ABSTRACT
Rationale: Chronic obstructive pulmonary disease (COPD) is a major public health problem. This study was performed to determine whether the low attenuation area (LAA) and visual score provided by low-dose computed tomography (CT) can be used to detect occult parenchymal disease, such as insidious COPD. Methods: Each participant underwent low-dose CT scan and pulmonary function tests. The LAA% of the corresponding lung area was calculated. The cut-off level between the normal lung density area and LAA was defined as –960 HU, and the severity of emphysematous change (visual score) and LAA% were evaluated on three same chest CT slices obtained at full inspiration. Results: Forty-eight of 2,247 individuals including 1058 non-smokers and 1189 smokers were diagnosed with COPD. Chest CT findings in individuals diagnosed with COPD showed centrilobular emphysema (50%), however, 17 of the subjects diagnosed with COPD had normal screening CT findings. Thirty-one subjects diagnosed with COPD showed a positive visual score, and 27 individuals with COPD showed LAA% of more than 30. Nine of 17 subjects with a negative visual score showed LAA% of more than 30. The visual score in smokers was significantly higher than that of non-smokers. The lung function in smokers was lower than that of non-smokers. Smokers also showed higher frequencies of chest CT abnormalities. Conclusion: Low-dose CT scans detected LAA and a positive visual score before COPD associated with an impaired lung function develops. Smokers with normal spirometry had a potential to develop an airflow obstruction accompanied with abnormal CT findings.
INTRODUCTION
Many hospitals in Japan have begun screening using low-dose chest CT for the detection of lung cancer (Citation1). Various abnormalities, such as a low attenuation area (LAA), bronchial wall thickness, bronchiectasis and fibrosis, are often identified in addition to cancerous or benign nodules. We tend to underestimate the importance of these abnormalities when screening for lung cancer. A quantitative method using digital data as well as a visual assessment of the scan is currently available for the analysis of CT images (Citation2–4).
Chronic obstructive pulmonary disease (COPD) is a major public health problem, and the associated chronic morbidity and mortality in Japan have increased as in the United States and Europe (Citation5). However, COPD has not attracted adequate attention from the health care community. In the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging system, the diagnosis of COPD is confirmed by spirometry, and is classified into four stages (Citation6). Pulmonary function tests are used for the initial diagnosis and assessment of clinical stage of COPD. COPD is an insidious disease, with many years between the development of pulmonary function abnormalities with an irreversible airflow limitation and the onset of serious respiratory symptoms, such as severe breathlessness. As much as 30% of the lung may be destroyed by emphysema before either symptoms or abnormalities become evident on pulmonary function tests (Citation7). Therefore, spirometry allows the recognition of a functional impairment caused by the disease, thus providing an indirect way of assessing disease severity.
Chest CT has been established as a sensitive diagnostic modality for the detection of early symptomatic and asymptomatic COPD (Citation8). In previous studies, the percentage of the low attenuation area (LAA%) on CT scans has been shown to correlate with macroscopic and microscopic emphysematous changes in the lung (Citation9). In understanding the potential long-term impact on the function with tiotropium (UPLIFT), the mean absolute improvements in forced expiratory volume in one second (FEV1) in the tiotropium group were maintained throughout the trial, in comparison to the placebo group. This therapy with tiotropium was associated with improvements in the lung function, quality of life, and exacerbations during a 4-year period, especially COPD patients with GOLD stage II (Citation10, 11). The detection of emphysematous changes thus emphasizes that early intervention, including the cessation of cigarette smoking and appropriate treatment, should be promoted in subjects with chest CT evidence of emphysematous changes. Many hospitals in Japan perform low-dose chest CT as a health check, but do not perform pulmonary function tests, thus, many asymptomatic COPD patients could be detected based on the findings of such low-dose CT images. The present study was performed to assess the ability of low-dose chest CT to detect emphysematous changes or airway disease such as a COPD diagnosed using both radiological evaluations and pulmonary function tests.
Subjects and methods
The research protocol, including each patient's data, the use of low-dose CT and pulmonary function tests, was approved by the human ethics committee of Azumi General Hospital. All subjects gave their informed consent at presentation to undergo chest CT scans and pulmonary function tests.
