ABSTRACT
Smoking associated COPD progression is likely to be directly linked to differential injury and repair dynamics in small airways (SA). Although IL8 is a well-accepted marker for injured airway epithelium, Clara cells [the predominant proliferating cells in SA] and SCGB1A1 protein [their major secretory product] have only recently emerged as potential SA repair markers. We therefore postulate that the SCGB1A1/IL8 ratio in the airways of smokers would be inversely associated with physiological, radiological and clinical measures of COPD. A cross-sectional cohort of 28 smokers undergoing surgery for peripheral nodule was recruited (24M/4F, age 61 ± 11 y, FEV1s 76 ± 20%, smoking 40 ± 12 p.y). SCGB1A1 and IL8 were measured by ELISA in the induced sputum (IS) 3 to 5 days prior to surgery as well as by immunohistochemistry from lung tissue (also assessed morphometrically) obtained distant to the cancer surgery site. COPD was assessed using standard clinical, functional and radiological parameters. Log-transformed IS-SCGB1A1 was linearly correlated with SCGB1A1-positive epithelial cells detected via immunohistochemistry (r = .533, p = .001), while IS-IL8 was positively related to SA infiltrating neutrophils (Elastase-positive cells). There was a striking negative correlation between IS-SCGB1A1/IL8 levels and whole airway thickness [SA < 2 mm] at morphometry (r = −0.83, p < 0.0001). IS-SCGB1A1/IL8 levels were also inversely associated with nitrogen slope [r = −0.52, p < 0.001] and HRCT SA score [r = −0.51, p < 0.001]. In a multivariate analysis the IS-SCGB1A1/IL8 ratio was a stronger predictor than both the physiological and radiological measures of SA disease assessed. The SCGB1A1/IL8 ratio measured in sputum is a potentially valuable biomarker for non-invasive assessment of SA remodelling in smokers.
INTRODUCTION
Cigarette smoking is the leading cause of COPD. Cigarette associated noxious agents injure airway epithelium and drive the key pathophysiological processes that lead to airway inflammation [essentially comprised of CD8 T-cells and neutrophils] and airway obstruction (Citation1). These “injury” changes are coupled to a repair process that ideally returns the airways to normal baseline conditions once the injurious agents are removed but may not be able to do so in the setting of ongoing injury. Around twenty percent of smokers may develop COPD (Citation2). In general, an imbalance between injury and repair processes, and particularly an inadequate repair process for any level of injury, are potential explanations for discrepancies among smokers who develop chronic airflow obstruction and those who do not.
Several studies have established that the major site of airway obstruction in COPD is in airways smaller than 2-mm internal diameter, and more recently that the decline in forced expiratory volume during the second (FEV1s) in COPD is related to thickening of the walls of these small conducting airways (Citation1). The bronchiolar airways are relatively susceptible to airway injury and obstruction given their characteristically simple epithelial lining and non-cartilaginous nature, respectively. Interleukin-8 (IL-8) from injured epithelium is the major chemoattractant for neutrophils at the site of inflammation which, in turn, are potent sources of inflammatory mediators (Citation3). IL-8 has been shown as a reliable marker of airway inflammation in COPD, at steady state and during exacerbation (Citation4–6). On the other hand, non-ciliated non-mucous Clara cells also reside in these airways and play a major role in airway integrity maintenance as they are major contributors to proliferating cells (Citation7) and, consequently, repair processes in the small airways (Citation5).
Clara cells are identified by their particular morphology (rich secretory apparatus and dome shaped apical side protruding within the lumen) and the expression of the homodimer Clara cell protein SCGB1A1 (for “secretoglobin1A1”, international nomenclature), also called CC10, CCSP, CC16, uteroglobin, UP-1 (Citation8, 9). SCGB1A1 has been shown to have anti-inflammatory and immunomodulatory activities and also antitoxidant properties (Citation10–15). Inhibition of phospholipase A2 (Citation16) and subsequent decrease of proinflammatory eicosanoids production, potent inhibition of TNF-α induced gamma-interferon release by lymphocytes and hydrophobic pocket binding for phospholipids and pollutants are known mechanisms of action of this protein.
