Abstract
The recovery of potentially pathogenic microorganisms (PPMs) from bronchial secretions is associated with a local inflammatory response in COPD patients. The objective of this study was to determine the relationships between bronchial colonisation and both bronchial and systemic inflammation in stable COPD. In COPD patients recruited on first admission for an exacerbation, bacterial sputum cultures, interleukin (IL)-1β, IL-6 and IL-8 levels, and blood C-reactive protein (CRP) were measured in stable condition. Bronchial colonisation was found in 39 of the 133 (29%) patients and was significantly related to higher sputum IL-1β (median [percentile 25–75]; 462 [121–993] vs. 154 [41–477] pg/ml, p = 0.002), IL-6 (147 [71–424] vs. 109 [50–197] pg/ml, p = 0.047) and IL-8 values (15 [9–19] vs. 8 [3–15] (×103) pg/ml, p = 0.002). Patients with positive cultures also showed significantly elevated levels of serum CRP (6.5 [2.5–8.5] vs. 3.5 [1.7–5.4] mg/l, p = 0.016). Bronchial colonisation by Haemophilus influenzae was associated with higher levels of IL-1β and IL-8 and clinically significant worse scores on the activity and impact domains of the St. George's Respiratory Questionnaire. In conclusion, bronchial colonisation is associated with bronchial inflammation and high blood CRP levels in stable COPD patients, being Haemophilus influenzae related to a more severe inflammatory response and impairment in health-related quality of life.
Introduction
In healthy subjects the bronchial tree and the pulmonary parenchyma are sterile, but potentially pathogenic microorganisms (PPMs) are often recovered from the bronchial secretions of patients with chronic obstructive pulmonary disease (COPD) during periods of clinical stability, with increased prevalence and higher loads appearing during episodes of exacerbation (Citation1,2). Bronchial colonisation is identified in one third of the stable COPD patients (Citation1), and has been related to an inflammatory response identifiable in bronchial secretions and blood, and quality of life impairment (Citation3–5). The mechanisms behind this relationship are not yet well understood, however, and may be different depending on the colonising PPM (Citation6–10).
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease with subtypes that has been described in accordance with epidemiological and clinical patterns (Citation11–13). More recently, factor and cluster analysis (Citation14–18) have been used for further assessment of the heterogeneity of the disease. The Phenotype and Course of Chronic Obstructive Pulmonary Disease (PAC-COPD) study has used these tools for the identification of COPD subtypes, in a cohort of 342 subjects hospitalised for the first time because of an exacerbation of the disease and examined three months after discharge, when clinically stable, validating prospectively three COPD subtypes against hospitalisations and mortality (Citation19). Bronchial colonisation was similarly prevalent in the subtypes defined in the PAC-COPD Study, ranging between 26% and 36%, a finding that suggest that the relationships between bronchial colonization and COPD may be independent of the phenotype of the disease.
Starting with the hypothesis that bronchial colonisation in COPD is associated with bronchial and systemic inflammation, this study investigates the relationships between colonisation, bronchial and systemic inflammation in stable patients recruited at a first admission for disease exacerbation and enrolled in the PAC-COPD study (Citation20), first focusing on the prevalence, typology and load of bronchial colonisation in COPD patients, and, second, investigating species-specific relationships between the colonising PPM and the observed inflammatory response, examining the associations between bronchial colonisation, inflammation and quality of life.
Methods
Design and participants
This cross-sectional analysis focusing on bronchial colonisation and inflammation in COPD was part of the population-based PAC-COPD study, that enrolled 342 COPD patients hospitalized for the first time for an exacerbation of their disease between January 2004 and March 2006 in 9 teaching hospitals in Spain, and further evaluated the patients in clinical stability. The recruitment process and the definitions of exacerbation, first admission, and COPD have been previously reported (Citation20, 21). Current and former smokers included in the PAC-COPD study who expectorated samples with low squamous cell content and had complete information on sputum microbiology, cell counts and inflammatory markers at the baseline evaluation in clinical stability were selected for the present analysis, under the assumption that samples with 20% squamous cells or less were representative of tracheobronchial secretions (Citation22). The research protocol was approved by the ethics committees of all the participating hospitals and written informed consent was obtained from all subjects.
Variables and measurements
Sociodemographic data were recorded at recruitment. Patients answered an epidemiologic questionnaire and performed all tests at least three months after hospital discharge and when clinically stable. Questions covered smoking habits, co-morbidity, respiratory symptoms, health-related quality of life with the St. George's Respiratory Questionnaire (SGRQ) and treatments. Patient functional characteristics included results of forced spirometry, reversibility testing, exercise capacity measured using the six minute walking test and body mass index (BMI).