Subjects
During a health screening program for the detection of lung cancer, individuals who enrolled after responding to advertisements for lung cancer screening received a general health check including a low-dose chest CT scan and pulmonary function tests. This program in Japan is a routine care program as a public health campaign and a portion of the costs is covered by each company and community which the enrolled individuals belonged to. The enrolled individuals were from a rural area of Japan; namely the Azumi or Kouhoku area around Azumi General Hospital, or they belonged to an agricultural cooperative association in Nagano Prefecture. These individuals were representative of the rural population, and many were a highly motivated for various reasons to worry about lung health, especially lung cancer. The subjects filled out a questionnaire about lifestyle, respiratory symptoms, smoking history, past medical history and demographic data. Questions about respiratory symptoms were related to cough, sputum and shortness of breath. The questions and ratings of responses related to the above symptoms included: 1) frequency of these symptoms (none, intermittently, almost every day), 2) duration (within 1 week, within 1 month, and over 3 months), 3) progressive or persistent symptoms. Any subjects with self-reported asthma, bronchiectasis, diffuse panbronchiolitis, or pulmonary tuberculosis with severe obstructive pulmonary function based on their clinical information, chest CT findings or pulmonary function tests were excluded.
Multislice CT scan
Each participant underwent low-dose non-enhanced Multislice CT (model Toshiba Asteion Multi, Tokyo, Japan; 25 mA, 120 kVp, 5-mm section thickness, 0.75 seconds of rotation, and pitch of 5.5). All CT images were viewed in cine-mode format on a computer workstation by one radiologist (S.S.) and one pulmonologist (K.T.) with 40 and 11 years of experience, respectively, and displayed under 3 display conditions to adequately examine the lungs, hilar bronchi and mediastinum (width 1000 Hounsfield units (HU), level –700 HU; width 1500 HU, level –550 HU; and width 300 HU, level 20 HU, respectively).
Severity of emphysema graded using visual score
The severity of emphysema change was scored subjectively by using the scoring method of Goddard et al. (Citation12). CT scans were independently reviewed by one blinded radiologist and one pulmonologist, and the mean of the two severity scores was used for diagnosis. Emphysematous destruction was identified as LAA and hypovascular regions in the lung (Citation12, 13). The lung sections were defined, in order to standardize the analysis as: the upper (cranial) section represented the area approximately 10 mm above the superior margin of the aortic arch; the middle section represented the area approximately 10 mm below the carina; and the lower (caudal) section was limited to the slice approximately 3 cm above the first recognition of the diaphragm (Citation14–16). The percentage of the lung with emphysematous changes was determined using the following 5-point scale: 0, less than 5%; 1, 5%–25% involvement; 2, 26%–50%; 3, 51%-75%; and 4, more than 76%. The evaluation was performed for each lung at each of the 3 selected right and left slices to obtain a maximum score of 24. A positive visual score was defined as any visual score greater than 0. The type of emphysema on the chest CT findings at the 3 selected slices was classified into centrilobular emphysema (CLE), paraseptal emphysema showing mainly subpleural emphysematous change excluding bullae and blebs (PSE), panlobular emphysema showing mainly panacinar emphysematous change (PLE), and combined pulmonary fibrosis and emphysema (CPFE) (Citation17).
Analysis of low attenuation area
Three similar sections to those used in the scoring method were analyzed. Each CT image was composed of 512 × 512 matrices with numerical data (CT numbers) in HU. The LAA% was computed using a previously reported method with minor modifications (Citation9). The cut-off level between the normal lung density area and LAA was defined as –960 HU (Citation18). The LAA% of each segment was averaged relative to the areas of the right and left lungs since the areas of the right and left lungs were not identical. The trachea, major bronchus, and vessels at the hilum were excluded from this analysis, and the lung parenchyma was outlined as the region of interest. The LAA% values of the upper, middle and lower sections (U-LAA%, M-LAA% and L-LAA%, respectively) were calculated. The maximum LAA% among the three sections was also selected.
Pulmonary function tests
Pulmonary function tests were performed using the routine method (Citation19). Spirometry was performed in all participants from 15–45 minutes after the inhalation of β2 stimulant. The vital capacity (VC), forced vital capacity (FVC), and FEV1 were measured using a Spiroshift SP-700 (Fukuda Denshi, Tokyo). The%VC,%FEV1 and FEV1/FVC were calculated according to the prediction equations of the Japanese Society of Chest Diseases.
