–55.
- Paladini D, Vassallo M, Sglavo G, Lapadula C, Martinelli P. The role of spatio-temporal image correlation (STIC) with tomographic ultrasound imaging (TUI) in the sequential analysis of fetal congenital heart disease. Ultrasound Obstet Gynecol 2006;27:555–561.
- Kalache KD, Bamberg C, Proquitte H, Sarioglu N, Lebek H, Esser T. Three-dimensional multi-slice view: new prospects for evaluation of congenital anomalies in the fetus. J Ultrasound Med 2006;25:1041–1049.
- Pooh RK. New application of B-flow sono-angiography in perinatology. Ultrasound Obstet Gynecol 2000;15:163.
- Goncalves LF, Espinoza J, Romero R, Lee W, Beyer B, Treadwell MC, Humes R. A systematic approach to prenatal diagnosis of transposition of the great arteries using 4- dimensional ultrasonography with spatiotemporal image correlation. J Ultrasound Med 2004;23:1225–1231.
- Moore JW, Wight NE, Jones MC, Krous HF. Truncus arteriosus associated with trisomy 18. Pediatr Cardiol 1994;15:154–156.
- Digilio MC, Angioni A, Giannotti A, Dallapiccola B, Marino B. Truncus arteriosus and duplication 8q. Am J Med Genet A 2003;121:79–81.
- Silengo M, Rulli I, Delmonaco AG, Ferrero GB, Pucci A, Sanna R. Truncus arteriosus and isochromosome 8q. Am J Med Genet A 2005;133:223–224.
- Finley BE, Seguin JH, Bennett TL, Ardinger R, Burlbaw J, Levitch L, Keifer C, Pasztor L. Terminal deletion of 7q presenting in utero with a truncus arteriosus and nonimmune hydrops. Am J Med Genet 1993;47:221–222.
- Harris RD. Obstetrics. 22q11 deletion syndrome with truncus arteriosus, hypoplastic left ventricle, VSD, clubfoot, sandal toes, cleft palate, butterfly vertebrae. Ultrasound Q 2005;21:107–108 (see also 125–126).
- McElhinney DB, Driscoll DA, Emanuel BS, Goldmuntz E. Chromosome 22q11 deletion in patients with truncus arteriosus. Pediatr Cardiol 2003;24:569–573.
- Mehraein Y, Wippermann CF, Michel-Behnke I, Nhan Ngo TK, Hillig U, Giersberg M, Aulepp U, BarthH, Fritz B, Rehder H. Microdeletion 22q11 in complex cardiovascular malformations. Hum Genet 1997;99:433–442.
- Momma K, Ando M, Matsuoka R. Truncus arteriosus communis associated with chromosome 22q11 deletion. J Am Coll Cardiol 1997;30:1067–1071.
- Marino B, Digilio MC, Toscano A, Anaclerio S, Giannotti A, Feltri C, de Ioris MA, Angioni A, Dallapiccola B. Anatomic patterns of conotruncal defects associated with deletion 22q11. Genet Med 2001;3:45–48.
- Brunet A, Gabau E, Perich RM, Valdesoiro L, Brun C, Caballin MR, Guitart M. Microdeletion and microduplication 22q11.2 screening in 295 patients with clinical features of DiGeorge/Velocardiofacial syndrome. Am J Med Genet A 2006;140:2426–2432.
- Chaoui R, Kalache KD, Heling KS, Tennstedt C, Bommer C, Korner H. Absent or hypoplastic thymus on ultrasound: a marker for deletion 22q11.2 in fetal cardiac defects. Ultrasound Obstet Gynecol 2002;20:546–552.
- Digilio MC, Marino B, Musolino AM, Giannotti A, Dallapiccola B. Familial recurrence of nonsyndromic interrupted aortic arch and truncus arteriosus with atrioventricular canal. Teratology 2000;61:329–331.