In addition, knockout mice for this gene are more susceptible to any lung injury (bacterial or viral infection, ozone, cigarette smoke) or sensitization and develop increased inflammatory and remodelling reactions, more frequent lung tumours and a more intense Th2 orientated immune response (Citation8, Citation14, Citation15). Genome-wide profiling using microarray techniques has identified the SCGB1A1 gene among other epithelial cell genes as being associated with COPD (Citation17). Proteomic analyses confirmed potential roles for SCGB1A1 in allergic rhinitis/asthma, COPD, ARDS and post-transplant bronchiolitis (Citation18–23); and the potential value of SCGB1A1 monitoring in these diseases (Citation24–27). A deficiency in SCGB1A1 protein in the BAL and in small airways of smokers (Citation29) and in patients with COPD (Citation28) has been previously reported although it was not related to any spirometric criteria.
A reliable non-invasive biomarker of small airways disease and progression has many advantages including the assessment of early changes that can help to identify smokers who will develop chronic airflow obstruction and the improved identification of “responders” to potential therapeutic interventions (Citation28); A potential biomarker such as this one will be helpful to better understand the various phenotypes related to the current definition of COPD.
We therefore postulate that combining airway specific inflammation (IL8) and repair (SCGB1A1) markers measured in the sputum supernatant will represent a potential non-invasive biomarker with an improved signal for disease and potentially disease progression in COPD.
To test our hypothesis, we related the balance between repair and injury in SA as represented by the SCGB1A1/IL8 ratio in IS and tissue to the morphometric analysis of small airways present in part of lung slices removed at surgery from smokers’ lungs as well as to expiratory gated spirometrically-triggered HRCT and pulmonary function tests as standard indices related to COPD.
MATERIALS AND METHODS
Patients and pulmonary function tests
Twenty-eight cigarette smokers with variable levels of airflow obstruction (smokers without COPD (n = 14) and smokers with COPD (n = 14)) who were eligible for lung resection because of peripheral nodules or proven malignancy were recruited as the study cohort after obtaining written informed consent. Smoking status, respiratory symptoms and history were recorded at the time of routine clinical examination pre-operatively. BODE score was calculated as previously described (Citation29). A symptomatic score was calculated as the sum of four items quoted from 0 to 2 (dyspnea, cough, daily sputum production and colour). In addition, Phase III (dN2) and closing volume were obtained during a single breath nitrogen washout test and routine pulmonary function tests including spirometry, total body plethysmography and DLCO measurement were also performed. Fourteen control subjects free of any known disease and not taking any medication were asked to produce an induce sputum in order to test for specificity of the results after obtaining their written informed consent.
CT scan
During the week prior to surgical intervention all patients had a HRCT with a particular expiratory gated spirometrically triggered protocol according to previously published methods (Citation30, 31). In particular, expiratory gating CT-scan was coupled with a CT-scan-spirometry trigger device and compared with inspiratory gated series. CT scan results were independently analyzed in a double-blinded manner with a scoring system for the following items for each lobe: cysts, bronchiectasis, centrolobular micronodules, mucoid impactions, atelectasia and thickened airway wall. Low and very low parenchymal attenuated zones were measured at −900 and −960 HU and expressed as a percentage of total lung volume.
Air trapping was expressed as a percentage of reduction and as a difference (Citation30) of very low attenuation area of the parenchymal density acquired during inspiratory and expiratory efforts, at determined 4 lung stairs. Results were expressed as a total score being the sum of the semi-quantitative assessments of small airway related lesions at each lobe and of a local score for the lesions occupying only the removed lobe. Indexes of emphysema were calculated for the removed lobe and for the whole lung as the ratio between low attenuation volumes (−900 and −960 HU respectively) and total lung volumes.
Induced sputum
All adequate samples were collected 3 to 5 days before surgery according to international guidelines and processed for analyses (Citation32). Before sputum induction, all subjects underwent spirometry, with FEV1 and vital capacity measurements, before and 10 min after inhalation of 200 μg salbutamol by metered-dose inhaler. Hypertonic saline (3, 4 and 5% saline) was nebulised with an ultrasonic nebuliser (DP 100 Syst’am; Paris, France). This nebuliser generates particles with a mean mass aerodynamic diameter of 4.5 μm and has an output of 2.4 mL·min−1. Subjects inhaled hypertonic saline solution for 5-min periods up to 30 min, and were asked to rinse their mouth out with water before induction to avoid salivary contamination of induced sputum samples as much as possible. The concentration of saline was increased, if possible, at intervals of 10 min (two nebulisations of each concentration) from 3% to 4% to 5%. Spirometry was repeated at 5-min intervals throughout the procedure and immediately after sputum induction was completed. At the end of the test, a nebulisation with bronchodilators was given.