COPD criteria
The diagnosis and staging of COPD was based on the clinical symptoms and the results of spirometry according the criteria of GOLD (Citation6). Four different stages were defined as: Stage I, II, III and IV.
Statistical analysis
The subjects were classified into two groups based on the diagnostic criteria of GOLD; non-COPD (normal) including non-smokers and smokers and COPD (Stage I – IV). The following characteristics were listed in each group: sex, age, smoking history, pulmonary function, and symptoms. Student's t-test was used to assess the significance of differences between two groups and to study the differences in LAA%, visual score, and pulmonary function variables between COPD and non-COPD subjects. The receiver operating characteristics (ROC) curves were used to determine the most accurate diagnostic thresholds for variables that correlated to FEV1/FVC or differed between COPD and non-COPD subjects. The obtained thresholds were used to transform the continuous variables into categorical variables, and Pearson's chi-square test was performed to study differences in the distributions of these categorical variables between COPD and non-COPD subjects. This analysis was used to calculate the probability of COPD using the following equation: P’ = ey/(1+ey), where y = constant + x1×LAA% + x2×visual score. The constant and parameter estimates (x1, x2) were determined by a logistic regression analysis with COPD as a dependent variable, and the LAA% and visual score as independent variables. The goodness of fit of the model was assessed using the Hosmer and Lemeshow test. The ROC curves of the LAA% and visual score were drawn based on the probability of COPD and the diagnostic characteristic of the logistic prediction model were evaluated. Finally, all individual data were analyzed to determine whether some ranges or combinations of ranges of variables were highly predictive of either the presence or absence of COPD. Finally, the best combination of the thresholds of two variables was selected to evaluate the diagnostic characteristics, i.e., sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV).
The results are expressed as the means ± standard deviation (SD) unless otherwise indicated. A p value < 0.05 was considered to be significant. The statistical analysis was performed with the SAS ver. 8.2 statistical software package (SAS Institute; Cary, NC).
RESULTS
A total of 48 subjects were diagnosed with COPD
A total of 1,359 men and 888 women underwent low-dose chest CT scans and pulmonary function testing at the Azumi General Hospital during 1 year. Their mean age was 53.7 ± 12.7 years. The numbers of never-, ex- and present smokers were 1,058 (47.1%), 457 (20.3%) and 732 (32.6%), respectively. The mean pack-years of ex- and present smokers were 22.7 and 30.7, respectively. The numbers of male and female smokers were 1,081 and 108, respectively. Sixty-nine (3.1%) subjects complained of chronic productive cough and mucus hypersecretion. Three patients with bronchiectasis and 6 with old tuberculosis with severe obstructive pulmonary function were excluded from this study. No difference was observed in the prevalence of bronchiectasis between smokers and non-smokers.
Forty-eight (2.1%) of 2,247 individuals (40 men and 8 women including 27 present smokers, 14 ex-smokers and 7 never-smokers) were diagnosed with Stage I to Stage III COPD. None of the screened individuals had been diagnosed previously to have COPD before this health check. The numbers of patients with GOLD Stage I, II and III disease were 22, 24 and 2, respectively. Two individuals with GOLD Stage III complained of progressive shortness of breath with cough and sputum. They were both ex-smokers (45 and 60 pack-years), and had never been treated for COPD.
Pulmonary function tests showed significant differences in smoker in comparison to non-smoker
As shown in , the mean age of COPD patients was significantly higher than that of non-smoker and smoker subjects. The number of pack-years and respiratory symptoms among the COPD patients were significantly higher than those among the smokers. Only 2.7% of smokers and 0.07% of non-smokers have a low FEV1 but a normal FEV1/FVC. No difference was observed in the spectrum of CT abnormalities in smokers. The VC, FEV1, FEV1/FVC and%FEV1 were also significantly lower than those of non-smoker and smoker subjects. The frequency of smokers was significantly higher than that of male non-smokers. The FEV1/FVC and%FEV1 in smokers were significantly lower than those of non-smoker.
