Abstract
Objective. Vaginal bleeding, placental abruption, and defective placentation are frequently observed in patients with preterm prelabor rupture of membranes (PROM). Recently, a role of vascular endothelial growth factor (VEGF) and its receptor, VEGF receptor (VEGFR)- 1 has been implicated in the mechanisms of membrane rupture. The purpose of this study was to determine whether the soluble form of VEGFR-1 and -2 concentrations in amniotic fluid (AF) change with preterm PROM, intra-amniotic infection/inflammation (IAI), or parturition.
Study design. This cross-sectional study included 544 patients in the following groups: (1) midtrimester (MT) (n = 48); (2) preterm labor (PTL) leading to term delivery (n = 143); (3) PTL resulting in preterm delivery with (n = 72) and without IAI (n = 100); (4) preterm PROM with (n = 46) and without IAI (n = 42); (5) term in labor (n = 48); and (6) term not in labor (n = 45). The concentrations of sVEGFR-1 and sVEGFR-2 were determined by ELISA. Non-parametric statistics and logistic regression analysis were applied.
Results. (1) Preterm PROM (with and without IAI) had a lower median AF concentration of sVEGFR-1 than patients with PTL who delivered at term (p < 0.001 for each comparison); (2) A decrease in AFsVEGFR-1 concentrations per each quartile was associated with PROM after adjusting for confounders (OR 1.8; 95%CI 1.4–2.3); (3) IAI, regardless of the membrane status, was not associated with a change in the median AF concentrations of sVEGFR-1 and sVEGFR-2 (p > 0.05 for each comparison); and (4) Spontaneous term and PTL did not change the median sVEGFR-1 and sVEGFR-2 concentrations (p > 0.05 for each comparison).
Conclusion. (1) This is the first evidence that preterm PROM is associated with a lower AF concentration of sVEGFR-1 than patients with PTL intact membranes. These findings cannot be attributed to gestational age, labor, or IAI; and (2) AF concentrations of sVEGFR-2 did not change with preterm PROM, IAI, or labor at term and preterm.
Introduction
Preterm prelabor rupture of membranes (PROM) is responsible for 30–40% of preterm deliveries and therefore is a leading cause of preterm birth, as well as a major contributor to perinatal morbidity and mortality worldwide [Citation1–12]. A previous study examining the placental lesions from patients with preterm PROM reported that such placentas have two major pathologic findings: acute inflammatory lesions (acute histologic chorioamnionitis and/or funisitis) and vascular lesions (multiple infarction, intervillous thrombosis, and thrombosis or narrowing of spiral arteries) [Citation13]. The mechanisms by which microbial invasion of the amniotic cavity and inflammation lead to membrane weakening and rupture have been extensively studied [Citation5,Citation12,Citation14–26]. In contrast, a few studies have examined the mechanism responsible for vascular pathology [Citation27–31].
A major risk factor for preterm PROM is vaginal bleeding [Citation32,Citation33]. Women presenting with preterm PROM are also at increased risk of developing placental abruption [Citation34]. Moreover, a failure of physiologic transformation of the myometrial segment of the spiral artery, suggestive of defective placentation, is frequently observed in preterm PROM [Citation35]. However, the precise mechanisms, by which, the vascular changes and disruption of vascular integrity lead to preterm PROM remain unknown.
Pregnancy is a state that requires extensive angiogenesis in both maternal and fetal compartments [Citation36–38]. Vascular endothelial growth factor (VEGF) is a potent angiogenic factor. Its primary site of action is vascular endothelium and its main function is to promote angiogenesis, a process by which new vessels form from pre-existing vasculature [Citation39], through endothelial cell proliferation, migration [Citation40,Citation41], and prevention of endothelial cell apoptosis [Citation42]. VEGF exerts its action through binding to specific receptors, i.e. VEGF receptor-1 and -2 (VEGFR-1 and 2) [Citation43–47]. VEGFR-2 regulates endothelial cell division and migration, whereas the function of VEGFR-1 remains unclear and is thought to be a decoy receptor for VEGF. Both receptors of VEGF have soluble forms which contain an extracellular domain without intracellular portion of these proteins. The soluble form of VEGFR-1 has been found to have anti-angiogenic activity by binding to VEGF and preventing VEGF from binding to VEGFR-2 [Citation40,Citation48]. Similarly, under experimental conditions, the recombinant sVEGFR-2 has anti-angiogenic activity [Citation49,Citation50].
Normal pregnancy requires a balance between angiogenic and anti-angiogenic factors. The loss of only one copy of the VEGF gene is uniformly lethal during embryonic life [Citation51,Citation52]. An imbalance of angiogenic and anti-angiogenic factors of VEGF-signaling system in maternal circulation has been implicated in the pathophysiology of several obstetrical syndromes including preeclampsia [Citation53–66], pregnancies with small for gestational age fetuses [Citation66–69], placental abruption [Citation70], fetal death [Citation71], twin to twin transfusion syndrome [Citation72], ‘mirror syndrome’ [Citation73], and spontaneous preterm parturition [Citation74].
Both VEGF and their receptors (VEGFR-1 and -2) are expressed in the human amnion [Citation75,Citation76]. The precise role of VEGF and its receptors in the amnion is unknown, however, they have been implicated in the regulation of amniotic fluid (AF) volume [Citation77,Citation78]. Recently, VEGF and VEGFR-1 in the fetal membranes have also been suggested to play a role in the pathophysiology of preterm PROM [Citation75]. Since VEGF and sVEGFR-1 are present in human AF [Citation59,Citation79,Citation80], it is likely that sVEGFR-1 and sVEGFR-2, the natural antagonists of VEGF, are involved in the regulation of VEGF activity in AF cavity and play a role in the maintenance of normal pregnancy. The purpose of this study was to determine whether sVEGFR-1 and sVEGFR-2 concentrations in AF change with gestational age, preterm PROM, intra-amniotic infection/inflammation (IAI) or preterm and term parturition.