The concentration of saline was not increased if the FEV1 fell by >10% from the postbronchodilator value. Sputum induction was discontinued if the FEV1 declined >20% or if troublesome symptoms occurred (i.e., dyspnoea, wheezing, severe cough). Selected sputum plugs from saliva were then analysed. The volume of the induced sputum plugs was determined and overlaid with an equal volume of 0.1% dithiothreitol (Sputalysin 10%; Behring Diagnostics Inc., Somerville, NJ, USA) (Citation33–35). The sample was then gently mixed by vortex mixer and placed in a shaking water bath at 37°C for 30 min to ensure complete homogenisation. The homogenised sample was centrifuged (GR4.22; Jouan, St Herblain, France) at 2,000 rpm (400 × g) for 10 min. The supernatant was aspirated and frozen at −80°C for later analysis.
Phosphate-buffered saline was added to the cell pellets, which remained after centrifugation, and resuspended. Total cell counts were obtained after staining with May-Grunwald Giemsa on cytospin (Cytospin 2; Shandon, Runcorn, UK), by two observers blind to specimen identity. ELISA tests were used in order to assess SCGB1A1 (Biovendor) and IL-8 (Diaclone) after adequate dilution of the samples (1:1000 and 1:2).
Lung histology
At the time of surgery, a lung slice at distance from the neoplastic lesion was provided by the surgeon. It was first inflated with a needle mounted on a syringe and then fixed by immersion in acetone then embedded in Glycol Methacrylate (GMA) (Citation36). Samples of fixed tissue were processed into GMA blocks, cut into sections that were 2 to 3 micrometers thick, and placed on glass slides. Blocks were fully cut until exhausted in order to reach 6 airways at least per patient. Hematoxylin eosin staining was used to perform small airway morphometry analysis. Briefly, adequately orientated small airways were delineated using an image analyzer (AnalySIS 7.2 for Windows, Olympus Soft Imaging Solutions, Muenster, Germany) linked to a CCD camera (Sony DXC950P, Sony Group, Tokyo, Japan) connected to a light microscope (Olympus BHS, Olympus Optical, Tokyo, Japan).
Morphometric indices related to small airway assessment were obtained by delineating five lines and area referred as internal lumen (PI and AI), basement membrane (PBM and ABM), internal and outer smooth muscle limits (PISM, AISM and POSM, AOSM), and the outer limit of the adventitia (Ptot and Atot). Epithelial area (EA), subepithelial area (SE), and Smooth Muscle Wall area (SMWA) were calculated (Citation1, Citation37). The whole thickness of the airway was calculated as (Atot-Ai)/Pi (Citation30). All lengths and areas measured were corrected respectively by the PBM and the ABM of each airway to adjust for differences in airway size (Citation30).
Immunohistochemistry
Sections of identified small airways were stained separately to identify polymorphonuclear (PMN) neutrophils (Neutrophil Elastase NE, Dako-cytomation, dilution 1:50), macrophages (CD68, Dako-cytomation, dilution 1:500), CD8 T-cells (Novocastra laboratories, dilution 1:100) and SCGB1A1 epithelial cells (dilution 1:10 000, Biovendor). Control sections were treated with mouse IgG1 isotype at the same dilution. Manual cells counts were performed within each bronchioli wall at 100 micrometers maximal depth and expressed as a number/mm2 of basement membrane for inflammatory cells. SCGB1A1 positive epithelial cells were counted and expressed as a number per mm2 of EA. Interobserver reproducibility for morphometry and immunohistochemistry was tested in 10 randomly selected patients.
Statistical analysis
Patient characteristics are presented as mean and confidence interval at 95% or median and interquartiles if the variable was not normally distributed. The number of subject included was based on previous reports (Citation35, 36). An a posteriori power of 63% based on observed results was estimated. Qualitative data are presented as absolute values and percentages. Comparisons were made using parametric tests if data passed normality test before or after log transformations, otherwise nonparametric tests were used. A Kruskall–Wallis test was used for comparison between control, smokers without COPD and COPD patients.