Table 1. Subject characteristics
LAA% and visual score were correlated with FEV1/FVC
As shown in , chest CT findings in 24 of 48 (50%) individuals diagnosed with COPD were consistent with centrilobular emphysema. On the other hand, 17 individuals with COPD showed normal CT findings. Approximately 90% of the patients with PSE were smokers, and the number of COPD patients with PSE was significantly lower than that of patients with CLE (p < 0.0001). The patients with CPFE showed pulmonary fibrosis in the lower lobes and emphysematous changes in the upper lobes.
Table 2. Chest computed tomography findings
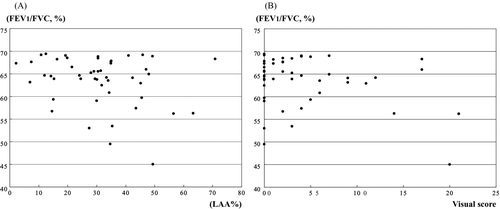
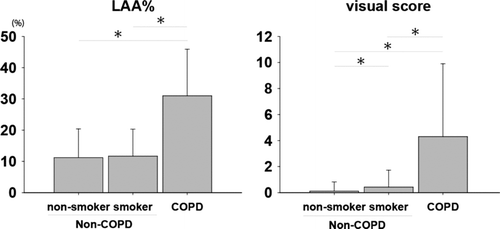
The mean LAA% in PLE pattern (45.7 ± 16) was much higher than that of the CPFE (8.6 ± 7.7) and PSE patterns (14.1 ± 10.4). The sensitivity and specificity of CLE and PSE on chest CT in COPD patients were 50% and 29%, and 94% and 95%, respectively (p < 0.0001 and p = 0.0002, respectively). As shown in , significant relationships were observed between the LAA% and FEV1/FVC (p = 0.05, r = −0.29; ), the visual score and FEV1/FVC (p = 0.044, r = −0.24; ), and the visual score and%FEV1 (p = 0.05, r = −0.28). There was no difference in LAA% between non-smokers and smokers, but the visual scores in smokers were significantly higher than those of non-smokers.
The results of the LAA% and positive visual score could detect COPD subjects
As shown in , the numbers of subjects with an LAA% <10% and ≧ 30% were 1,159 and 123, respectively. Forty-five of 1,159 individuals with an LAA% <10% were diagnosed as having COPD (38 men including 36 smokers, 7 women including 2 smokers, respectively). Twenty-seven of 123 individuals with an LAA% ≧30% were diagnosed with COPD (25 men including 23 smokers, 2 women including 1 smoker, respectively).
Table 3. Characteristics of individuals with LAA%, including those diagnosed having COPD
The number of subjects with a positive visual score was 249. Thirty-one of 249 subjects with a positive score were diagnosed to be COPD patients (29 men including 27 smokers and 2 women including 1 smoker, respectively). An LAA% < 10% and an LAA% ≧30% were noted in 7 and 9 of 17 COPD individuals with a negative visual score, respectively. The subjects with LAA% ≧30% and a negative visual score had severe airflow limitations without emphysematous change, or the so-called non-emphysematous pattern.
Table 4. Threshold for COPD using the combination of LAA% and visual score
The LAA% and visual score were shown to be statistically significant independent variables
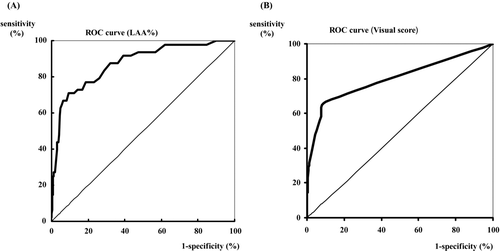
The areas under the ROC curves were 0.87 (LAA%) and 0.80 (visual score), respectively (). The LAA% and visual score were shown to be statistically significant independent variables. The combination of the cut-off values between the LAA% and visual score were measured. As shown in , if the LAA% ≧ 10 and visual score ≧ 1 are positive, the sensitivity and specificity are 62.5% and 94.0%, respectively.
When both positive scores the detection of COPD, the best cut-off values for the LAA% and visual score were ≧ 10% and ≧ 1, respectively. If the LAA% ≧ 10 or visual score ≧ 1 are positive, the sensitivity, specificity, PV and NPV are 97.9%, 48.6%, 4.0% and 99.9%, respectively. If the LAA% ≧ 30 and visual score ≧ 1 are positive, 43 smokers and 49 non-smokers showed positive LAA%. Twenty-one smokers (1.8%) with normal spirometry and 11 non-smokers (1.0%) showed positive findings for both LAA% and the visual score.