Materials and methods
Study design and population
A cross sectional study was designed by searching our clinical database and bank of biological samples, and included 544 patients in the following groups: (1) women in the midtrimester (MT) of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n = 48); (2) patients with an episode of spontaneous preterm labor (PTL) and intact membranes who delivered at term (n = 143); (3) patients with PTL and intact membranes who delivered preterm (<37 weeks gestation) with IAI (n = 72) and without IAI (n = 100); (4) patients with preterm PROM with IAI (n = 46) and without IAI (n = 42); (5) normal pregnant women at term with spontaneous labor (n = 48); and (6) normal pregnant women at term without spontaneous labor (n = 45).
All women provided written informed consent before the collection of AF. The collection and utilization of the samples were approved by the Human Investigation Committee of the Sotero del Rio Hospital, Santiago, Chile (a major affiliate of the Catholic University of Santiago), the Institutional Review Boards of the Wayne State University, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been used in previous studies.
Definitions
Preterm PROM was diagnosed by sterile speculum examination confirming pooling of AF in the vagina in association with positive nitrazine and ferning tests when necessary, before 37 weeks of gestation and in the absence of labor. Spontaneous PTL was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 min associated with cervical change before 37 completed weeks of gestation that required hospitalization. Intra-amniotic infection was defined as a positive AF culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an AF interleukin (IL)-6 concentration ≥2.6 ng/ml [Citation81]. The patients were considered to have a normal pregnancy outcome if they did not have any medical, obstetrical, or surgical complications, and delivered a term neonate (≥37 weeks) of appropriate birth weight for gestational age [Citation82,Citation83] without complications. Small for gestational age was defined as birthweight below the 10th percentile according to the reference range proposed by Alexander et al. [Citation82] or Gonzalez et al. [Citation83], depending on ethnicity.
Sample collection
AF samples were obtained by transabdominal amniocentesis performed for genetic indications, evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity in patients approaching term. Women at term in labor consisted of women who were admitted for suspected PTL because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering that these patients were at term in labor was derived retrospectively, if the following criteria were met: (1) spontaneous labor; (2) delivery within 24 h from amniocentesis; (3) analysis of AF consistent with fetal lung maturity; (4) birthweight >2500 g; (5) absence of respiratory distress syndrome or other complications of prematurity; and (6) physical examination of the newborn by the pediatricians consistent with a term neonate. Samples of AF were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell count, glucose concentration, and gram-stain were also performed shortly after collection as previously described [Citation84–86]. The results of these tests were used for clinical management. AF IL-6 concentrations were used only for research purposes. AF not required for clinical assessment was centrifuged for 10 min at 4°C and the supernatant was stored at −70°C until analysis.
Determination of soluble VEGFR-1 and VEGFR-2 immunoassay in amniotic fluid
AF concentrations of sVEGFR-1 and sVEGFR-2 were determined by sensitive and specific immunoassays obtained from R&D Systems (Minneapolis, MN). The immunoassay systems were validated for using human AF before the conduction of this study. Briefly, the immunoassay utilized the quantitative sandwich enzyme immunoassay technique and their concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for sVEGFR-1 were 1.4% and 3.9%, respectively; and for sVEGFR-2, 2%, and 4%, respectively. The sensitivities of the assays were 0.016 ng/ml for sVEGFR-1, and 0.019 ng/ml for sVEGFR-2.
Statistical analysis
The normality of the data was tested using Shapiro–Wilk and Kolmogorov–Smirnov tests. Because AF concentrations of sVEGFR-1 and sVEGFR-2 were not normally distributed, even after logarithmic transformation, non-parametric statistics were used for analysis. Comparisons between proportions were performed with contingency tables, Chi-square and Fisher's Exact test. Kruskal–Wallis and Mann–Whitney U tests were used to determine the differences of the median among and between groups, respectively. Multivariate logistic regression (step-wise) was applied to examine the association between AF concentrations of sVEGFR-1 and the presence of preterm PROM while adjusting for potential confounders. Spearman rank correlation was utilized to assess correlations between two continuous variables. Analysis was conducted with SPSS v.12 (SPSS, Chicago, IL). A p value of <0.05 was considered significant.
Results
Demographic and clinical characteristics of the study population
presents the demographic and clinical characteristics of patients in the MT, term not in labor and term in labor groups. and display the demographic and clinical characteristics of patients with spontaneous PTL and intact membranes and those with preterm PROM, respectively. Among patients with PTL with intact membranes, those with IAI had a significantly lower median gestational age at amniocentesis than those without IAI who delivered preterm and those who delivered at term (p < 0.001 for all; ). Similarly, the median gestational age at amniocentesis of patients with preterm PROM with IAI was lower than that of those without IAI (p = 0.02; ).
Table I. Demographic and clinical characteristics of patients in the midtrimester and those at term with and without spontaneous labor.
Table II. Demographic and clinical characteristics of patients presenting with spontaneous preterm labor with intact membranes.
Table III. Demographic and clinical characteristics of patients presenting with preterm prelabor rupture of membranes.
