Abstract
Objective: Inflammation is a mechanism of host response to infection, which can be harmful when inappropriately modulated. Soluble ST2 (sST2) is a decoy receptor of interleukin (IL)-33, and this complex modulates the balance in the Th1/Th2 immune response. Moreover, sST2 inhibits the production of pro-inflammatory cytokines in cooperation with an anti-inflammatory cytokine, IL-10. The objectives of this study were to: (1) determine whether umbilical cord plasma sST2 concentration differs between preterm neonates with and without funisitis and between those with and without the fetal inflammatory response syndrome (FIRS); and (2) evaluate the relationship between sST2 and IL-10 among neonates with funisitis and/or FIRS.
Methods: Umbilical cord plasma was collected from neonates delivered prematurely due to preterm labor or preterm prelabor rupture of membranes with (n = 36), and without funisitis (n = 30). FIRS (umbilical cord IL-6 concentration ≥17.5 pg/mL) was identified in 29 neonates. Plasma sST2 and IL-10 concentrations were determined by enzyme linked immune sorbent assay.
Results: The median umbilical cord plasma sST2 concentration was 6.7-fold higher in neonates with FIRS than in those without FIRS (median 44.6 ng/mL, interquartile range (IQR) 13.8–80.3 ng/mL versus median 6.7 ng/mL, IQR 5.6–20.1 ng/mL; p < 0.0001). Similarly, the median umbilical cord plasma sST2 concentration was 2.7-fold higher in neonates with funisitis than in those without funisitis (median 19.1 ng/mL; IQR 7.1–75.0 ng/mL versus median 7.2 ng/mL; IQR 5.9–23.1 ng/mL; p = 0.008). There was a strong positive correlation between sST2 and IL-10 in neonates with funisitis and/or FIRS (Spearman’s Rho = 0.7, p < 0.0001).
Conclusion: FIRS and funisitis are associated with an elevation of umbilical cord plasma concentrations of soluble ST2. This protein represents an important mediator of the immune response in neonates diagnosed with FIRS by promoting an anti-inflammatory effect in association with IL-10.
Introduction
Intra-amniotic infection is associated with the spontaneous onset of labor [Citation1–9] in patients with preterm labor (PTL) and intact membranes [Citation10–27], and patients with preterm prelabor rupture of membranes (PROM) [Citation28–38]. Moreover, this condition has been identified in a subset of patients with a short cervix [Citation39–42], cervical insufficiency [Citation43,Citation44], and other conditions which confer increased risk for preterm delivery, such as an intrauterine device in pregnancy [Citation45], vaginal bleeding [Citation46] and placenta previa [Citation47].
Microbial invasion of the amniotic cavity (MIAC) can lead to fetal infection [Citation21,Citation48–51]. Indeed, fetal bacteremia detected in blood obtained by cordocentesis has been reported in 30% of patients who have intra-amniotic infection [Citation51], and one-fifth of preterm neonates born before 32 weeks of gestation have evidence of bacteremia in umbilical cord blood [Citation52,Citation53]. Subsequently, fetal microbial invasion may lead to a systemic inflammatory response, which we have termed the fetal inflammatory response syndrome (FIRS) [Citation21,Citation54]. This can be detected by the presence of elevated concentrations of cytokines, such as interleukin (IL) -6, in umbilical cord blood [Citation21,Citation55,Citation56] or alternatively, by the presence of inflammation in the umbilical cord (funisitis) [Citation55–58] or chorionic vasculitis [Citation58].
FIRS is associated with the impending onset of labor [Citation59], as well as multi-systemic involvement and high risk of short- and long-term complications [Citation21,Citation54,Citation56,Citation60]. The fetal organ systems involved include the skin [Citation61–63], heart [Citation64–66], lungs [Citation67–74], eyes [Citation75], kidneys [Citation76], adrenal glands [Citation77], hematologic system [Citation78–80], thymus [Citation81–83] and the central nervous system [Citation84–97]. Although FIRS is frequently found in patients with intra-amniotic infection/inflammation (IAI), it can also be observed in fetuses with congenital viral infection [Citation98–104] or alloimmunization [Citation105].
Inflammation is a host defense mechanism elicited by insults such as infection [Citation106], trauma [Citation107], ischemia-reperfusion injury [Citation108,Citation109], necrosis [Citation110], and tissue injury [Citation111,Citation112]. The innate immune system provides the first line of defense against infection through the engagement of pattern recognition receptors (i.e. Toll-like receptors, TLRs) [Citation113–117], which recognize microbial products [Citation118–120] and induce an inflammatory response [Citation121–123] through the production of both chemokines and cytokines [Citation123–125]. The crucial balance between pro- and anti-inflammatory responses is regulated, in part, by an inhibitory system activated by the anti-inflammatory limb of the immune response [Citation126–129]. IL-10 is considered a key player in this process, serving as a major anti-inflammatory mediator, since it is produced mainly by monocytes and inhibits the transcription of pro-inflammatory cytokines [Citation126,Citation130,Citation131]. Inappropriate modulation of this process may result in immunosuppression or an exaggerated inflammatory response; both can be harmful to the host, as shown in experimental and observational studies [Citation56,Citation67,Citation132]. Indeed, a major cause of death in patients with sepsis is immunosuppression [Citation133].