Detailed information about the methods and the sources of the questionnaires and standardisation of the tests used in the PAC-COPD study has been previously published (Citation20, 21).
Sputum induction
In patients unable to produce sputum spontaneously a sample was induced according to standard methods (Citation23–25) and processed within 60 minutes of collection to guarantee cell viability (Citation26). In brief, the patient was pre-treated with an inhaled ß-adrenergic agent 10 minutes before the nebulisation of increasing concentrations of hypertonic saline (0.9%, 3%, 4% and 5%), for 7 minutes each. Subjects were asked to blow their nose, rinse their mouth, and swallow water to minimize contamination from postnasal drip and saliva and the nebulisation procedure was interrupted when the sputum volume collected was 1 ml or more (Citation27). After every induction the patient attempted to cough up sputum into a sterile plastic dish. The sputum sample obtained after the first induction was taken for microbiologic exam. The sputum samples obtained after a second induction were used for the analysis of inflammatory markers.
Microbiological exam
The sputum sample was weighed and processed with an equal volume of dithiothreitol (DTT) (Sputasol, Oxoid Ltd., Hants, UK), and cultured; the microbial load grown was then determined as described elsewhere (Citation28). Microbiological processing included determination of microbial typology and load through serial dilutions and culture in selective media for potentially pathogenic microorganisms (PPMs), according to standard methods (Citation29) with quantitative cultures expressed as colony-forming units (cfu) per millilitre.
In patients with polymicrobial cultures, the highest PPM load recovered was considered for the quantitative analysis. Cultures were considered positive for bronchial colonisation if they grew PPMs such as Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, Pseudomonas aeruginosa, enterobacteria and/orStaphylococcus aureus according to previously defined criteria (Citation30,31), at loads of at least 100 cfu/ml. Haemophilus parainfluenzae was not considered as a PPM in the present study. This bacteria has shown in cell cultures a low adherence to the bronchial mucosa (Citation32), and in patients with chronic bronchitis has demonstrated poor ability to induce an airway inflammatory response (Citation33).
Airway inflammatory markers and differential cell count
The sputum was separated from contaminating saliva by macroscopic examination. A Neubauer haemocytometer was used to determine cell viability using trypan blue exclusion. The remaining sputum was mixed with 4 times its weight of DTT solution, vortexed for 15 seconds, and then rocked for 15 minutes. A weight of phosphate-buffered saline solution equal to that of DTT was then added and the whole mixture was further vortexed for 15 seconds. The total inflammatory cell count, expressed as the absolute number of cells per gram of sputum, was calculated by subtracting squamous cells from the total cell count. Absolute and differential cell counts for neutrophils, lymphocytes and eosinophils were calculated by counting 400 non-squamous cells in Wright-stained slides. The remaining suspension was filtered through 48-μm nylon gauze and centrifuged at 750g.
The supernatant was decanted and stored at –80ºC for later analyses. Cytokine concentrations (interleukin [IL] -1β, IL-6, IL-8) were measured in this sample using a cytokine bead array (BD Biosciences, San Diego CA, USA) following the manufacturer's instructions. The detection limits of these assays were 7.2 pg/ml for IL-1β, 2.5 pg/ml for IL-6 and 3.6 pg/ml for IL-8. All assays were performed in duplicate and, because intra-assay variation was always <10%, reported values correspond to the average of the 2 determinations.
Blood inflammatory markers
At the same visit for all participants, a peripheral venous blood sample (20 ml) was collected into tubes containing ethylene diamine triacetic acid after overnight fasting. The sample was centrifuged at 2000 rpm for 10 minutes, and the serum was stored at –80ºC until analysis. Serum fibrinogen levels were measured by the Klauss method and C-reactive protein (CRP) levels by nephelometry. IL-6, IL-8 and tumour necrosis factor α (TNF-α) levels were determined with a high sensitivity enzyme-linked immunosorbent assay kit (Biosource, Camarillo, CA, USA). The lower limits of detection of these assays in serum samples were 0.16 mg/l, 0.104 pg/ml, 0.2 pg/ml and 0.09 pg/ml for CRP, IL-6, IL-8 and TNF-α respectively. All assays were performed in duplicate and reported values correspond to the average of the 2 determinations.