Changes of amniotic fluid concentrations of sVEGFR-1 and sVEGFR-2 in pregnancy
Although sVEGFR-1 was detected in all samples, sVEGFR-2 was detected in 99% (541/544) of patients. Three AF samples (two from patients with PTL who delivered at term and one from a patient at term in labor) had sVEGFR-2 concentrations below the limit of detection of the immunoassay. Women with PTL and intact membranes who delivered at term had a significantly higher median AF sVEGFR-1 concentration (median 101 ng/ml; range 0.1–595.6 ng/ml) than those in the MT (median 33 ng/ml; range 10.7–74.3 ng/ml; p < 0.001) and than those at term not in labor (median 64 ng/ml; range 10.6–373.4 ng/ml; p = 0.01; ). Similar to sVEGFR-1, the changes of AF concentrations of sVEGFR-2 as a function of gestational age followed the same trend as sVEGFR-1. Women with PTL who delivered at term had a significantly higher median AF sVEGFR-2 concentration (median 0.7 ng/ml; range 0–3.7 ng/ml) than those in the MT (median 0.4 ng/ml; range 0.1–1.5 ng/ml; p = 0.002) and than those at term not in labor (median 0.5 ng/ml; range 0.1–9.3 ng/ml; p = 0.03; ). Collectively, the AF concentrations of sVEGFR-1 and sVEGFR-2 increased from the MT to 24–28 weeks of gestation (sVEGFR-1: median 140.6 ng/ml; range 1.4–454 ng/ml; and for sVEGFR-2: median 1.2 ng/ml; range 0.2–3.9 ng/ml), and then declined until term.
Figure 1. Amniotic fluid concentrations of sVEGFR-1 in normal pregnant women at midtrimester, patients with preterm labor (PTL) with intact membranes who delivered at term, and women at term without labor. Patients with PTL who delivered at term (median: 101 ng/ml; range 0.1–595.6 ng/ml) had a significantly higher median sVEGFR-1 concentration in amniotic fluid than women in the midtrimester (median: 33 ng/ml; range: 10.7–74.3 ng/ml; p < 0.001) and than those at term not in labor (median: 64 ng/ml; range: 10.6–373.4 ng/ml; p = 0.01). Women at term not in labor had a significantly higher median amniotic fluid concentration of sVEGFR-1 than those in the midtrimester (p < 0.001). LOD: limit of detection. *p < 0.05.
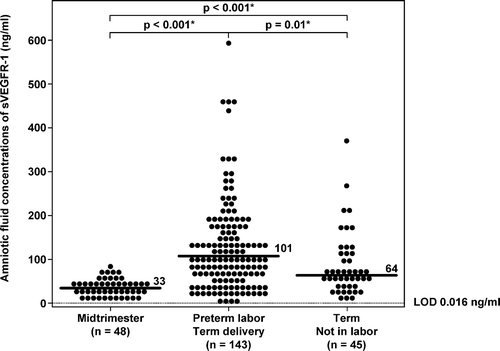
Figure 2. Amniotic fluid concentrations of sVEGFR-2 in normal pregnant women at midtrimester, patients with PTL with intact membranes who delivered at term, and women at term without labor. Patients with PTL who delivered at term (median: 0.7 ng/ml; range: 0–3.7 ng/ml) had a significantly higher median amniotic fluid concentration of sVEGFR-2 than women in midtrimester (median: 0.4 ng/ml; range: 0.1–1.5 ng/ml; p = 0.002) and than women at term not in labor (median: 0.5 ng/ml; range: 0.1–9.3 ng/ml; p = 0.03). There was no significant difference in the median amniotic fluid concentration of sVEGFR-2 between normal pregnant women at term not in labor and those in the midtrimester (p = 0.4). LOD, limit of detection. *p < 0.05.
Preterm PROM (with and without IAI) is associated with a decrease in amniotic fluid concentrations of sVEGFR-1
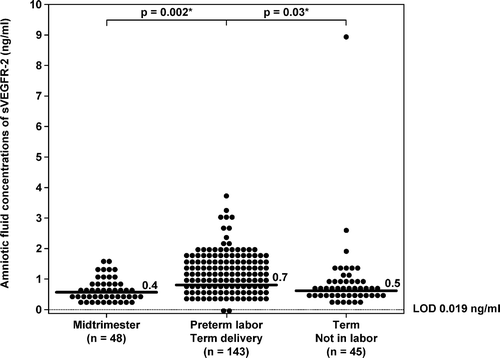
There was no significant difference in the median gestational age at amniocentesis between patients with PTL who delivered at term and those with preterm PROM with and without IAI (p > 0.05 for each comparison). Patients with preterm PROM, regardless of IAI, had a significantly lower median AF concentration of sVEGFR-1 than those with PTL who delivered at term (preterm PROM without IAI: median 56 ng/ml; range: 8.9–398.2 ng/ml, preterm PROM with IAI: median 45 ng/ml; range: 1.4–255.6 ng/ml and PTL who delivered at term: median 101 ng/ml; range: 0.1–595.6 ng/ml; p = 0.002 and p < 0.001, respectively; ). In contrast, there was no significant difference in the median AF sVEGFR-2 concentrations among the three groups (preterm PROM without IAI: 0.7 ng/ml; range: 0.1–2.0 ng/ml, preterm PROM with IAI: 0.6 ng/ml; range: 0.1–3.7 ng/ml, and PTL who delivered at term: 0.7 ng/ml; range: 0.0–3.7 ng/ml; p = 0.8 and p = 0.5, respectively; ).
Figure 3. Amniotic fluid concentrations of sVEGFR-1 in patients with preterm labor (PTL) and intact membranes who delivered at term, and in those with preterm prelabor rupture of membranes (PROM) with and without intra-amniotic infection/inflammation (IAI). Patients with preterm PROM with (median: 45 ng/ml; range:1.4–255.6 ng/ml) and without IAI (median: 56 ng/ml; range: 8.9–398.2 ng/ml) had a significantly lower median amniotic fluid concentration of sVEGFR-1 than those with PTL who delivered at term (median: 101 ng/ml; range: 0.1–595.6 ng/ml) (p < 0.001 and p = 0.002; respectively). Amniotic fluid sVEGFR-1 concentrations did not change with the presence of IAI (p = 0.3). LOD, limit of detection. *p < 0.05.