ST2 is a member of the IL-1 receptor super-family [Citation134] and exists in four isoforms. The two best characterized isoforms are: (1) ST2L, a membrane receptor; and (2) soluble ST2 (sST2) [Citation134,Citation135]. The ligand for ST2L and sST2 is IL-33 [Citation136]. Upon binding to ST2L, IL-33 is capable of stimulating the Th2-type immune response and cytokine production [Citation137–139]. In contrast, sST2 acts as a decoy receptor for IL-33 and is thought to inhibit IL-33 function, thus favoring a shift toward the Th1 immune response [Citation140–143]. Besides its regulatory properties on the type of the adaptive immune response, sST2 also plays a role in innate immunity [Citation132,Citation144–146]. Elevated sST2 production has been observed during inflammatory conditions, such as lipopolysaccharide (LPS)-induced inflammation [Citation147,Citation148] and ultraviolet light irradiation [Citation149]. However, several studies have demonstrated that sST2 has anti-inflammatory properties [Citation132,Citation145,Citation146]. This beneficial effect of sST2 is thought to be mediated by IL-10 [Citation132]. During pregnancy, plasma sST2 concentrations were higher in women with preeclampsia than in those with uncomplicated pregnancies [Citation150,Citation151]. In contrast, among women with PTL, those with IAI had a lower median amniotic fluid sST2 concentration than those without IAI [Citation152]. Interestingly, IL-33 expression was also found in macrophages of the chorioamniotic membranes in acute chorioamnionitis [Citation153].
The objectives of this study were to: (1) determine whether umbilical cord plasma sST2 concentration differs between preterm neonates with and without funisitis (the histologic counterpart of FIRS) and between those with and without FIRS (defined as umbilical cord IL-6 concentration ≥17.5 pg/mL); and (2) evaluate the relationship between sST2 and IL-10 in neonates with funisitis and/or FIRS.
Patients and methods
Study design and population
A retrospective cross-sectional study was conducted by searching the Detroit Medical Center/Wayne State University/Perinatology Research Branch (NICHD/NIH) clinical database and bank of biological samples. Sixty-six pregnant women with spontaneous preterm delivery (either PTL or preterm PROM) between 27 and 34 weeks of gestation with (n = 36) and without (n = 30) funisitis were included. Multiple gestations and pregnancies with fetal chromosomal and/or structural anomalies were excluded. Umbilical cord blood was collected immediately after birth. Placentas underwent histopathologic examination after delivery.
All women provided written informed consent before the collection of biological samples. The collection and utilization of the samples were approved by the Institutional Review Board of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/DHHS). Many of these samples have been used in previous studies.
Clinical definitions
The diagnosis of PTL was made in the presence of regular uterine contractions (at least 3 in 30 min) and documented cervical change. Preterm PROM was diagnosed with sterile speculum examination by the combination of vaginal pooling, nitrazine and/or ferning test. Funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel wall or Wharton’s jelly, according to criteria previously described [Citation58]. FIRS was defined as an umbilical cord blood IL-6 concentration ≥17.5 pg/mL [Citation55,Citation154].
Sample collection and determination of sST2 in umbilical cord plasma
Umbilical cord blood was collected immediately after birth into tubes containing EDTA. Blood was centrifuged at 1300 g for 10 min at 4 °C. The samples were stored at −70 °C until analysis. Specific enzyme-linked immunoassays were used for the determination of umbilical cord plasma concentrations of sST2, IL-6, and IL-10 (R&D Systems, Minneapolis, MN). The quantitative sandwich enzyme immunoassay was employed. The inter- and intra-assay coefficients of variation were: (1) sST2 4.6% and 3.9%, respectively; (2) IL-10 6.9% and 4.4%, respectively and (3) IL-6 8.7% and 4.6%, respectively. The sensitivity was 17.5 pg/mL for sST2, 0.65 pg/mL for IL-10, and 0.09 pg/mL for IL-6.
Histopathologic examination
Tissue sections for histopathologic evaluation included one chorioamniotic membrane roll, two full-thickness sections from the placental disc, and one section of the umbilical cord. Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin and stained with hemotoxylin and eosin. Histopathologic examination was performed by a perinatal pathologist who was blinded to the clinical information.
Statistical analysis
The Shapiro–Wilk test was used to determine if the data were normally distributed. The Mann–Whitney U test was used to compare continuous non-parametric variables between groups. Comparison between proportions was performed using the Chi-square or Fisher’s exact tests. Correlation between two continuous variables was determined using Spearman’s rank correlation test. Multivariable general linear models including effect modification terms were constructed to examine whether the relationship between sST2 and IL-10 differed significantly as a function of either preterm PROM or gestational age (GA) at delivery. Plasma sST2 concentrations were log base 2 transformed to meet the assumptions of linear regression. A p value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 19 (IBM Corp, Armonk, NY) and SAS version 9.3 (Cary, NC).
Results
Demographic and clinical characteristics of the study population
Demographic and clinical characteristics of the study population are presented in . There were no significant differences in maternal age, GA at delivery and birth weight between neonates with and without funisitis (). The frequency of patients presenting with spontaneous PTL and intact membranes or preterm PROM was not significantly different between these groups (p = 0.6; ). Similarly, when comparing neonates with and without FIRS, there were no significant differences in maternal age, GA at delivery or birth weight (). Neither umbilical cord plasma concentrations of sST2 nor IL-10 were correlated with GA at delivery (Spearman’s Rho −0.07, p = 0.6 and 0.01, p = 0.9, respectively). Two of 29 neonates with FIRS did not have funisitis.
Table 1. Demographic and clinical characteristics of the study population with and without funisitis.