Statistical analysis
Data were analysed using the SPSS statistical software package version 15 (SPSS Inc., Chicago, IL, USA). Results for categorical variables are expressed as absolute and relative frequencies. Results for continuous variables are expressed as means (standard deviation [SD]) or medians (percentile 25–75 [P25-P75]) when the distribution was not normal.
First, sociodemographic, clinical and functional characteristics of no colonised and colonised patients were compared and the relationships between bronchial colonisation and inflammation were assessed in these patients using chi-square, Fisher exact, t-tests or Kruskal-Wallis tests, as required. Associations between sputum characteristics and systemic inflammation were examined through bivariate and multivariate analysis.
When CRP was considered as the outcome, the level was categorised using 7.06 mg/l as the cut-off, given that CRP levels over that value have been shown to be related to all-cause and cardiovascular mortality in COPD patients (Citation34). Bivariate logistic regression was used to assess the associations between bronchial colonisation and high CRP levels, and covariate variables that showed a bivariate association (p < 0.20) were included in the multivariate analysis that assessed the relationship between bronchial colonisation and the outcome. Results were expressed as crude and adjusted odds ratios (OR) with 95% confidence intervals (95% CI). Finally, differential relationships between bronchial colonisation and patient characteristics, bronchial and systemic inflammation, and quality of life were assessed.
Levels of bronchial and systemic inflammatory markers and quality-of-life scores in patients colonized by Haemophilus influenzae were compared with results in non-colonised patients, repeating this comparison in patients colonised by other PPMs for this assessment. Correlations between bacterial loads and cytokine levels is sputum were also assessed in patients colonized by Haemophilus influenzae, using Spearman's rank correlation coefficients for the identification of dose-response relationships between bacterial load and bronchial inflammation. All statistical tests were two-sided, and a p-value of 0.05 or less was reported as statistically significant.
Results
Sputum quality and microbiology
One-hundred fifty-five participants in the PAC-COPD study had a complete microbiologic, cytological and inflammatory sputum characterisation available at baseline. One-hundred thirty-three of them (85.8%) produced sputum samples that showed a low proportion of squamous cells, and were considered as representative of tracheobronchial secretions. These samples also showed higher cell viability, counts and cytokine concentrations when compared with samples that contained more than 20% squamous cells on examination (data not shown, p = 0.002, Kruskal-Wallis test). In patients expectorating representative samples spontaneous (n = 76) and induced sputum (n = 57) gave similar results on cell counts and cytokine concentrations (data not shown, p > 0.05, Kruskal-Wallis test), and both samples were considered as equivalent for the purposes of this study.
Accordingly with these results, the 133 patients who had representative sputum samples were selected and included in the present analysis. They had a mean age of 70 (SD 9) years, a moderate lung function impairment (mean post-bronchodilator forced expiratory volume in 1 second [FEV1] 52% [SD 16] of predicted), and a well-defined neutrophilic inflammatory pattern in sputum (median 72% [48%–84%]) (). PPMs were cultured from the sputum of 39 of these patients (29%). The bacterial load was high in most cases, with a median count over 106 colony forming units per millilitre. Forty-seven PPMs were recovered from these 39 patients, Haemophilus influenzae (n = 22), Pseudomonas aeruginosa (n = 8), Moraxella catarrhalis (n = 6) and Streptococcus pneumoniae (n = 5) being the bacteria most often cultivated ().
Table 1. Sociodemographic and sputum characteristics (microbiology, cytology and inflammatory markers) of PAC-COPD patients (n = 133)*
Bronchial colonisation and pulmonary and systemic inflammation
Clinical and sputum characteristics of no colonised (n = 94) and colonised (n = 39) COPD patients are compared in . Sociodemographic, clinical and functional differences between the two groups were not significant, except for higher cumulative smoking in colonised patients. A similar neutrophilic bronchial inflammatory response was observed in both no colonised and colonised patients, but higher values of IL-1β (p = 0.002, Kruskall-Wallis test), IL-6 (p = 0.047) and IL-8 (p = 0.002) were found in colonised. Bronchial colonisation was also significantly related to sputum eosinophilia in both absolute and relative counts (p = 0.015).
Table 2. Patient characteristics, sputum characteristics and plasma inflammatory markers according to bronchial colonisation*, in PAC-COPD patients (n = 133)
Inflammatory markers in sputum did not show significant associations with inhaled corticosteroid therapy in the studied patients. Eosinophil% in sputum samples from patients using inhaled corticosteroids (median [percentile 25–75] 1.2 [0–3.3] showed statistically non-significant differences when compared with the level of this inflammatory marker in patients not using this treatment (0.4 [0–3.9], p = 0.337, Kruskal-Wallis test). Similar results were obtained on the levels of IL-1β and IL-8 (data not shown, p = 0.567 and p = 0.530, respectively).