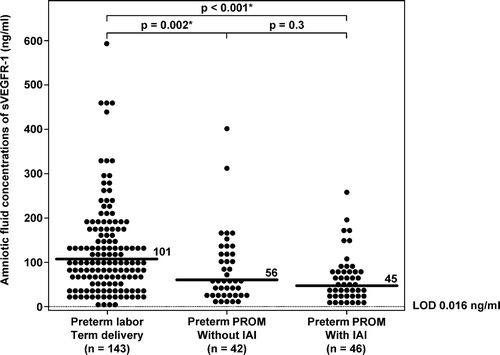
Figure 4. Amniotic fluid concentrations of sVEGFR-2 in patients with preterm labor (PTL) and intact membranes who delivered at term, and in those with preterm prelabor rupture of membranes (PROM) with and without intra-amniotic infection/inflammation (IAI). There were no significant differences in the median amniotic fluid sVEGFR-2 concentrations between patients with PTL who delivered at term (median: 0.7 ng/ml; range: 0.0–3.7 ng/ml) and those with preterm PROM without IAI (median: 0.7 ng/ml; range: 0.1–2.0 ng/ml; p = 0.8) and between patients with PTL and those with preterm PROM with IAI (median: 0.6 ng/ml; range: 0.1–3.7 ng/ml; p = 0.5). LOD, limit of detection.
Among patients with preterm gestations (PTL and preterm PROM), the frequency of preterm PROM increased as AF concentrations of sVEGFR-1 decreased from the 4th to the 1st quartile (Q4: 9.9% (10/101), Q3: 11.8% (12/102), Q2: 28% (28/100) and Q1: 38% (38/99); chi-square for trend p < 0.001). The association between a decrease in AF sVEGFR-1 concentrations per quartile and preterm PROM remained significant after adjusting for gestational age at amniocentesis (weeks), maternal age (years), smoking, nulliparity, the presence of small-for-gestational age neonates, AF concentrations of IL-6 (ng/ml), and duration of sample storage (years) (OR 1.8; 95% CI 1.4–2.4).
Intra-amniotic infection/inflammation is not associated with changes in amniotic fluid concentrations of sVEGFR-1 and sVEGFR-2
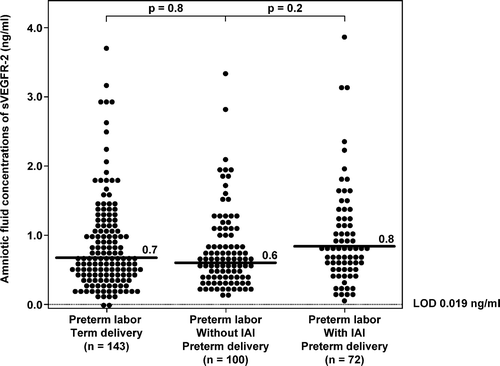
IAI in patients with preterm PROM did not significantly change the median AF concentration of sVEGFR-1 (p = 0.3; ) or that of sVEGFR-2 (p = 0.5; ). Similarly, among patients with PTL who delivered preterm, there was no significant difference in the median AF concentration of sVEGFR-1 or sVEGFR-2 between patients with and without IAI (sVEGFR-1; PTL without IAI: median 99.4 ng/ml; range: 0.7–459.0 ng/ml vs. PTL with IAI: median 98.7 ng/ml; range: 2.0–544.8 ng/ml; p = 0.1; ; and sVEGFR-2; PTL without IAI: median 0.6 ng/ml; range: 0.2–3.3 ng/ml vs. PTL with IAI: median 0.8 ng/ml; range: 0.1–3.9 ng/ml; p = 0.2; ). There was no association between AF concentrations of sVEGFR-1 or sVEGFR-2 with IAI in either patients with PTL or those with preterm PROM after adjusting for gestational age at amniocentesis by logistic regression analysis (p > 0.05).
Figure 5. Amniotic fluid concentrations of sVEGFR-1 among women with spontaneous preterm labor (PTL) and intact membranes. There were no significant differences in the median amniotic fluid sVEGFR-1 concentrations among the subgroups of patients with PTL (PTL with term delivery: median: 101 ng/ml; range: 0.1–595.6 ng/ml; PTL who delivered preterm without IAI: median: 99.4 ng/ml; range: 0.7–459 ng/ml; PTL who delivered preterm with IAI: median: 98.7 ng/ml; range: 2.0–544.8 ng/ml; all p > 0.05). LOD, limit of detection.
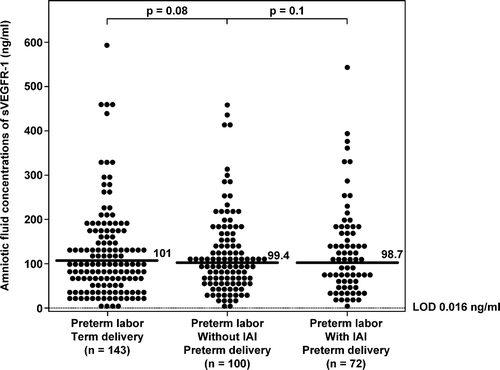
Figure 6. Amniotic fluid concentrations of sVEGFR-2 among women with spontaneous preterm labor (PTL) and intact membranes. There were no significant differences in the median amniotic fluid sVEGFR-2 concentrations among the subgroups of patients with PTL (PTL with term delivery: median: 0.7 ng/ml; range: 0.0–3.7 ng/ml; PTL who delivered preterm without IAI: median: 0.6 ng/ml; range: 0.2–3.3 ng/ml; PTL who delivered preterm with IAI: median: 0.8 ng/ml; range: 0.1–3.9 ng/ml; all p > 0.05). LOD, limit of detection.