Table 2. Demographic and clinical characteristics of the study population with and without FIRS.
Funisitis was associated with an increase in the umbilical cord plasma sST2 concentration
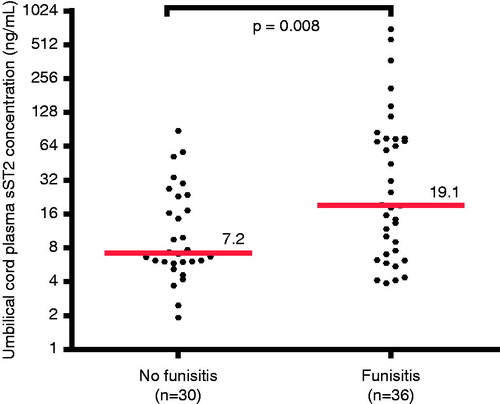
Soluble ST2 was detected in all samples. The median umbilical cord plasma concentration of sST2 was 2.7-fold higher in neonates with funisitis than in those without funisitis (19.1 ng/mL; interquartile range (IQR) 7.1–75.0 ng/mL versus 7.2 ng/mL; IQR 5.9–23.1 ng/mL; p = 0.008; ). IL-10 was below the detection limit of the assay in 16 patients; among these cases, three neonates had funisitis and none had FIRS. The median umbilical cord plasma concentration of IL-10 was also significantly greater in neonates with funisitis, than in those without funisitis (4.2 pg/mL; IQR 2.8–7.8 pg/mL versus 1.9 pg/mL; IQR 0–2.7 pg/mL; p < 0.0001).
Figure 1. Umbilical cord plasma sST2 concentrations in neonates with and without funisitis. The median plasma concentration of sST2 in umbilical cord was greater in neonates with funisitis than in those without funisitis (median 19.1 ng/mL; IQR 7.1–75.0 ng/mL versus median 7.2 ng/mL; IQR 5.9–23.1 ng/mL; p = 0.008). The y-axis is expressed in log2 scale.
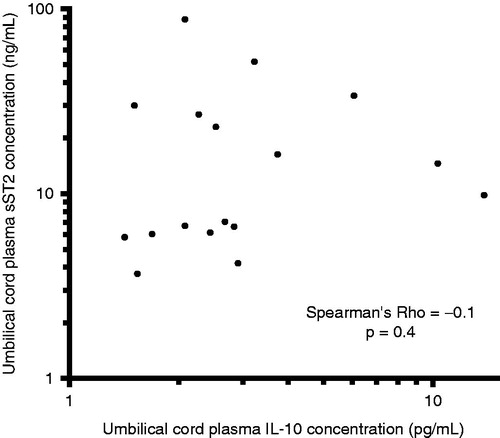
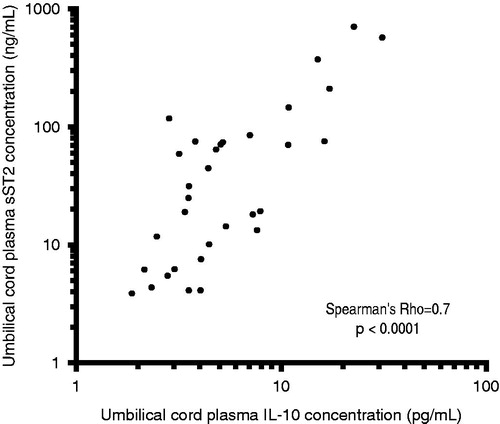
Among neonates with funisitis, there was a significant positive correlation between umbilical cord plasma concentrations of sST2 and IL-10 (Spearman’s Rho = 0.7, p < 0.0001; ). In contrast, there was no significant correlation between umbilical cord plasma concentrations of sST2 and IL-10 among neonates without funisitis (Spearman’s Rho = −0.1, p = 0.4; ).
Figure 2. Correlation between umbilical cord plasma concentrations of sST2 and IL-10 in neonates with funisitis. There was a significant positive correlation between umbilical cord plasma concentrations of sST2 and IL-10 (Spearman’s Rho = 0.7, p < 0.0001). The y- and x-axes are expressed in log10 scale.
FIRS was associated with an increase in the umbilical cord plasma sST2 concentration
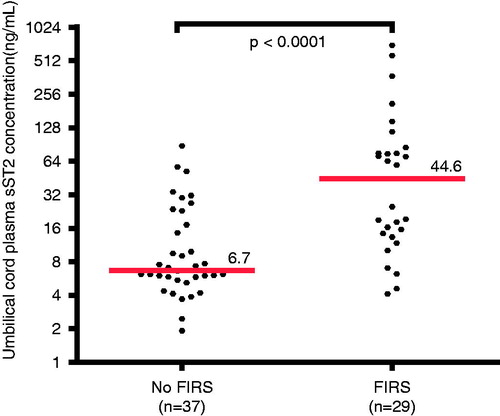
The median plasma concentration of sST2 in the umbilical cord was 6.7-fold higher in neonates with FIRS, than in those without FIRS (44.6 ng/mL; IQR 13.8–80.3 ng/mL versus 6.7 ng/mL; IQR 5.6–20.1 ng/mL; p < 0.0001; ). The median plasma IL-10 concentration in the umbilical cord of neonates with FIRS was significantly greater than in those without FIRS (5.3 pg/mL; IQR 3.5–10.9 pg/mL versus 2.5 pg/mL; IQR 2.1–3.6 pg/mL; p < 0.0001).