Spearman's rho and Pearson's r coefficient of correlation were computed to test for correlations. Linear regression, 95% confidence and prevision curves are proposed. A paired t-test was used in order to compare local and general data obtained at HRCT. To further test our main hypothesis, wall airway thickness (Atot) was related to all small airway related factors tested (IS cellularity and different mediator levels, relevant indices from the pulmonary function tests, HRCT criteria and clinical score) using a correlation matrix. The strongest correlates with small airway thickness (p value below .15) were then included simultaneously in a multiple linear-regression model. Reproducibility was computed according to linear regression (r-square value) and Bland-Altman graphical analysis. Analyses were performed with the SPSS software v14.0.
RESULTS
Patients
The demographic profiles of the study cohort are represented in . The patients were mostly male and had moderate airway limitation and relatively low BODE scores [all being eligible for surgery]. At time of inclusion in this study, 100% were weaned from smoking, but 39% of them only a month ago. All patients were studied at least, at 6 weeks from their last exacerbation. The most frequent cardiovascular co-morbidities were hypertension and stable coronary heart disease. 24 patients were affected by tumors classified as pT1 (77%) and pT2 (23%), and 2 patients were found to be pN1. Histology was adenocarcinoma in 65%, squamous cell carcinoma in 31% and a carcinoid tumor in 1 patient (4%). 4 patients were free of tumor (organizing pneumonia in two, hamartochondroma in one and anthracotic node in one).
Table 1. Patient characteristics
Nitrogen washout slope recorded increased dN2 values above 125% of predicted values in 19/28 patients, while closing volumes when expressed as ratios of vital capacity and reported to predicted values were mostly within normal ranges.
Induced sputum
An adequate sputum sample was obtained in all patients. Mean Coefficient of variation for SCGB1A1 and IL8 assays done in triplicate were 1.4% and 2.1% respectively. Analysis of the differential cell counts and cytokines levels () demonstrated significant correlations between IL-8 and PMN counts (rho = .857, p = .0007) and between macrophages and SCGB1A1 levels (rho = .643, p = .002). A second sputum induction between three and five days later was possible in 12 patients and reproducibility was considered correct according to the r-square value (.91 for SCGB1A1 and .82 for IL8) and Bland Altman graphical analysis (not shown). Cigarette smoke weaning time (1 month vs. former) and presence or absence of tumor in the resected lung did not affect statistically any value especially SCGB1A1 and IL8 concentrations or their ratio. Patients with COPD had significantly lower SCGB1A1/IL8 ratio when compared to smokers without airflow obstruction and controls ().
Figure 1. Box-plot comparison for log-transformed IS SCGB1A1 / IL8 ratio in controls, smokers and COPD patients.
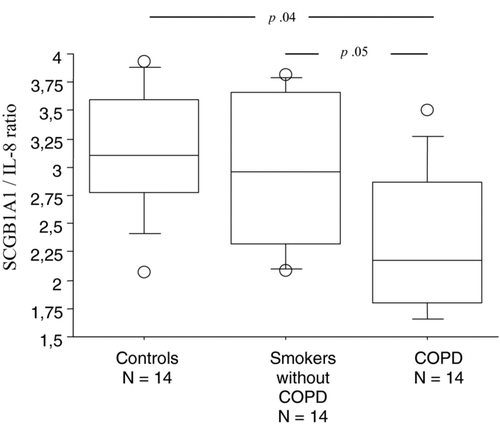
Table 2. Induced sputum (IS) results. Cellularity is shown as absolute cell number and % of cells after correction for epithelial cells
Small airway morphometry
Measurements and immunostaining results are presented in . Inter-observer reproducibility was computed in a sample of ten patients and found satisfactory for all parameters (for example: SCGB1A1 positive epithelial cell counts: r-square .78, WAtot: r-square .88). SCGB1A1 immunostaining was restricted to epithelial areas (). Airways with a diameter greater than 2 mm were not included in the analysis. Lengths are in mm and areas in mm2 but all values are presented after correction respectively by length and area of basement membrane according to previously published reports (Citation1, Citation37) .
Figure 2. Correlation between SCGB1A1/IL-8 ratio and the wall thickness of the airways assessed by morphometry.