Labor in preterm or term gestation is not associated with changes in amniotic fluid concentrations of sVEGFR-1 and sVEGFR-2
There was no significant difference in the median AF concentration of sVEGFR-1 between women with PTL who delivered preterm and those who delivered at term (p = 0.08; ). Similar results were observed for sVEGFR-2. (p = 0.8; ).
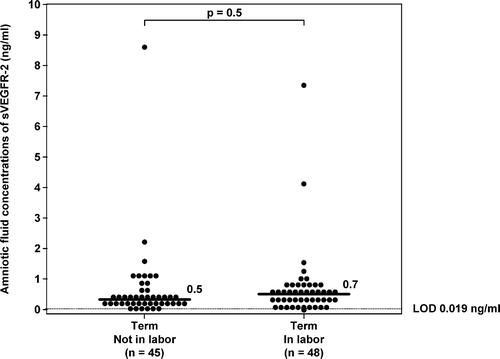
There was no significant difference in the median AF concentration of sVEGFR-1 and sVEGFR-2 between women at term with and without labor (sVEGFR-1; term in labor 65 ng/ml, range 4.7–243.8 ng/ml vs. term not in labor median 64 ng/ml, range 10.6–373.4 ng/ml; p = 0.6; and for sVEGFR-2; term in labor median 0.7 ng/ml, range 0.0–7.9 ng/ml vs. term not in labor median 0.5 ng/ml, range 0.1–9.3 ng/ml; p = 0.5; ).
Figure 7. Amniotic fluid concentrations of sVEGFR-1 in normal pregnant women at term with and without labor. There was no significant difference in the median amniotic fluid sVEGFR-1 concentrations between patients with spontaneous labor and those not in labor (term in labor: median: 65 ng/ml; range: 4.7–243.8 ng/ml; vs. term not in labor: median: 64 ng/ml; range: 10.6–373.4 ng/ml; p = 0.6). LOD, limit of detection.
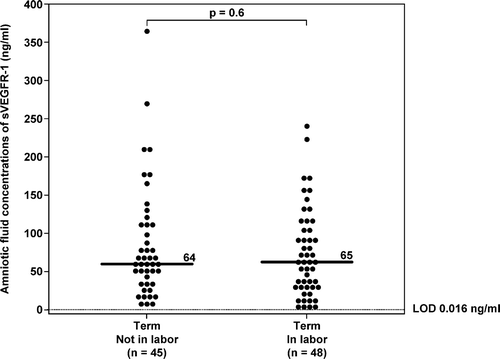
Figure 8. Amniotic fluid concentrations of sVEGFR-2 in normal pregnant women at term with and without labor. There was no significant difference in the median amniotic fluid sVEGFR-2 concentrations between patients with spontaneous labor at term and those at term not in labor (term in labor: median: 0.7 ng/ml; range: 0.0–7.9 ng/ml; vs. term not in labor: median: 0.5 ng/ml; range: 0.1–9.3 ng/ml; p = 0.5). LOD, limit of detection.
Discussion
Principal findings of this study
(1) Preterm PROM, regardless of the presence or absence of IAI was associated with lower median AF concentrations of sVEGFR-1, but not sVEGFR-2 than pregnancies with PTL with intact membranes; (2) this association had a dose–response relationship as described by an increased risk of preterm PROM with descending quartiles of sVEGFR-1 concentrations in AF; (3) the soluble forms of VEGFR-1 and VEGFR-2 are physiologic constituents of AF and their concentrations change with gestational age, with the highest concentration observed at 24–28 weeks of gestation; and (4) AF concentrations of sVEGFR-1 and sVEGFR-2 did not change with IAI or spontaneous labor in preterm or term gestation.
Changes of amniotic fluid concentrations of sVEGFR-1 and sVEGFR-2 in pregnancy
In this study, we described the changes of sVEGFR-1 and sVEGFR-2 concentrations in AF as a function of gestational age. The concentrations of both soluble receptors increased from MT until 24–28 weeks, and then decreased as the gestational age approached term. Since both soluble forms of VEGF can bind to and inhibit VEGF, the changes of AF concentrations of sVEGFR-1 and sVEGFR-2 with gestational age could reflect the changes of VEGF concentrations in the AF cavity. The role of VEGF in AF cavity during pregnancy remains unclear but could be related to fetal growth, placental development [Citation87] and the regulation of AF volume [Citation88].
The sources of sVEGFR-1 and sVEGFR-2 in AF are unknown. The concentration of sVEGFR-1 in normal pregnancy at term was the highest in the AF (median 51,040 pg/ml) compared to those observed in maternal blood (median 3,417 pg/ml) [Citation89] with the lowest concentration observed in umbilical artery serum (mean 188 pg/ml) [Citation90]. The results for sVEGFR-1 concentrations in AF of normal pregnant women at term not in labor (median 64 ng/ml) in this study were within the same range as those reported by others [Citation89,Citation90]. The concentration gradients of sVEGFR-1 do not support a maternal or fetal origin for sVEGFR-1 in AF. It is possible that sVEGFR-1 in AF could be derived from the placenta, fetal membranes, or uterine decidua. In contrast, the concentration of sVEGFR-2 in normal pregnancy at term was the highest in maternal blood (mean 6.4 ng/ml) and in umbilical vein serum (mean 6.8 ng/ml) [Citation90] with the lowest concentration observed in the AF (median 0.5 ng/ml at term, from our study). These observations indicate that sVEGFR-2 in AF may be derived from different sources than those of sVEGFR-1, or that the regulation of the expression of these soluble receptors in amniotic cavity is different.