Figure 4. Umbilical cord plasma sST2 concentrations in neonates with and without the FIRS. The median plasma concentration of sST2 in umbilical cord was greater in neonates with FIRS than in those without FIRS (median 44.6 ng/mL; IQR 13.8–80.3 ng/mL versus median 6.7 ng/mL; IQR 5.6–20.1 ng/mL; p < 0.0001). The y-axis is expressed in log2 scale.
Among neonates with FIRS, a significant positive correlation between umbilical cord plasma concentrations of sST2 and IL-10 (Spearman’s Rho = 0.7, p < 0.0001) was observed. In contrast, among patients without FIRS, there was no correlation between umbilical cord plasma concentrations of sST2 and IL-10 (Spearman’s Rho = −0.2, p = 0.1).
Use of a general linear models to examine whether the relationship between IL-10 and sST2 differed as a function of preterm PROM revealed no evidence of effect modification, neither overall (p = 0.27), in the presence of FIRS/funisitis (p = 0.43), nor in the absence of FIRS/funisitis (p = 0.32). Similarly, the relationship between FIRS/funisitis and sST2 did not vary as a function of preterm PROM (p = 0.57).
Discussion
Principal findings of the study
(1) Preterm neonates with FIRS and/or funisitis had greater umbilical cord plasma concentrations of sST2 than neonates without FIRS and/or funisitis; (2) the median umbilical cord plasma sST2 concentration was 6.7-fold higher in FIRS and 2.7-fold higher in funisitis, compared to those without these conditions; and (3) there was a strong positive correlation between sST2 and IL-10 concentrations in the umbilical cord plasma in neonates with FIRS and/or funisitis, but not in neonates without these conditions.
The role of ST2 in infection/inflammation
Emerging evidence suggests that ST2 plays a role in regulating the innate limb of the immune response through inhibition of TLRs signaling [Citation145,Citation146,Citation155]. TLRs are members of the Toll/IL-1R (TIR) superfamily, which also includes ST2 along with IL-1 and IL-18 [Citation156]. TLR signaling can activate many genes identical to those induced by IL-1 [Citation157], and, thus, are important for the initiation and development of the pro-inflammatory response. Nevertheless, a balance between activation and inhibition is required in order to avoid detrimental inflammation. Thus, negative regulators of TIR signaling are essential to achieve an immunological balance. Indeed, in contrast to other TIR family members which induce the inflammatory response through the activation of NF-κB, the TIR domain of the ST2 receptor activates mitogen-activated protein kinases [Citation158]. Moreover, ST2 has been shown to sequester the adaptor proteins, myeloid differentiation primary response (MyD)88 and MyD88-adapter-like protein through its TIR domain, resulting in TLR4 down-regulation [Citation146,Citation155]. Similarly, another study has reported negative regulation of TLR2 by ST2, affecting the formation of TLR2-MyD88 and MyD88-IL-1R associated kinase immune-complexes [Citation155].
There is also evidence that ST2 is an important selective negative regulator of the TIR domain containing receptor function [Citation146]. Thus, it is not surprising that macrophages from ST2-deficient mice produce more pro-inflammatory cytokines in response to LPS or bacterial lipoprotein than wild-type mice. This leads to a sustained pro-inflammatory cytokine production by ST2 deficient macrophages [Citation146]. On the other hand, sST2 has an inhibitory effect on pro-inflammatory cytokine production. Evidence in support of this is: (1) sST2 inhibits pro-inflammatory cytokine production from LPS-stimulated macrophages [Citation145]; and (2) in animal models of sepsis and ischemia-reperfusion injury, the administration of sST2-Fc fusion protein suppresses the production of TNF-α, IL-6 and IL-12 [Citation144,Citation145], and increases survival [Citation132].
ST2 is also involved in endotoxin tolerance [Citation146]. Wild-type mice remain healthy after priming with a sub-lethal dose of LPS, and subsequent challenging with a lethal dose of LPS; whereas ST2-deficient mice were unable to develop endotoxin tolerance, had an exaggerated inflammatory response and died after the LPS challenge [Citation146]. An increased susceptibility to polymicrobial infection with impaired bacterial clearance is associated with altered phagosome maturation and nitrogen oxide-2 derived production of reactive oxygen species in ST2-deficient mice [Citation159].
Biology of IL-10
Traditionally, resolution of inflammation was thought to occur passively. However, it is now clear that resolution is an active process which consists mainly of a decreased production of pro-inflammatory components and removal of inflammatory cells. Both activation and resolution of the inflammatory response are of fundamental importance for survival. IL-10 is one of the most important anti-inflammatory cytokines, having a crucial role as a promoter of negative feedback in the inflammatory response [Citation131,Citation160,Citation161]. IL-10 knock-out mice have an exaggerated Th1 immune activation in the presence of infection [Citation162,Citation163], and die quickly due to a massive and sustained inflammatory response [Citation164,Citation165]. The over-production of IL-10 is associated with persistent infection [Citation166–170]. Moreover, mice receiving IL-10 or transgenic mice over-expressing IL-10 have decreased production of pro-inflammatory cytokines [Citation171,Citation172]. IL-10 production can be stimulated by administration of pro-inflammatory cytokines such as IL-6, IL-12, IL-27 and transforming growth factor-β, which stimulate T-helper cells [Citation173–177], or by endotoxin stimulation of macrophages and dendritic cells [Citation178].