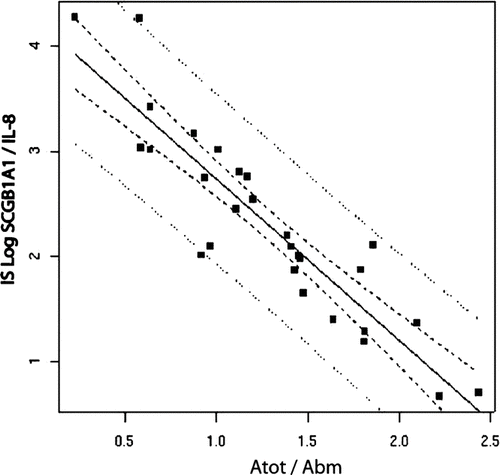
Table 3. Lung morphometry analysis and immunohistochemistry results
Table 4. HRCT parameters
HRCT (Table 4)
Intra-observer and inter-observer reproducibility for semi-quantitative scores were assessed in 8 patients and were found satisfactory (r-square 0.83 and r-square 0.78, respectively). Semi-quantitative scores and air trapping were not significantly different when comparing the removed lobe with the entire lungs, as confirmed by paired t-tests (p .47 and p .83 respectively) while the pairing was effective (r = .67, p <.00001 and r = .64, p < .00001, respectively). There was no statistical correlation between the semi-quantitative scores and any parameter derived from parenchymal attenuation (difference of attenuation and percentage of reduction of attenuation). −900 and −960HU indexes of emphysema were strongly correlated (r = .772, p < .0001), but were not related to any other parameter.
Wall thickness determinants
Univariate analysis () identified five criteria statistically linked to the whole airway thickness: the SCGB1A1/IL-8 ratio in the IS (after log transformation, Pearson's coefficient of correlation r = −.83, p < .0001; ) and the number of SCGB1A1 positive epithelial cells at histology (r = −.434; p .02), the phase III of the single breath nitrogen washout slope (r = .511, p .01) and the remodeling score of the lobe involved by the peripheral nodule at HRCT (r = .510, p .037). IS SCGB1A1 levels were also significant but to a lesser extent (r = .−399, p .034). As SCGB1A1 in both the IS and in histological assessment were related (after log transformation, Pearson's r = .591, p .001; ) we used only the sputum data in the multivariate analysis. The multivariate analysis indicated that SCGB1A1/IL-8 ratio in the IS had the strongest association with the small airway wall thickness.
Figure 3. Correlation between SCGB1A1 in the IS and the number of SCGB1A1 positive epithelial cells in small airways.
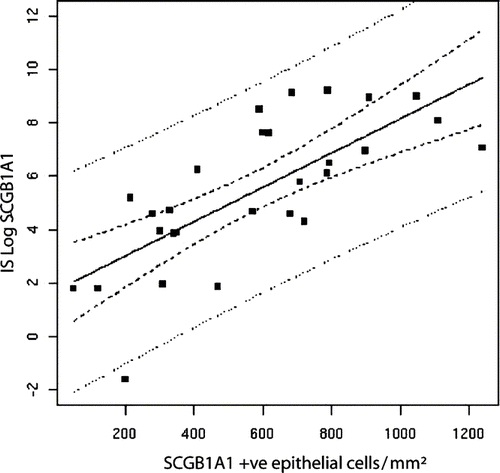
Table 5. Correlation matrix between whole small airway wall thickness and non-invasive tests
The correlation between IS IL-8 concentrations and neutrophils (elastase positive cells) at histology was significant (rho.43, p.01). IS SCGB1A1/IL-8 levels were also inversely associated with dN2 (r −.52, p < .001) and HRCT SA score (r −.51, p < .0001).
DISCUSSION
In the present study, we have shown for the first time that SCGB1A1 and IL-8 could potentially be mediators of interest in a dynamic vision of inflammatory/repair process in small airway pathogenesis in COPD. We demonstrated that the SCGB1A1/IL-8 ratio in the induced sputum was decreased in COPD patients. IL8 and SCGB1A1 levels in the IS of smokers were strongly related to immunostaining signals for these proteins in the small airways. We also demonstrated that the IS SCGB1A1/IL-8 composite ratio was a potentially useful non-invasive index related to distal lung remodeling in smokers. An a posteriori power calculation of 63% and a good reproducibility of our results gave us confidence in our results, given the complexity of the hypothesis tested in human beings. Small airways dedicated pulmonary function tests and expiratory HRCT were also related to distal lung remodeling as measured by lung morphometry but to a lesser extent. Importantly, the neoplastic processes present in some of these patients as a result of the study design all involved early stage tumors [pT1 or pT2] and did not seem to negatively influence the positive relationships found.