- Werner P, Raducha MG, Prociuk U, Ostrander EA, Spielman RS, Kirkness EF, Patterson DF, Henthorn PS. The keeshond defect in cardiac conotruncal development is oligogenic. Hum Genet 2005;116:368–377.
- Marino B, Digilio MC. Congenital heart disease and genetic syndromes: specific correlation between cardiac phenotype and genotype. Cardiovasc Pathol 2000;9:303–315.
- Tikkanen J, Heinonen OP. Risk factors for conal malformations of the heart. Eur J Epidemiol 1992;8:48–57.
- Abushaban L, Uthaman B, Kumar AR, Selvan J. Familial truncus arteriosus: a possible autosomal-recessive trait. Pediatr Cardiol 2003;24:64–66.
- Raatikka M, Rapola J, Tuuteri L, Louhimo I, Savilahti E. Familial third and fourth pharyngeal pouch syndrome with truncus arteriosus: DiGeorge syndrome. Pediatrics 1981;67:173–175.
- Goodyear JE. Persistent truncus arteriosus in two siblings. Br Heart J 1961;23:194–196.
- Brunson SC, Nudel DB, Gootman N, Aftalion B. Truncus arteriosus in a family. Am Heart J 1978;96:419–420.
- Shapiro SR, Ruckman RN, Kapur S, Chandra R, Galioto FM, Perry LW, Scott LP III. Single ventricle with truncus arteriosus in siblings. Am Heart J 1981;102:456–459.
- Mas C, Delatycki MB, Weintraub RG. Persistent truncus arteriosus in monozygotic twins: case report and literature review. Am J Med Genet 1999;82:146–148.
- Lang MJ, Aughton DJ, Riggs TW, Milad MP, Biesecker LG. Dizygotic twins concordant for truncus arteriosus. Clin Genet 1991;39:75–79.
- Kreutner AK, Levine J, Thiede H. A double truncus arteriosus in thoracopagus twins. N Engl J Med 1963;268:1388–1390.
- Miller ME, Smith DW. Conotruncal malformation complex: examples of possible monogenic inheritance. Pediatrics 1979;63:890–893.
- Patterson DF, Pexieder T, Schnarr WR, Navratil T, Alaili R. A single major-gene defect underlying cardiac conotruncal malformations interferes with myocardial growth during embryonic development: studies in the CTD line of keeshond dogs. Am J Hum Genet 1993;52:388–397.
- Pierpont ME, Gobel JW, Moller JH, Edwards JE. Cardiac malformations in relatives of children with truncus arteriosus or interruption of the aortic arch. Am J Cardiol 1988;61:423–427.
- Kuribayashi T, Roberts WC. Tetralogy of Fallot, truncus arteriosus, abnormal myocardial architecture and anomalies of the aortic arch system induced by bis- diamine in rat fetuses. J Am Coll Cardiol 1993;21:768–776.
- Fujiwara K, Yokota Y, Okamoto F, Kiyota Y, Sugawara E, Iemura J, Makino S. Successful surgical repair of truncus arteriosus with interrupted aortic arch in infancy by an anterior approach. Ann Thorac Surg 1988;45:441–444.
- McKay R, Miyamoto S, Peart I, Battistessa SA, Wren C, Cunliffe M, Robles A. Truncus arteriosus with interrupted aortic arch: successful correction in a neonate. Ann Thorac Surg 1989;48:587–589.
- Raudkivi PJ, Sutherland GR, Edwards JC, Manners JM, Keeton BR, Monro JL. Truncus arteriosus with type B interrupted aortic arch: correction in the neonate. Pediatr Cardiol 1990;11:117–119.
- Sano S, Brawn WJ, Mee RB. Repair of truncus arteriosus and interrupted aortic arch. J Card Surg 1990;5:157–162.
- Rao IM, Swanson JS, Hovaguimian H, McIrvin DM, King DH, Starr A. Anterior pulmonary translocation for repair of truncus arteriosus with interrupted arch. Ann Thorac Surg 1995;59:216–218.