The anti-inflammatory action of IL-10 is elicited mainly through inhibition of pro-inflammatory cytokine synthesis through the direct effect on LPS-activated macrophages and/or down-regulation of T cells [Citation130,Citation131,Citation179]. However, IL-10 also prevents the release of reactive oxygen intermediates from monocytes/macrophages [Citation180,Citation181]. IL-10 may also lead to down-regulation of major histo-compatibility complex (MHC) class II antigens [Citation182] and cell-adhesion molecules, such as intercellular adhesion molecule (ICAM)-1 on LPS-activated macrophages [Citation183], resulting in the reduced production of pro-inflammatory cytokines.
The link between sST2 and IL-10
In an intestinal ischemic reperfusion model, the administration of sST2-Fc fusion protein (before reperfusion) resulted in a reduced local and systemic inflammatory response (reduced neutrophil influx and cytokine production) and elevation of IL-10 concentration, leading to a reduction in the mortality rate of treated mice [Citation132]. This protective effect of sST2 did not persist in IL-10 knock-out mice, suggesting that IL-10 is essential for the beneficial effect of sST2. The mechanisms and target cells on which sST2 exerts its action and induces IL-10 production remain to be elucidated, although T-regulatory cells [Citation184] and direct action on macrophages have been proposed [Citation145].
Preterm neonates with FIRS and/or funisitis have a higher median concentration of sST2 in umbilical cord plasma
We found that the median umbilical cord plasma concentration of sST2 in neonates with FIRS was 6.7-fold higher than in neonates without FIRS. Similarly, but to a lesser extent, the median umbilical cord plasma concentration of sST2 was 2.7-fold higher in neonates with funisitis than in those without funisitis. FIRS is associated with an increase in pro-inflammatory cytokines (e.g. IL-1β, TNF-α) [Citation87], chemokine (IL-8) [Citation54] and C-reactive protein [Citation185] in umbilical cord blood, which may lead to short- and long-term complications in preterm neonates [Citation21,Citation55,Citation57,Citation58,Citation67,Citation86,Citation87,Citation89,Citation185–191]. On the other hand, as confirmed by the study herein, FIRS is also associated with an increased umbilical cord plasma concentration of IL-10 [Citation192,Citation193]. The protective anti-inflammatory effect of IL-10 in pregnancy has been confirmed by several studies [Citation194–198]. Elevated concentrations of both sST2 and IL-10 have also been described among patients with sepsis [Citation199]. Therefore, high plasma concentrations of sST2 and IL-10 in the umbilical cord of preterm neonates with FIRS and/or funisitis most likely reflect an anti-inflammatory host response mounted to counteract pro-inflammatory cytokines. The strong positive correlation between sST2 and IL-10 in neonates with FIRS, but not in those without FIRS, is consistent with the observation that sST2 exerts its action in cooperation with IL-10 [Citation132,Citation199]. On the other hand, a sustained elevation of serum sST2 concentration in septic patients is correlated with disease severity and mortality [Citation200]. One possible explanation of this observation is that after the initial hyper-inflammatory phase, patients with sepsis can become immunosuppressed due to activation of the anti-inflammatory limb of the immune system [Citation201–203]. Loss of delayed hypersensitivity, inability to clear infection, and predisposition to nosocomial infections can often be observed in these patients [Citation203].
Strengths and limitations of the study
This is the first study to report the changes in umbilical cord plasma concentrations of sST2 and IL-10 in neonates with FIRS and/or funisitis. The diagnosis of FIRS was defined stringently by the IL-6 concentrations in umbilical cord plasma. However, a limited sample size precluded analysis of an association between umbilical cord plasma sST2 concentration and adverse neonatal outcome. The cross-sectional nature of this study also precludes a clear determination of the temporal relationship between observed sST2 concentrations and the occurrence of FIRS and/or funisitis.
Conclusion
FIRS is associated with an elevation of umbilical cord plasma concentrations of sST2. This protein may represent an important mediator of the immune response in neonates diagnosed with this condition by promoting an anti-inflammatory response in cooperation with IL-10. Future studies examining the association between neonatal plasma sST2 concentration and adverse neonatal outcomes appear warranted.