Decreased SCGB1A1 and increased IL-8 concentrations had already been reported in COPD also our findings confirm these data (Citation3–6, Citation28, 29). Factors potentially influencing these levels like ongoing smoking, neoplastic process, exacerbation, and others were mostly controlled in this study conducted in a preoperative time in patients free of infection, weaned from tobacco smoking and for some of them without cancer. The specificity of such finding is clearly unknown as decreased SCGB1A1 levels and high IL-8 concentrations have also been reported in different respiratory conditions including bronchiectasis, severe asthma, ARDS, and others. It is to note that small airway structural changes have also been described in these diseases.
Although pathobiologic considerations of injury and repair dynamics and our results strongly support the SCGB1A1/IL8 ratio as a biomarker for airways disease that can be extrapolated from this specific cohort of smokers with lung malignancy to COPD patients in general, further detailed validation studies are both warranted and essential. In particular, our cross-sectional analysis and results need to be followed up by a longitudinal prospective cohort study to assess the predictive power of this ratio. In addition, the sensitivity, specificity and response characteristics of the SCGB1A1/IL-8 ratio biomarker in the setting of various COPD risk factors and therapeutic interventions will need to be evaluated. We observed a good reproducibility in our data which may provide confidence in the stability of the ratio.
We consistently observed a correlation between IS IL8 and SCGB1A1 levels with their major targets – neutrophils; and sources—Clara cells in the tissue.
We are providing evidence relating the ratio measured in IS and small airway tissue immunostaining. This fact enabled us to consider IS as a non invasive approach valid to investigate SA in COPD.
Obviously, it would have been ideal to measure BAL and plasma levels for these proteins at the same time but this was unfortunately not included in the study design. Nevertheless, the observation that IS SCGB1A1 levels reflected SCGB1A1 immuno-positive epithelial cells in the small airways despite a sampling interval of 3–5 days suggests that: 1) the protein levels in both compartments are relatively stable unlike perhaps serum levels that are prone to many confounding factors (Citation38–40); 2) that the major contributor of IS IL8 and SCGB1A1 levels in these “clinically stable” smokers is the small airway; or that inflammatory changes could occur in a same fashion along the airway tree as suggested by others in asthma (Citation41); 3) that there may not be a great deal of immunopositivity in different parts of the lung in this cohort of patients [also supported by the absence of significant differences between small airways scores obtained at HRCT (local and total) suggesting that small airway lesions were more or less evenly distributed]; and, 4) that the relationship between SCGB1A1 positive cells and secreted SCGB1A1 protein levels is tight suggesting unique cell production that is tightly distributed. Our study was not designed to formally assess any of these points. IS IL-8 levels were also related to neutrophils infiltrated the small airways. Altogether, these observations strengthened the confidence in the results.
The demonstration that COPD severity as assessed by morphometric, physiological and radiological measures of small airway status was associated with lower SCGB1A1 contents in the small airway epithelium and in the IS was a striking result given the relatively small sample size. Although the morphometric analysis of small airways in COPD is considered the “gold standard” for COPD pathology (Citation42, 43), the single breath nitrogen washout test [to assess the unevenness of ventilation in small airways] and the expiratory CT scan [to assess differential air trapping abnormalities] provide excellent functional counterparts. According to FEV1s values (GOLD classification), SCGB1A1/IL-8 ratio in the IS could discriminate among patients and controls (), even though there was no correlation with FEV1s values. Aiming to study the small airway compartment didn't support to go on with this dichotomy but to consider the cohort as a whole group.
Although many non-invasive biomarkers have been evaluated in COPD including biomarkers in the exhaled air, the exhaled breath condensate and in the IS, these studies do not usually include histopathological and morphometric analysis of the distal small airways which was a particular strength of the present study. Our focus on SCGB1A1 protein as a small airways repair marker which is specifically secreted by Clara cells (Citation40) was also, we believe, a strong point of advantage for this work despite other small airway related proteins also being of potential interest in an assessment of injury/repair dynamics in this compartment [including: surfactant proteins (SP) A, B and D which are also secreted specifically in the distal part of the lung (Citation28)].
By analyzing our SCGB1A1 levels according to the level of injury present [as reflected by IL-8 levels], the relationship with other measures of small airways remodeling was significantly improved. Nevertheless, we are not able to go beyond the observed association and prescribe any cause/effect relationship or mechanistic explanation for our results. Given that Clara cells are specifically targeted by cigarette smokingand that their supposed protective role in airway biology, we would speculate that SCGB1A1 levels may be functionally protective in COPD. With this in mind, we note that acute cigarette exposure tends to increase SCGB1A1 levels in the blood whereas SCGB1A1 positive epithelial cells are decreased in COPD (Citation43–45). In addition, genetic polymorphism of the SCGB1A1 gene has been associated with COPD development in smokers (Citation17).