Preterm PROM is associated with decreased amniotic fluid concentrations of sVEGFR-1, but not sVEGFR-2
The findings that women with preterm PROM, regardless of IAI, had lower AF concentrations of sVEGFR-1 than those with PTL with intact membranes are consistent with a previous study demonstrating that the expression of VEGF gene was increased in the fetal membranes and decidua of patients with preterm PROM regardless of the inflammatory status [Citation79]. It has been proposed that localized over-expression of VEGF gene in the fetal membranes plays a role in induction and activation of matrix metalloproteinase (MMP) through tissue plasminogen activator [Citation79]. It is possible that the decreased AF concentrations of sVEGFR-1 led to increased free VEGF concentrations/activity in AF cavity. VEGF, in turn, would stimulate its own expression in the fetal membranes and induce the activation of matrix-degrading enzymes leading to membrane rupture [Citation48,Citation91].
Alternatively, a decrease in AF concentrations of sVEGFR-1 in patients with preterm PROM could be a consequence of degradation of sVEGFR-1 by increased concentrations of MMPs, a group of enzymes implicated in the mechanism of membrane rupture. The degradation of sVEGFR-1 by MMPs has been demonstrated for MMP-7, MMP-2, and MMP-9 [Citation15,Citation23,Citation26,Citation92–94]. Previous studies have shown that preterm PROM was associated with increased AF concentrations of MMP-1, MMP-8 and MMP-9 and decreased AF MMP-2 concentrations [Citation15,Citation16,Citation20,Citation23,Citation26,Citation92–94]. However, the finding that AFsVEGFR-1 concentrations in preterm PROM were decreased regardless of the inflammatory status of the amniotic cavity contradicts this hypothesis. Since IAI was also associated with elevated MMPs concentrations in AF [Citation16,Citation23,Citation26], and thus, preterm PROM with IAI would have lower AFsVEGFR-1 concentrations than those without IAI. Future studies focusing on the temporal relationships between sVEGRF-1 and related MMPs in AF could help to clarify this issue.
Intra-amniotic infection/inflammation is not associated with changes in amniotic fluid concentrations of sVEGFR-1 and sVEGFR-2
VEGF is known as a vascular permeability factor. Such activity underlies the significance of this molecule in inflammation and other pathologic conditions, such as rheumatoid arthritis and atherosclerosis [Citation40]. Angiogenesis requires the participation of hematopoietic progenitors, endothelial progenitors, and inflammatory cells [Citation40,Citation95]. Monocytes express VEGFR-1 and the migration of monocytes in response to VEGF requires the tyrosine kinase domain of VEGFR-1 (membrane isoform) [Citation96]. Moreover, monocytes from healthy donors can release the soluble form of VEGFR-1 [Citation97]. Recently, angiogenic/anti-angiogenic factors have been implicated in the pathophysiology of sepsis [Citation98–102]. In experimental models of sepsis (endotoxemia and/or cecal ligation puncture) [Citation100–102] and observational studies in septic patients [Citation98,Citation99,Citation103], an increase in the plasma concentrations of VEGF [Citation98,Citation101,Citation103], placental growth factor (PlGF) [Citation102], and sVEGFR-1 [Citation99,Citation100] has been reported. The changes in sVEGFR-1 are considered to be an adaptive response to infection and to have survival value [Citation100,Citation102]. Indeed, the administration of adenovirus encoding for sVEGFR-1 [Citation101] or exogenous sVEGFR-1 [Citation100] attenuates the inflammatory response and reduces morbidity/mortality in mice [Citation100,Citation101]. Furthermore, an increased expression of VEGFR-1 mRNA and protein has been localized to macrophages and neutrophils infiltrating the chorionic plate of the placenta in patients with histologic chorioamnionitis [Citation104]. Collectively, these observations suggest a role of VEGF and VEGFR-1 in the mechanisms of maternal infection/inflammation. Our study did not find significant changes of AF sVEGFR-1 and sVEGFR-2 concentrations in IAI. It is possible that the AF white blood cells (which originate from the fetus), in contrast to adult monocytes, did not release sVEGFR-1 upon stimulation with proinflammatory cytokines or endotoxin.
Spontaneous labor in preterm or term gestation is not associated with changes in amniotic fluid concentrations of sVEGFR-1 and sVEGFR-2
VEGF has been implicated in the mechanisms of human parturition. Indeed, a previous study using microarrays demonstrated up-regulation of several angiogenic factors, including VEGF, in both membranes and choriodecidual tissue in women with term labor compared with those without labor [Citation105]. Another study also found the increased expression of VEGF mRNA in the fetal membranes in women at term in labor, a process thought to be an inflammatory-like condition [Citation106]. Our study did not find significant changes of AF sVEGFR-1 and sVEGFR-2 concentrations in human parturition both in preterm and term gestation.
Strengths and limitations
This is the first study to report the changes of AF concentrations of sVEGFR-1 in patients with preterm PROM. Moreover, the finding of a dose–response relationship between AF concentrations of sVEGFR-1 and preterm PROM strengthens the importance of the observation.
There are two potential limitations of this study. First, since performing amniocentesis in normal pregnant women without any indications is not ethical, the control group for preterm PROM included patients with PTL without IAI who delivered at term. This is the best control group that can be obtained in pregnant women. Second, this study is cross-sectional in nature, and thus, the temporal relationship between the change of AF sVEGFR-1 concentrations and preterm PROM cannot be established. It remains to be determined whether or not the decrease in AF concentrations of sVEGFR-1 precedes or follows the rupture of fetal membranes.
We conclude that preterm PROM is associated with a decrease in AF concentrations of sVEGFR-1. The soluble form of VEGFR-1 and 2 are physiologic constituents of human AF cavity and their concentrations change with gestational age. However, AF concentrations of sVEGFR-1 and sVEGFR-2 are relatively stable because they did not change with IAI or labor at term or preterm.