Declaration of interest
This project has been funded in whole or in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN275201300006C.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24
- Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79
- Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol 1993;169:1299–303
- Romero R, Gomez R, Chaiworapongsa T, et al. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15:41–56
- Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–23
- Newton ER. Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol 2005;32:571–600
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400
- Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20; discussion 203–20
- Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41
- Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140:947–52
- Hameed C, Tejani N, Verma UL, Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol 1984;149:726–30
- Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol 1984;148:739–43
- Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol 1986;67:500–6
- Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229–37
- Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. Am J Obstet Gynecol 1989;161:813–16
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–7
- Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16
- Coultrip LL, Lien JM, Gomez R, et al. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1994;171:901–11
- Yoon BH, Yang SH, Jun JK, et al. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol 1996;87:231–37
- Markenson GR, Martin RK, Tillotson-Criss M, et al. The use of the polymerase chain reaction to detect bacteria in amniotic fluid in pregnancies complicated by preterm labor. Am J Obstet Gynecol 1997;177:1471–7
- Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202
- Kara M, Ozden S, Arioglu P, Cetin A. The significance of amniotic fluid interleukin-6 levels in preterm labour. Aust N Z J Obstet Gynaecol 1998;38:403–6
- Greci LS, Gilson GJ, Nevils B, et al. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol 1998;179:172–8
- Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol 1998;92:77–82
- Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, et al. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med 1999;8:155–8
- Locksmith GJ, Clark P, Duff P, Schultz GS. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol 1999;94:1–6
- Rizzo G, Capponi A, Vlachopoulou A, et al. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol 1998;12:86–92
- Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–6
- Cotton DB, Hill LM, Strassner HT, et al. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol 1984;63:38–43
- Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol 1985;66:316–21
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. Qualitative amniotic fluid volume versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstet Gynecol 1986;67:579–83
- Feinstein SJ, Vintzileos AM, Lodeiro JG, et al. Amniocentesis with premature rupture of membranes. Obstet Gynecol 1986;68:147–52
- Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol 1979;54:226–30
- Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol 1992;167:1092–5
- Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51
- Font GE, Gauthier DW, Meyer WJ, et al. Catalase activity as a predictor of amniotic fluid culture results in preterm labor or premature rupture of membranes. Obstet Gynecol 1995;85:656–8
- Hussey MJ, Levy ES, Pombar X, et al. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. Am J Obstet Gynecol 1998;179:650–6
- Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 2000;183:1130–7
- Gomez R, Romero R, Nien JK, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2005;192:678–89
- Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–9
- Vaisbuch E, Romero R, Mazaki-Tovi S, et al. The risk of impending preterm delivery in asymptomatic patients with a nonmeasurable cervical length in the second trimester. Am J Obstet Gynecol 2010;203:446 e441–9
- Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol 2010;202:433 e431–8
- Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992;167:1086–91
- Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol 2008;198:633 e631–8
- Kim SK, Romero R, Kusanovic JP, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinat Med 2010;38:45–53
- Gomez R, Romero R, Nien JK, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005;18:31–7
- Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med 2010;38:275–9
- Carroll SG, Papaioannou S, Ntumazah IL, et al. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. Br J Obstet Gynaecol 1996;103:54–9
- Ferrazzi E, Muggiasca ML, Fabbri E, et al. Assessment of fetal inflammatory syndrome by “classical” markers in the management of preterm labor: a possible lesson from metabolomics and system biology. J Matern Fetal Neonatal Med 2012;25:54–61
- Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16:56--70
- Carroll SG, Papioannou S, Ntumazah IL, Philpott-Howard J, Nicolaides KH. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. Br J Obstet Gynaecol 1996;103:54--9
- Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, Hauth JC. The Alabama Preterm Birth Study: umbilical cord Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm new born infants. Am J Obstet Gynecol 2008;198:43.e1--5
- Romero R, Garite TJ. Twenty percent of very preterm neonates (23–32 weeks of gestation) are born with bacteremia caused by genital mycoplasmas. Am J Obstet Gynecol 2008;198:1–3
- Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007;50:652–83
- Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–9
- Murthy V, Kennea NL. Antenatal infection/inflammation and fetal tissue injury. Best Pract Res Clin Obstet Gynaecol 2007;21:479–89
- Kim CJ, Yoon BH, Park SS, et al. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol 2001;32:623–9
- Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25
- Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–93
- Arad I, Ergaz Z. The fetal inflammatory response syndrome and associated infant morbidity. Isr Med Assoc J 2004;6:766–9
- Kim YM, Romero R, Chaiworapongsa T, et al. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology 2006;49:506–14
- Zhang L, Saito M, Jobe A, et al. Intra-amniotic administration of E coli lipopolysaccharides causes sustained inflammation of the fetal skin in sheep. Reprod Sci 2012;19:1181–9
- Kemp MW, Saito M, Kallapur SG, et al. Inflammation of the fetal ovine skin following in utero exposure to Ureaplasma parvum. Reprod Sci 2011;18:1128–37
- Romero R, Espinoza J, Goncalves LF, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16:146–57
- Letti Muller AL, Barrios Pde M, Kliemann LM, et al. Tei index to assess fetal cardiac performance in fetuses at risk for fetal inflammatory response syndrome. Ultrasound Obstet Gynecol 2010;36:26–31
- Yanowitz TD, Jordan JA, Gilmour CH, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 2002;51:310–16
- Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–9
- Mittendorf R, Covert R, Montag AG, et al. Special relationships between fetal inflammatory response syndrome and bronchopulmonary dysplasia in neonates. J Perinat Med 2005;33:428–34
- Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 2001;79:205–9
- Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210–15
- Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol 2005;25:S31–5
- Ghezzi F, Gomez R, Romero R, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10
- Jobe AH, Newnham JP, Willet KE, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 2000;182:401–8
- Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 1997;99:2992–9
- Sood BG, Madan A, Saha S, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res 2010;67:394–400
- Yoon BH, Kim YA, Romero R, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1999;181:784–8
- Yoon BH, Romero R, Jun JK, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 1998;179:1107–14
- Romero R, Savasan ZA, Chaiworapongsa T, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med 2011;40:19–32
- Berry SM, Romero R, Gomez R, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol 1995;173:1315–20
- Chaiworapongsa T, Romero R, Berry SM, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med 2011;39:653–66
- De Felice C, Toti P, Santopietro R, et al. Small thymus in very low birth weight infants born to mothers with subclinical chorioamnionitis. J Pediatr 1999;135:384–6
- Toti P, De Felice C, Stumpo M, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol 2000;31:1121–8
- Di Naro E, Cromi A, Ghezzi F, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol 2006;194:153–9
- Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81
- Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol 2008;198:466.e1–11
- Mittendorf R, Montag AG, MacMillan W, et al. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am J Obstet Gynecol 2003;188:1438–4
- Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996;174:1433–40
- Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997;42:1–8
- Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res 1999;46:566–75
- Patrick LA, Smith GN. Proinflammatory cytokines: a link between chorioamnionitis and fetal brain injury. J Obstet Gynaecol Can 2002;24:705–9
- Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG 2005;112:16–18
- Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med 2006;34:5–12
- Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003;110:124–7
- Elovitz MA, Brown AG, Breen K, et al. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci 2011;29:663–71
- Moon JB, Kim JC, Yoon BH, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med 2002;30:301–6
- Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26
- Yoon BH, Romero R, Kim CJ, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 1997;177:406–11
- Gabrielli L, Bonasoni MP, Lazzarotto T, et al. Histological findings in foetuses congenitally infected by cytomegalovirus. J Clin Virol 2009;46:S16–21
- Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol 2011;65:110–17
- Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol 2010;185:1248–57
- McCarthy M, Auger D, Whittemore SR. Human cytomegalovirus causes productive infection and neuronal injury in differentiating fetal human central nervous system neuroepithelial precursor cells. J Hum Virol 2000;3:215–28
- Von Herzen JL, Benirschke K. Unexpected disseminated herpes simplex infection in a newborn. Obstet Gynecol 1977;50:728–30
- Hyde SR, Giacoia GP. Congenital herpes infection: placental and umbilical cord findings. Obstet Gynecol 1993;81:852–5
- Heifetz SA, Bauman M. Necrotizing funisitis and herpes simplex infection of placental and decidual tissues: study of four cases. Hum Pathol 1994;25:715–22
- Vaisbuch E, Romero R, Gomez R, et al. An elevated fetal interleukin-6 concentration can be observed in fetuses with anemia due to Rh alloimmunization: implications for the understanding of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2011;24:391–6
- Bhatia M, He M, Zhang H, Moochhala S. Sepsis as a model of SIRS. Front Biosci 2009;14:4703–11
- Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury 2007;38:1336–45
- Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000;190:255–66
- Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc Res 1996;32:743–51
- Franco-Pons N, Casas J, Fabrias G, et al. Fat necrosis generates proinflammatory halogenated lipids during acute pancreatitis. Ann Surg 2012; Sept 7 e-pub ahead of print
- Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol 2009;20:517–27
- Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost 2011;105:S13–33
- Matzinger P. The danger model: a renewed sense of self. Science 2002;296:301–5
- Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 1988;52:269–79
- Medzhitov R, Janeway C, Jr. The Toll receptor family and microbial recognition. Trends Microbiol 2000;8:452–6
- Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol 2005;25:503–10
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 2004;6:1382–7
- Castellheim A, Brekke OL, Espevik T, et al. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol 2009;69:479–91
- Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 2001;16:83–96
- Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol 2003;74:479–85
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365:63–78
- Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med 2001;29:S2–6; discussion S6–7
- Beutler B. Science review: key inflammatory and stress pathways in critical illness – the central role of the Toll-like receptors. Crit Care 2003;7:39–46
- Beutler B, Cerami A. Cachectin/tumor necrosis factor: an endogenous mediator of shock and inflammation. Immunol Res 1986;5:281–93
- Beutler B. Innate immune sensing of microbial infection: the mechanism and the therapeutic challenge. Wien Med Wochenschr 2002;152:547–51
- Keel M, Ungethum U, Steckholzer U, et al. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood 1997;90:3356–63
- Neidhardt R, Keel M, Steckholzer U, et al. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma 1997;42:863–70; discussion 870–61
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71–109
- Scumpia PO, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit Care Med 2005;33:S468–71
- Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–22
- Moore KW, O'Garra A, de Waal Malefyt R, et al. Interleukin-10. Annu Rev Immunol 1993;11:165–90
- Fagundes CT, Amaral FA, Souza AL, et al. ST2, an IL-1R family member, attenuates inflammation and lethality after intestinal ischemia and reperfusion. J Leukoc Biol 2007;81:492–9
- Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res 2001;24:273–87
- Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett 1989;258:301–04
- Takagi T, Yanagisawa K, Tsukamoto T, et al. Identification of the product of the murine ST2 gene. Biochim Biophys Acta 1993;1178:194–200
- Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–90
- Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol 2007;179:2051–4
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, et al. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood 2009;113:1526–34
- Bourgeois E, Van LP, Samson M, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol 2009;39:1046–55
- Xu D, Chan WL, Leung BP, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med 1998;187:787–94
- Lohning M, Stroehmann A, Coyle AJ, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci USA 1998;95:6930–5