In conclusion, we demonstrated that assessing the SCGB1A1/IL-8 ratio in the IS of smokers is a potential non-invasive marker of small airway remodeling. Whether smokers with high SCGB1A1/IL-8 levels could be better protected against COPD and whether exogenous SCGB1A1 supplementation could reverse those remodeling features will require further investigation in longitudinal study cohorts.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The author thanks La Direction de la Recherche Clinique (DRC) of the CHU de Montpellier, France, and L’APARD – Montpellier, France, Nicolas Molinari, PhD, BESPIM—CHU Nîmes, France and Dr. Nicole Mifsud, Monash University, Melbourne for their support. This work was supported by a grant from Direction de la Recherche Clinique (DRC) of the CHU de Montpellier, France, and l’APARD, Montpellier, France.
REFERENCES
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(26):2645–2653.
- Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006; 61(11):935–939.
- Kim V, Rogers TJ, Criner GJ. 2008. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 5(4):478–485.
- Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996; 153(2):530–534.
- Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med 1997; 155(2):542–548.
- Yamamoto C, Yoneda T, Yoshikawa M, Fu A, Tokuyama T, Tsukaguchi K, Narita N. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest 1997; 112(2):505–510.
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of clara cells in normal human airway epithelium. Am J Respir Crit Care Med 1999; 159(5 Pt 1):1585–1591.
- Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3(8):718–725.
- Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 2002; 161(1):173–182.
- Antico G, Lingen MW, Sassano A, Melby J, Welch RW, Fiore S, Pilon AL, Miele L. Recombinant human uteroglobin/CC10 inhibits the adhesion and migration of primary human endothelial cells via specific and saturable binding to fibronectin. J Cell Physiol 2006; 207(2):553–561.
- Singh G, Katyal SL. 1997. Clara cells and Clara cell 10 kD protein (CC10). Am J Respir Cell Mol Biol 2006; 17(2):141–143.
- Singh G, Katyal SL. Clara cell proteins. Ann N Y Acad Sci 2000; 923:43–58.
- Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. 2003. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol 171(2):1051–1060.
- Yang Y, Zhang Z, Mukherjee AB, Linnoila RI. 2004. Increased susceptibility of mice lacking Clara cell 10-kDa protein to lung tumorigenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a potent carcinogen in cigarette smoke. J Biol Chem 279(28):29336–29340.
- Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in CCSP-null mice suggests immunoregulatory roles for Clara cells. Am J Physiol Lung Cell Mol Physiol 2001; 281(6):L1523–1530.
- Durham SK, Selig WM. Pathophysiologic changes induced by phospholipase A2 in the isolated, perfused guinea pig lung. Exp Lung Res 1990; 16(4):323–338.
- Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, Pinsky DJ, Watkins SC, Pilewski JM, Sciurba FC, Peters DG, Hogg JC, Choi AM. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 2004; 101(41):14895–14900.
- Lindahl M, Irander K, Tagesson C, Stahlbom B. Nasal lavage fluid and proteomics as means to identify the effects of the irritating epoxy chemical dimethylbenzylamine. Biomarkers 2004; 9(1):56–70.
- Wattiez R, Noel-Georis I, Cruyt C, Broeckaert F, Bernard A, Falmagne P. Susceptibility to oxidative stress: proteomic analysis of bronchoalveolar lavage from ozone-sensitive and ozone-resistant strains of mice. Proteomics 2003; 3(5):658–665.
- Merkel DW, Rist W, Seither P, Weith A, Lenter MC. Proteomic study of human bronchoalveolar lavage fluids from smokers with chronic obstructive pulmonary disease by combining surface-enhanced laser desorption/ionization-mass spectrometry profiling with mass spectrometric protein identification. Proteomics 2005; 5(11):2972–2980.
- Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomics 2006; 6(3):1001–1010.
- de Torre C, Ying SX, Munson PJ, Meduri GU, Suffredini AF. Proteomic analysis of inflammatory biomarkers in bronchoalveolar lavage. Proteomics 2006; 6(13):3949–3957.