- Berdjis F, Wells WJ, Starnes VA. Truncus arteriosus with total anomalous pulmonary venous return and interrupted arch. Ann Thorac Surg 1996;61:220–222.
- Yasukochi S, Satomi G. Fetal diagnosis of common arterial trunk with interrupted aortic arch using color power Doppler angiography. Cardiol Young 2000;10:54–56.
- Ishizaka T, Allen SW, Strouse PJ, Ohye RG. Postductal origin of the left carotid, left subclavian, and aberrant retroesophageal right innominate arteries in truncus arteriosus with interrupted aortic arch. Pediatr Cardiol 2003;24:581–584.
- Konstantinov IE, Karamlou T, Blackstone EH, MoscaRS, Lofland GK, Caldarone CA, Williams WG, Mackie AS, McCrindle BW. Truncus arteriosus associated with interrupted aortic arch in 50 neonates: a congenital heart surgeons society study. Ann Thorac Surg 2006;81: 214–222.
- Pacileo G, Palma G, Russo MG, Vosa C, Calabro R. Truncus arteriosus and double aortic arch associated with DiGeorge syndrome. Tex Heart Inst J 1991;18:206–208.
- Alboliras ET, Lombardo S, Antillon J. Truncus arteriosus with double aortic arch: two-dimensional and color flow Doppler echocardiographic diagnosis. Am Heart J 1995;129: 415–417.
- Paul JF, Serraf A. Images in cardiovascular medicine. Truncus arteriosus and double aortic arch. Circulation 2002;105:e170.
- Bhan A, Gupta M, Kumar MJ, Kothari SS, Gulati GS. Persistent truncus arteriosus with double aortic arch. Pediatr Cardiol 2006;27:378–380.
- Lenox CC, Debich DE, Zuberbuhler JR. The role of coronary artery abnormalities in the prognosis of truncus arteriosus. J Thorac Cardiovasc Surg 1992;104: 1728–1742.
- Areias JC, Lopes JM. Common arterial trunk associated with absence of one atrioventricular connexion. Int J Cardiol 1987;17:329–332.
- Diogenes TC, Atik E, Aiello VD. Common arterial trunk associated with absence of right atrioventricular connexion. Int J Cardiol 1990;27:385–388.
- Rao PS, Levy JM, Nikicicz E, Gilbert-Barness EF. Tricuspid atresia: association with persistent truncus arteriosus. Am Heart J 1991;122:829–835.
- Sharma D, Mehta AB, Bharati S, Lev M. Tricuspid atresia with persistent truncus arteriosus. Chest 1981;79:363–365.
- Tandon R, Moller JH, Edwards JE. Persistent truncus arteriosus associated with tricuspid atresia. Minn Med 1974;57:448–450.
- Wang JN, Wu MH, Wang JK, Lue HC. Tricuspid atresia with persistent truncus arteriosus. J Formos Med Assoc 1999;98:290–291.
- Atik E, Soares AM, Aiello VD. Common arterial trunk associated with atrioventricular septal defect. Cardiol Young 1999;9:617–620.
- Arai H, Harada K, Tamura M, Okamura T, Takada G. Polysplenia syndrome with common atrioventricular canal and persistent truncus arteriosus. Tohoku J Exp Med 1995;177:171–177.
- Sousa-Uva M, Serraf A, Cloez JL, Lacour-Gayet F, Roux D, Bruniaux J, Piot D, Petit J, Planche C. Repair of truncus arteriosus and complete atrioventricular canal defect. J Thorac Cardiovasc Surg 1994;108:385–387.
- Paris YM, Bhan I, Marx GR, Rhodes J. Truncus arteriosus with a single left ventricle: case report of a previously unrecognized entity. Am Heart J 1997;133:377–380.