Acknowledgements
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Santolaya-Forgas J, Romero R, Espinoza J, Erez O, Friel L, Kusanovic JP, Bahado-Singh R, Nien JK. Prelabor rupture of the membranes. In: Reece EA, Hobbins JC, editors. Clinical obstetrics: The fetus and the mother, 3rd ed. New York (NY): Lippincott Williams & Wilkins. pp 1130–1188.
- Gibbs RS, Blanco JD. Premature rupture of the membranes. Obstet Gynecol 1982;60:671–679.
- Parry S, Strauss JF, III. Premature rupture of the fetal membranes. N Engl J Med 1998;338:663–670.
- Gunn GC, Mishell DR, Jr., Morton DG. Premature rupture of the fetal membranes. A review. Am J Obstet Gynecol 1970;106:469–483.
- Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am 2005;32:411–428.
- Keirse MJ, Ohlsson A, Treffers PE, Kanhani HHH. Prelabour rupture of the membranes preterm. In: Chalmers I, Enkin M, Keirse MJ, editors. Effective care in pregnancy and childbirth. Oxford University Press; 1989. p 666.
- Christensen KK, Christensen P, Ingemarsson I, Mardh PA, Nordenfelt E, Ripa T, Solum T, Svenningsen N. A study of complications in preterm deliveries after prolonged premature rupture of the membranes. Obstet Gynecol 1976;48:670–677.
- Daikoku NH, Kaltreider DF, Khouzami VA, Spence M, Johnson JW. Premature rupture of membranes and spontaneous preterm labor: maternal endometritis risks. Obstet Gynecol 1982;59:13–20.
- Johnson JW, Daikoku NH, Niebyl JR, Johnson TR, Jr., Khouzami VA, Witter FR. Premature rupture of the membranes and prolonged latency. Obstet Gynecol 1981;57:547–556.
- Lebherz TB, Hellman LP, Madding R, Anctil A, Arje SL. Double-blind study of premature rupture of the membranes. A report of 1,896 cases. Am J Obstet Gynecol 1963;87:218–225.
- Shubert PJ, Diss E, Iams JD. Etiology of preterm premature rupture of membranes. Obstet Gynecol Clin North Am 1992;19:251–263.
- Kelly T. The pathophysiology of premature rupture of the membranes. Curr Opin Obstet Gynecol 1995;7:140–145.
- Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol 1993;168:585–591.
- Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, Maymon E, Berry S. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002;187:1125–1130.
- Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane rupture. J Perinat Med 1999;27:362–368.
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 2000;183:94–99.
- Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, Gomez R, Yoon BH, Romero R. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med 2003;31:12–22.
- Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 2002;12:237–246.
- Edwin SS, Romero R, Rathnasabapathy CM, Athaydel N, Armant DR, Subramanian MG. Protein kinase C stimulates release of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by human decidual cells. J Matern Fetal Neonatal Med 2002;12:231–236.
- Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:914–920.
- Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intra-amniotic infection. Infect Dis Clin North Am 1997;11:135–176.
- Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998;179:1248–1253.
- Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, Seubert DE. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol 2000;182:1545–1553.
- Rechavaleta-Velasco F, Mayon-Gonzalez J, Gonzalez-Jimenez M, Hernandez-Guerrero C, Vadillo-Ortega F. Association of type II apoptosis and 92-kDa type IV collagenase expression in human amniochorion in prematurely ruptured membranes with tumor necrosis factor receptor-1 expression. J Soc Gynecol Investig 2002;9:60–67.
- Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, Strauss JF, III. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod 2002;8:494–501.
- Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, Chaiworapongsa T, Kim JC, Yoon BH, Menon R, et al A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med 2001;29:308–316.
- Stephenson CD, Lockwood CJ, Ma Y, Guller S. Thrombin-dependent regulation of matrix metalloproteinase (MMP)- 9 levels in human fetal membranes. J Matern Fetal Neonatal Med 2005;18:17–22.
- Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med 2002;11:11–17.
- Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med 2001;10:297–300.
- Erez O, Espinoza J, Chaiworapongsa T, Gotsch F, Kusanovic JP, Than NG, Mazaki-Tovi S, Vaisbuch E, Papp Z, Yoon BH, et al A link between a hemostatic disorder and preterm PROM: a role for tissue factor and tissue factor pathway inhibitor. J Matern Fetal Neonatal Med 2008;21:732–744.
- Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:368–373.
- Harger JH, Hsing AW, Tuomala RE, Gibbs RS, Mead PB, Eschenbach DA, Knox GE, Polk BF. Risk factors for preterm premature rupture of fetal membranes: a multicenter case–control study. Am J Obstet Gynecol 1990;163:130–137.
- Spinillo A, Nicola S, Piazzi G, Ghazal K, Colonna L, Baltaro F. Epidemiological correlates of preterm premature rupture of membranes. Int J Gynaecol Obstet 1994;47:7–15.
- Ananth CV, Oyelese Y, Srinivas N, Yeo L, Vintzileos AM. Preterm premature rupture of membranes, intrauterine infection, and oligohydramnios: risk factors for placental abruption. Obstet Gynecol 2004;104:71–77.
- Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002;187:1137–1142.
- Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003;110 (Suppl 1):S10–S18.
- Demir R, Yaba A, Huppertz B. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem 2009; May 28 [Epub ahead of print].
- Demir R, Seval Y, Huppertz B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem 2007;109:257–265.
- Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 1989;84:1470–1478.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–676.
- Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep 2008;41:278–286.
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998;273:30336–30343.
- Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta 1997;18:657–665.
- Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T. Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors 1995;12:235–243.
- Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum Reprod 1996;11:1090–1098.
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993;72:835–846.
- Shiraishi S, Nakagawa K, Kinukawa N, Nakano H, Sueishi K. Immunohistochemical localization of vascular endothelial growth factor in the human placenta. Placenta 1996;17:111–121.
- Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 2006;9:225–230.
- Lin P, Sankar S, Shan S, Dewhirst MW, Polverini PJ, Quinn TQ, Peters KG. Inhibition of tumor growth by targeting tumor endothelium using a soluble vascular endothelial growth factor receptor. Cell Growth Differ 1998;9:49–58.
- McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/Flk-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2002;43:474–482.
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380:439–442.
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, et al Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–439.
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–1547.
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005;17:3–18.
- Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, et al Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med 2008;21:41–52.
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–2351.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658.
- Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol 2005;193:984–989.
- Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol 1999;106:1019–1022.
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23.
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 2003;188:177–182.
- Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 1998;179:1539–1544.
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in pre-eclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–5563.
- Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol 2007;109:1368–1374.
- Schlembach D, Wallner W, Sengenberger R, Stiegler E, Mortl M, Beckmann MW, Lang U. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2007;29:407–413.
- Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40.
- Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;31:303–309.
- Savvidou MD, Noori M, Anderson JM, Hingorani AD, Nicolaides KH. Maternal endothelial function and serum concentrations of placental growth factor and soluble endoglin in women with abnormal placentation. Ultrasound Obstet Gynecol 2008;32:871–876.
- Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol 2006;108:338–344.
- Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, Kusanovic JP, Erez O, Bujold E, Goncalves LF, et al Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med 2007;20:495–507.
- Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, Edwin S, Erez O, Gotsch F, Mazaki-Tovi S, et al Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol 2008;198:382–388.
- Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, et al A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne's syndrome). J Matern Fetal Neonatal Med 2006;19:607–613.
- Chaiworapongsa T, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med 2009;22:1122–1139.
- Daneshmand SS, Chmait RH, Moore TR, Bogic L. Preterm premature rupture of membranes: vascular endothelial growth factor and its association with histologic chorioamnionitis. Am J Obstet Gynecol 2002;187:1131–1136.
- Astern JM, Gowin-Brown J, Kendal-Wright CE. The expression of vascular endothelial growth factor and its receptors in primary human amniotic epithelial cells (AEC). Reproductive Sciences 2009;16:116A, Abstract 165.
- Cheung CY, Brace RA. Developmental expression of vascular endothelial growth factor and its receptors in ovine placenta and fetal membranes. J Soc Gynecol Investig 1999;6:179–185.
- Cheung CY. Vascular endothelial growth factor activation of intramembranous absorption: a critical pathway for amniotic fluid volume regulation. J Soc Gynecol Investig 2004;11:63–74.
- Tranquilli AL, Giannubilo SR, Bezzeccheri V, Ciavattini A, Scagnoli C, Mazzanti L. Amniotic levels of nitric oxide and vascular endothelial growth factor in pregnancy with subsequent intrauterine fetal death. Eur J Obstet Gynecol Reprod Biol 2004;114:162–165.
- Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmaki E. Amniotic fluid – soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol 2000;95:353–357.
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136.
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–168.
- Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000]. Rev Med Chil 2004;132:1155–1165.
- Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intra-amniotic infection. Am J Obstet Gynecol 1988;159:114–119.
- Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–974.
- Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–830.
- Cheung CY. Vascular endothelial growth factor: possible role in fetal development and placental function. J Soc Gynecol Investig 1997;4:169–177.
- Cheung CY. Vascular endothelial growth factor activation of intramembranous absorption: a critical pathway for amniotic fluid volume regulation. J Soc Gynecol Investig 2004;11:63–74.
- Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 2005;122:33–39.
- Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, Schlembach D. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112:51–57.
- Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. VEGF-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb 2008;15:26–33.
- Ito TK, Ishii G, Saito S, Yano K, Hoshino A, Suzuki T, Ochiai A. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood 2009;113:2363–2369.
- Lei H, Vadillo-Ortega F, Paavola LG, Strauss JF, III. 92-kDa gelatinase (matrix metalloproteinase-9) is induced in rat amnion immediately prior to parturition. Biol Reprod 1995;53:339–344.
- Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, Lei H, Muschel RJ, Stetler-Stevenson WG, Strauss JF, III. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol 1995;146:148–156.
- Risau W. Mechanisms of angiogenesis. Nature 1997;386:671–674.
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996;87:3336–3343.
- Barleon B, Reusch P, Totzke F, Herzog C, Keck C, Martiny-Baron G, Marme D. Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis 2001;4:143–154.
- Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock 2005;24:508–512.
- Shapiro NI, Yano K, Okada H, Fischer C, Howell M, Spokes KC, Ngo L, Angus DC, Aird WC. A prospective, observational study of soluble FLT-1 and vascular endothelial growth factor in sepsis. Shock 2008;29:452–457.
- Tsao PN, Chan FT, Wei SC, Hsieh WS, Chou HC, Su YN, Chen CY, Hsu WM, Hsieh FJ, Hsu SM. Soluble vascular endothelial growth factor receptor-1 protects mice in sepsis. Crit Care Med 2007;35:1955–1960.
- Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, et al Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med 2006;203:1447–1458.
- Yano K, Okada Y, Beldi G, Shih SC, Bodyak N, Okada H, Kang PM, Luscinskas W, Robson SC, Carmeliet P, et al Elevated levels of placental growth factor represent an adaptive host response in sepsis. J Exp Med 2008;205:2623–2631.
- van der FM, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock 2005;23:35–38.
- Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol 2002;33:1069–1077.
- Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol 2002;187:728–734.
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195:394–324.