DISCUSSION
This is the first report to determine whether it is possible to detect insidious COPD using the LAA% and visual score on low-dose CT conducted as part of an annual health check. Several studies designed to detect and quantify pulmonary emphysema using CT have been reported (Citation9). These observations raise the question of whether low-dose CT can be applicable as a method of early intervention in COPD in addition to pulmonary function tests. This study attempted to clarify whether the patterns of emphysema in smokers with normal spirometry findings were different from the clinical data of non-smokers.
The maximum LAA% was selected using three sections to minimize the radiation dose according to the report of Nakano et al. (Citation18). This Nakano's study set –960 HU as the cut-off level between the normal lung density area and LAA. Although the preliminary data were not shown in this report, the mean LAA% in 24 patients with COPD was approximately 46% using a cut-off level of –960 HU on low-dose CTs. These cut-off levels may be due to differences between conventional and HRCT scans, or between CT machines with different reconstruction algorithms.
Although not shown here, the detection of LAA was similar in 10 mm and 2 mm collimation on CTs. However, the detection of micro LAA and abnormalities in the lung apex was superior with 2 mm collimation. Seventeen (35.4%) of the 48 participants with COPD had normal CT findings without any emphysematous changes. As much as 30% of the lung may be destroyed by emphysema before either symptoms or abnormalities become evident on pulmonary function tests (Citation7). The screening technique should be positive in all cases.
Figure 3. Receiver operating characteristic (ROC) curves for COPD using the low attenuation area (LAA%; A) and visual score (B). The area under the ROC curve for the LAA% is 0.87, and that for visual score is 0.80. The sensitivity and specificity of these individual variables for the screening of COPD were: threshold of the LAA% (range, 0–100%); 10% (sensitivity 93.8%, specificity 52.9%), 20% (sensitivity 72.9%, specificity 83.1%), and 30% (sensitivity 58.3%, specificity 95.7%) and threshold of visual score (range 0–24); 1 (sensitivity 66.7%, specificity 90.1%), 2 (sensitivity 58.3%, specificity 92.2%), and 3 (sensitivity 47.9%, specificity 95.8%).
However, it is important to exclude false positives; i.e., cases in which the results of the screening are positive but the individual does not have the disease. These 17 participants may not have shown emphysematous changes due to the 5-mm slice thickness and low-resolution due to the low-dose CT; this is a limitation of these methods. It is necessary to perform thin-section CT or higher-dose CT to allow better visual assessment. Thin-section CT has been validated for the assessment of emphysematous change generally.
Because low-dose CT is commonly performed to screen for lung cancer in Japan, its utility was examined for detection of emphysema. It is probably necessary, statistically, to use low LAA% because low-dose CT with 5-mm collimation showed indistinct emphysematous changes. However, by using LAA% ≧ 30%, 8 of the 17 COPD individuals with a negative visual score were identified as COPD individuals.
COPD is defined by functional criteria according to GOLD, thus pulmonary emphysema is one type of COPD, and there are COPD subjects without apparent emphysematous change (Citation20). The visual score assesses only pulmonary emphysema. LAA% may be included in not only COPD with emphysema type but also COPD with non-emphysematous type. The visual score alone has been shown to be of limited reliability in the analysis of emphysematous change (Citation21). Nine of 17 participants with a negative visual score showed an LAA% of more than 30% in that study. As shown in , an LAA%≧ 30 shows a high specificity for COPD, although the pathological and clinical significance of a high LAA% has not been precisely determined.
These combinations of LAA% and positive visual scores were sufficient to be useful in detecting COPD although these values do not reveal pulmonary function. As shown in , the LAA% and visual score were correlated with FEV1/FVC, and visual score was correlated with%FEV1. It is therefore significant to detect these COPD patients based on the LAA% and visual score on low-dose chest CT. The detection of emphysematous changes should be emphasized because only symptomatic treatment is available once the patient presents with a functional impairment.