- Coyle AJ, Lloyd C, Tian J, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med 1999;190:895–902
- Townsend MJ, Fallon PG, Matthews DJ, et al. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med 2000;191:1069–76
- Leung BP, Xu D, Culshaw S, et al. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol 2004;173:145–50
- Sweet MJ, Leung BP, Kang D, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol 2001;166:6633–9
- Brint EK, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol 2004;5:373–79
- Saccani S, Polentarutti N, Penton-Rol G, et al. Divergent effects of LPS on expression of IL-1 receptor family members in mononuclear phagocytes in vitro and in vivo. Cytokine 1998;10:773–80
- Mildner M, Storka A, Lichtenauer M, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res 2010;87:769–77
- Kumar S, Tzimas MN, Griswold DE, Young PR. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem Biophys Res Commun 1997;235:474–8
- Granne I, Southcombe JH, Snider JV, et al. ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS One 2011;6:e24463
- Stampalija T CT, Chaemsaithong P, Korzeniewski S, et al. Maternal plasma concentrations of sST2 and angiogenic/anti-angiogenic factors in preeclampsia. J Matern Fetal Neonatal Med (in press)
- Stampalija T CT, Romero R, Tarca AL, et al. Soluble ST2, a modulator of the inflammatory response, in preterm and term labor. J Matern Fetal Neonatal Med (submitted)
- Topping V, Romero R, Than NG, et al. Interleukin-33 in the human placenta. J Matern Fetal Neonatal Med 2013;26:327–38
- Savasan ZA, Chaiworapongsa T, Romero R, et al. Interleukin-19 in fetal systemic inflammation. J Matern Fetal Neonatal Med 2012;25:995--1005
- Liu J, Buckley JM, Redmond HP, Wang JH. ST2 negatively regulates TLR2 signaling, but is not required for bacterial lipoprotein-induced tolerance. J Immunol 2010;184:5802–8
- Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003;117:re3
- O'Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today 2000;21:206–9
- Brint EK, Fitzgerald KA, Smith P, et al. Characterization of signaling pathways activated by the interleukin 1 (IL-1) receptor homologue T1/ST2. A role for Jun N-terminal kinase in IL-4 induction. J Biol Chem 2002;277:49205–11
- Buckley JM, Liu JH, Li CH, et al. Increased susceptibility of ST2-deficient mice to polymicrobial sepsis is associated with an impaired bactericidal function. J Immunol 2011;187:4293–9
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765
- Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med 2007;204:239–43
- Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 1999;67:4435–42
- Deckert M, Soltek S, Geginat G, et al. Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infect Immun 2001;69:4561–71
- Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 1996;157:798–805
- Hunter CA, Ellis-Neyes LA, Slifer T, et al. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol 1997;158:3311–16
- Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol 2007;28:378–84
- Lilic D, Gravenor I, Robson N, et al. Deregulated production of protective cytokines in response to Candida albicans infection in patients with chronic mucocutaneous candidiasis. Infect Immun 2003;71:5690–9
- Mancilla-Diaz JM, Escartin-Perez RE, Lopez-Alonso VE, et al. Role of 5-HT1A and 5-HT1B receptors in the hypophagic effect of 5-HT on the structure of feeding behavior. Med Sci Monit 2005;11:BR74–9
- Pagliari C, Fernandes ER, Guedes F, et al. Role of mast cells as IL10 producing cells in paracoccidioidomycosis skin lesions. Mycopathologia 2006;162:331–5
- Fornari MC, Bava AJ, Guereno MT, et al. High serum interleukin-10 and tumor necrosis factor alpha levels in chronic paracoccidioidomycosis. Clin Diagn Lab Immunol 2001;8:1036–8
- Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med 1993;177:1205–8
- Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol 2002;168:3402–11
- Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nat Immunol 2007;8:1281–3
- Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol 2007;8:1372–9
- Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 2007;8:1380–9
- Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 2007;8:1363–71
- McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 2007;8:1390–97
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol 2006;6:379–86
- Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991;146:3444–51
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 1989;170:2081–95
- Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med 1991;174:1549–55
- de Waal Malefyt R, Abrams J, Bennett B, et al. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991;174:1209–20
- Willems F, Marchant A, Delville JP, et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol 1994;24:1007–9
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531–62
- Yoon BH, Romero R, Shim JY, et al. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90
- Santana C, Guindeo MC, Gonzalez G, et al. Cord blood levels of cytokines as predictors of early neonatal sepsis. Acta Paediatr 2001;90:1176–81
- Nishimaki S, Sato M, An H, et al. Comparison of markers for fetal inflammatory response syndrome: fetal blood interleukin-6 and neonatal urinary beta(2)-microglobulin. J Obstet Gynaecol Res 2009;35:472–6
- Romero R, Gomez R, Galasso M, et al. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol 1994;171:912–21
- Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20
- Paananen R, Husa AK, Vuolteenaho R, et al. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr 2009;154:39–43 e33
- Chaiworapongsa T, Romero R, Kim JC, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol 2002;186:1178–82
- Savasan ZA, Chaiworapongsa T, Romero R, et al. Interleukin-19 in fetal systemic inflammation. J Matern Fetal Neonatal Med 2012;25:995–1005
- Wirbelauer J, Seidenspinner S, Thomas W, et al. Funisitis is associated with increased interleukin-10 gene expression in cord blood mononuclear cells in preterm infants </=32 weeks of gestation. Eur J Obstet Gynecol Reprod Biol 2011;155:31–5
- Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol 2006;177:4888–96
- Terrone DA, Rinehart BK, Granger JP, et al. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol 2001;98:476–80
- Fortunato SJ, Menon R, Swan KF, Lombardi SJ. Interleukin-10 inhibition of interleukin-6 in human amniochorionic membrane: transcriptional regulation. Am J Obstet Gynecol 1996;175:1057–65
- Froen JF, Munkeby BH, Stray-Pedersen B, Saugstad OD. Interleukin-10 reverses acute detrimental effects of endotoxin-induced inflammation on perinatal cerebral hypoxia-ischemia. Brain Res 2002;942:87–94
- Meyer U, Murray PJ, Urwyler A, et al. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry 2008;13:208–21
- Brunner M, Krenn C, Roth G, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med 2004;30:1468–73
- Hoogerwerf JJ, Tanck MW, van Zoelen MA, et al. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med 2010;36:630–7
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–50
- van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis 2008;8:32–43
- Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to ‘immunoparalysis'. Chem Immunol 2000;74:162–77