- Shaddy RE, McGough EC. Successful diagnosis and surgical treatment of single ventricle, truncus arteriosus. Ann Thorac Surg 1989;48:298–300.
- Siddoway JL Jr., Chernish SM. Truncus arteriosus associated with single ventricle. AMA Am J Dis Child 1952;84:706–717.
- Schofield DE, Anderson RH. Common arterial trunk with pulmonary atresia. Int J Cardiol 1988;20:290–294.
- Tongsong T, Sirichotiyakul S, Sukpan K, Sittiwangkul R. Prenatal features of a truncus arteriosus with pulmonary atresia and pulmonary circulation derived from the ductus arteriosus. J Ultrasound Med 2004;23:1221–1224.
- Van Mierop LH, Kutsche LM. Cardiovascular anomalies in DiGeorge syndrome and importance of neural crest as a possible pathogenetic factor. Am J Cardiol 1986;58:133–137.
- Gumbiner CH, McManus BM, Latson LA. Associated occurrence of persistent truncus arteriosus and asplenia. Pediatr Cardiol 1991;12:192–195.
- Cunat V, Stranak Z, Pycha K, Tlaskal T, Melichar J, Miletin J, Janota J, Kucera J, Velebil P. Congenital diaphragmatic hernia associated with esophageal atresia, tracheoesophageal fistula, and truncus arteriosus in a premature newborn. Pediatr Surg Int 2005;21:684–686.
- Waldman JD, Wernly JA. Cyanotic congenital heart disease with decreased pulmonary blood flow in children. Pediatr Clin North Am 1999;46:385–404.
- Victorica BE, Krovetz LJ, Elliott LP, Van Mierop LH, Bartley TD, Gessner IH, Schiebler GL. Persistent truncus arteriosus in infancy. A study of 14 cases. Am Heart J 1969;77:13–25.
- Westmoreland D. Critical congenital cardiac defects in the newborn. J Perinat Neonatal Nurs 1999;12:67–87.
- Williams JM, de LM, Black MD, Freedom RM, Williams WG, McCrindle BW. Factors associated with outcomes of persistent truncus arteriosus. J Am Coll Cardiol 1999;34:545–553.
- Bosatra MG, Passarani S, Marino MR, Marcolin R, Fumagalli R, Pesenti A. Caesarean delivery of a patient with truncus arteriosus. Int J Obstet Anesth 1997;6:279–284.
- Carvalho G, Silva AA, Bestetti RB, Leme-Neto AC. Long-term survival in truncus arteriosus communis type A1 associated with Ehlers-Danlos syndrome – a case report. Angiology 2002;53:363–365.
- Gutierrez PS, Binotto MA, Aiello VD, Mansur AJ. Chest pain in an adult with truncus arteriosus communis. Am J Cardiol 2004;93:272–273.
- Haskal ZJ. SIR 2005 annual meeting film panel case: hemoptysis and bronchial artery embolization in an adult with uncorrected truncus arteriosus and Eisenmenger syndrome. J Vasc Interv Radiol 2005;16:635–638.
- Hanley FL, Heinemann MK, Jonas RA, Mayer JE Jr., Cook NR, Wessel DL, Castaneda AR. Repair of truncus arteriosus in the neonate. J Thorac Cardiovasc Surg 1993;105:1047–1056.
- Brizard CP, Cochrane A, Austin C, Nomura F, KarlTR. Management strategy and long-term outcome for truncus arteriosus. Eur J Cardiothorac Surg 1997;11: 687–695.
- Thompson LD, McElhinney DB, Reddy M, Petrossian E, Silverman NH, Hanley FL. Neonatal repair of truncus arteriosus: continuing improvement in outcomes. Ann Thorac Surg 2001;72:391–395.
- McElhinney DB, Reddy VM, Rajasinghe HA, Mora BN, Silverman NH, Hanley FL. Trends in the management of truncal valve insufficiency. Ann Thorac Surg 1998;65: 517–524.