Our data are considered to provide a chance to examine the CT scans of the smokers with normal lung function and compare these results to the results of a similar analysis in the non-smokers. As shown in , smokers with normal spirometry tended demonstrate larger quantities of sputum in comparison to non-smoker, and the difference was significant. The FEV1/FVC and%FEV1 in the smokers with normal spirometry showed significantly lower findings than those of non-smokers. Chest CT findings showed abnormalities, such as PSE, CLE and CPFE and the visual score was also higher than that of non-smokers. Therefore, smokers with normal spirometry and abnormal CT findings may have potentials to develop an airflow obstruction in the future in comparison to non-smokers.
The prevalence rate of COPD in the present study in individuals aged over 40 years was 2.4%, thus the logistic regression model, using a probability of 0.009, sensitivity of 95.8% and specificity of 58.2%, would predict the presence or absence of COPD with a positive predictive value of 5.3% and a negative predictive value of 99.8%. In the Nippon COPD epidemiology study in Japan, the prevalence rate of COPD in subjects aged over 40 years was 10.9% (Citation22). The differences between the two studies were likely to be due to differences in the prevalence rate among the individuals participating in the two studies.
The Nippon COPD study was based on random sampling of a general population regardless of symptoms. Although the Nippon COPD study was based on the results of pulmonary function tests and self reports, the prevalence of COPD may have included bronchial asthma, diffuse panbronchiolitis, or pulmonary tuberculosis with severe obstructive pulmonary function. However, the prevalence rate of Nippon COPD study excluding bronchial asthma was 8.6% (Citation22). The current participants were recruited from a population in which the mean age was about 10 years younger than that of the general population in the same area that undergoes an annual health examination, and they included few elderly subjects and none with obvious COPD.
The current study mainly examined active workers employed in various industries living in Azumi and Taihoku areas rather than conducting a field screening survey. The male smoking rate in this study was high, similar to a previous report, possibly because the people who live in this area had not been educated about the harm caused by cigarettes (Citation23). Therefore, if the present study had been performed based on individuals from the true general population, it may have detected individuals with COPD at a higher rate, and smoking subjects may develop to COPD 10 years later based on our visual score and chest CT findings.
Screening is dependent on a high NPV but a PPV that is too low will give a lot of false positives resulting in a substantial cost. As shown in , if the cut-off values were set for the LAA% and visual score were >10% and ≧1, respectively, this cut-off value of low LAA% was sufficiently useful to allow detection of many false positive cases, including non-COPD subjects. As shown in and , these values will not be adaptable to clinical use for the detection of COPD subjects. For example, when selecting patients based on risk factors such as the smoking history, their detection rates according to the ROC under the area of LAA% and visual score arise as 0.87 and 0.80 to 0.88 and 0.82, respectively.
The current software based algorithms for disease quantification are more reproducible than the visual score, but they lack the capacity to integrate subtleties that only the human eye can detect. As shown in , there was no difference in LAA% between smokers and non-smokers. However, a visual analysis in smokers showed a significantly higher score than that of non-smokers. A visual analysis would therefore be helpful to identify the differences in smokers with a normal lung function. For different aims (to select a much more strongly suspected COPD group or a more distinctly suspected COPD group), it would therefore be possible to perform early intervention in groups according to the selected cut-off points of LAA% and visual score. This raises the question of how many values can be adapted to clinical use. Therefore, the best cut-off values of COPD require an LAA% ≧ 30 and a positive visual score (≧ 1) in order to identify COPD patients, respectively.
CONCLUSION
In subjects suffering from insidious COPD, low-dose CT scans showed the LAA and positive visual score before the subjects with COPD develop an impaired lung function. Smokers with normal spirometry findings are thus considered to have a potential to develop an airflow obstruction with abnormal CT findings.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sone S, Takashima S, Li F, Yang ZG, Honda T, Maruyama Y, Hasegawa M, Yamanda T, Kubo K, Hanamura K, Asakura K. Mass screening for lung cancer with a mobile spiral computed tomography scanner. Lancet 1998; 351:1242–1245.
- Dechman G, Mishima M, Bates JHT. Assessment of acute pleural effusion in dogs by computed tomography. J Appl Physiol 1994; 76:1993–1998.
- Gevenois PA, De Vuyst P, Sy M, Scillia P, Chaminade L, de Maertelaer V, Zanen J, Yernault JC. Pulmonary emphysema: quantitative CT during expiration. Radiology 1996; 199:825–829.