- Nord M, Schubert K, Cassel TK, Andersson O, Riise GC. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation 2002; 73(8):1264–1269.
- Lesur O, Langevin S, Berthiaume Y, Legare M, Skrobik Y, Bellemare JF, Levy B, Fortier Y, Lauzier F, Bravo G, Nickmilder M, Rousseau E, Bernard A. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med 2006; 32(8):1167–1174.
- Bernard A, Carbonnelle S, Nickmilder M, de Burbure C. Non-invasive biomarkers of pulmonary damage and inflammation: Application to children exposed to ozone and trichloramine. Toxicol Appl Pharmacol 2005; 206(2):185–190.
- Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, Hays SM. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers 2007; 12(5):445–467.
- Wang SX, Liu P, Wei MT, Chen L, Guo Y, Wang RY, Tu ZG, Liang XC. Roles of serum clara cell protein 16 and surfactant protein-D in the early diagnosis and progression of silicosis. J Occup Environ Med 2007; 49(8):834–839.
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, York E, Mainra RR, Ramesh W, Melenka LS, Wilde E, Cowie RL, Williams D, Gan WQ, Rousseau R. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177(11):1207–1214.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 32–34; 50(10):1005–1012.
- Berger P, Laurent F, Begueret H, Perot V, Rouiller R, Raherison C, Molimard M, Marthan R, Tunon-de-Lara JM. Structure and function of small airways in smokers: relationship between air trapping at CT and airway inflammation. Radiology 2003; 228(1):85–94.
- Tunon-de-Lara JM, Laurent F, Giraud V, Perez T, Aguilaniu B, Meziane H, Basset-Merle A, Chanez P. Air trapping in mild and moderate asthma: effect of inhaled corticosteroids. J Allergy Clin Immunol 2007; 119(3):583–590.
- Djukanovic R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl 2002; 37:1s–2s.
- Woolhouse IS, Bayley DL, Stockley RA. Effect of sputum processing with dithiothreitol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax 2002; 57(8):667–671.
- Louis R, Shute J, Goldring K, Perks B, Lau LC, Radermecker M, Djukanovic R. The effect of processing on inflammatory markers in induced sputum. Eur Respir J 1999; 13(3):660–667.
- Stockley RA, Bayley DL. Validation of assays for inflammatory mediators in sputum. Eur Respir J 2000; 15(4):778–781.
- Beckstead JH. Optimal antigen localization in human tissues using aldehyde-fixed plastic-embedded sections. J Histochem Cytochem 1985; 33(9):954–958.
- Kim V, Criner GJ, Abdallah HY, Gaughan JP, Furukawa S, Solomides CC. Small airway morphometry and improvement in pulmonary function after lung volume reduction surgery. Am J Respir Crit Care Med 2005; 171(1):40–47.
- Helleday R, Segerstedt B, Forsberg B, Mudway I, Nordberg G, Bernard, A, Blomberg A. Exploring the time dependence of serum clara cell protein as a biomarker of pulmonary injury in humans. Chest 2006; 130(3):672–675.
- Hermans C, Aly O, Nyberg BI, Peterson C, Bernard A. Determinants of Clara cell protein (CC16) concentration in serum: a reassessment with two different immunoassays. Clin Chim Acta 1998; 272(2):101–110.
- Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med 1999; 159(2):646–678.
- Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993; 147(2):405–410.
- Braido F, Riccio AM, Guerra L, Gamalero C, Zolezzi A, Tarantini F, De Giovanni B, Folli C, Descalzi D, Canonica GW. Clara cell 16 protein in COPD sputum: a marker of small airways damage? Respir Med 2007; 101(10):2119–2124.
- Shijubo N, Itoh Y, Yamaguchi T, Shibuya Y, Morita Y, Hirasawa M, Okutani R, Kawai T, Abe S. Serum and BAL Clara cell 10 kDa protein (CC10) levels and CC10-positive bronchiolar cells are decreased in smokers. Eur Respir J 1997; 10(5):1108–1114.
- Pilette C, Godding V, Kiss R, Delos M, Verbeken E, Decaestecker C, De Paepe K, Vaerman JP, Decramer M, Sibille Y. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163(1):185–194.
- Van Miert E, Dumont X, Bernard A. CC16 as a marker of lung epithelial hyperpermeability in an acute model of rats exposed to mainstream cigarette smoke. Toxicol Lett 2005; 159(2):115–123.