- McLean AN, Sproule MW, Cowan MD, Thomson NC. High resolution computed tomography in asthma. Thorax 1998; 53:308–314.
- National Heart, Lung, and Blood Institute. Morbidity and Mortality: chartbook on cardiovascular, lung, and blood diseases. Bethesda, MD: US Department. Of Health and Human Services, Public Health Service, National Institutes of Health, 1988.
- Global Initiative for Chronic Obstructive Lung Disease. (GOLD) http://www.gddcopd.com (accessed May 2008).
- Pratt PC, Kilburn KH. A modern concept of the emphysema based as correlations of structure and function. Hum Pathol 1970; 1:443–463.
- Thurlbeck WM, Muller DC. Emphysema. Definition, imaging and quantification. AJR Am J Roentgenol 1994; 163:1017–1025.
- Kuwano K, Matsuba K, Ikeda T, Murakami J, Araki A, Nishitani H, Ishida T, Yasumoto K, Shigematsu N. The diagnosis of mild emphysema: correlation of computed tomography and pathology scores. Am Rev Respir Dis 1990; 141:169–178.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M; UPLIFT Study Investigators. A 4-Year Trial of Tiotropium in Chronic Obstructive Pulmonary Disease. N Engl J Med 2008; 359:1543–1554.
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP; UPLIFT investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374:1171–1178.
- Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol 1982; 33:379–387.
- Gurney JW, Jones KK, Robbins RA, Gossman GL, Nelson KJ, Daughton D, Spurzem JR, Rennard SI. Regional distribution of high-resolution CT with pulmonary function tests in unselected smokers. Radiology 1992; 183:457–463.
- Mishima M, Oku Y, Kawakami K, Sakai N, Fukui M, Hirai T, Chin K, Ohi M, Nishimura K, Itoh H, Tanemura M, Kuno K. Quantitative assessment of the spatial distribution of low attenuation areas on X-ray CT using texture analysis in patients with chronic pulmonary emphysema. Front Med Biol Eng 1997; 8:19–34.
- Mishima M, Hirai T, Jin Z, Oku Y, Sakai N, Nakano Y, Sakai H, Chin K, Ohi M, Kawakami K, Shimada K, Itoh H, Yamaguchi K, Sawa T, Kuno K. Standardization of low attenuation area versus total lung area in chest X-ray CT as an indicator of chronic pulmonary emphysema. Front Med Biol Eng 1997; 8:1993–1998.
- Coxson HO, Rogers RM, Whittall KP, D’yachkova Y, Pare PD, Sciurba FC, Hogg JC. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 1999; 159:851–856.
- Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF; Groupe d’Etude et de Recherche sur les Maladies Orphelines Pulmonaires (GERM O P). Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005; 26:586–593.
- Nakano Y, Sakai H, Muro S, Hirai T, Oku Y, Nishimura K, Mishima M. Comparison of low attenuation areas on CT between inner and outer segments of the lung in COPD patients: incidence and contribution to lung function. Thorax 1999; 54:384– 389.
- Kubo K, Yamazaki Y, Masubuchi T, Takamizawa A, Yamamoto H, Koizumi T, Fujimoto K, Matsuzawa Y, Honda T, Hasegawa M, Sone S. Pulmonary infection with mycobacterium avium-intracellulare leads to air trapping distal to the small airway. Am J Respir Crit Care Med 1998; 27: 979– 984.
- Fujimoto K, Kitaguchi Y, Kubo K, Honda T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology 2006; 11:731–740.
- Bankier AA, Maertelaer VD, Keyzer C, Gevenois PA. Pulmonary emphysema: Subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999; 211:851–858.
- Fukuchi Y, Nishimura M, Ichinose M, Adachi M, Nagai A, Kuriyama T, Takahashi K, Nishimura K, Ishioka S, Aizawa H, Zaher C. COPD in Japan: the Nippon COPD epidemiology study. Respirology 2004; 9:458–465.
- Sone S, Li, F, Yang ZG, Honda T, Maruyama Y, Takashima S, Hasegawa M, Kawakami S, Kubo K, Haniuda M, Yamanda T. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Brit J Cancer 2001; 84:25–32.