Bronchial colonisation was associated with systemic inflammation identifiable through an increase in blood CRP concentration (median 6.5 [2.5–8.5] in colonised vs. 3.5 [1.7–5.4] mg/l in non colonised patients, p = 0.016). The proportion of patients with plasma levels of CRP over 7.06 mg/l was also significantly higher in colonised patients (36% vs. 18%, p = 0.030, chi-square test). No significant differences, however, were detected in fibrinogen and plasma cytokine (IL-6, IL-8, and TNF-α) levels between no colonised and colonised patients.
The relationship found between bronchial colonisation and high CPR plasma levels was statistically significant (OR 2.52, 95% CI 1.08–5.92, p = 0.033) (), and maintained after the adjustment for age and FEV1%. The risk of having a CRP concentration over 7.06 mg/l more than doubled in COPD patients who were colonised by PPMs in the multivariate model (adjusted OR 2.57, 95% CI 1.07–6.18).
Table 3. Risk factors for high levels of plasma C-reactive protein (CRP) in PAC-COPD patients (n = 133)
Species-specific relationships of bronchial colonisation
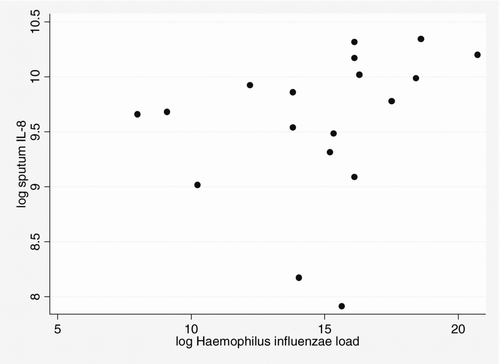
H. influenzae was the most prevalent colonising PPM. When the characteristics of patients colonised by this PPM (n = 22) were compared with no colonised patients, post bronchodilator FEV1% and frequency of exacerbation in the previous year were similar, even though patients colonised by H. influenzae had significantly more cumulative smoking (103 [62–138] vs. 58 [36–90] pack-years; p = 0.003, Kruskall-Wallis test). H. influenzae-colonised patients had significantly higher median cytokine values for both IL-1β and IL-8 (p = 0.001, Kruskal-Wallis test). The level of IL-8 were significantly correlated with the load of H. influenzaein the sputum sample (correlation coefficient r = 0.529, p = 0.024, Spearman rank test); see
Figure 1. Scarplot of total bacterial count (colony forming units (cfu)/ml−1) and sputum interleukin (IL)-8 (pg/ml−1) (Spearman's correlation coefficient r = 0.529, p = 0.024) in patients colonised by Haemophilus influenzae.
Bronchial colonisation by H. influenzae was associated with a worse health-related quality of life when compared with no colonised patients (SGRQ total score 42 [SD 20] versus 35 [SD 17], p = 0.105, Student's t-test), a comparison that reached statistically significance in the activity (58 [SD 26] versus 47 [SD 24], p = 0.049, Student t test) and impact (34 [SD 18] versus 25 [SD 18], p = 0.041, Student's t-test) domains of the questionnaire. These results were not found when other PPMs are the colonisers of the lower airway ().
Table 4. Species-specific relationships of bronchial colonisation (n = 133)
Discussion
We have investigated the characteristics of bronchial colonisation and its relationships with bronchial and systemic inflammation in a large, well-characterised sample of COPD patients in stable clinical condition about 3 months after recruitment during their first admission for an exacerbation. Sputum samples with a low proportion of squamous cells were recovered from most patients (85.8%). These samples were of high quality, showed high cell viability, and were considered representative of tracheobronchial secretions. Bronchial colonisation by PPMs was identified in 29% of these sputum samples, and was associated with a well-defined local inflammatory response, as shown by higher levels of IL-1β, IL-6 and IL-8.
Colonised patients additionally showed a systemic inflammatory response, identifiable through higher concentrations of blood CRP. A species-specific relationship between bronchial colonisation by H. influenzae and IL-1β and IL-8 sputum levels was also detected, a bronchial inflammatory pattern that emerged to be related to worse health-related quality of life. Higher scores in the activity and impact domains of the St. George's Respiratory Questionnaire were observed in patients colonised by H. influenzae, suggesting that this PPM may have more far-reaching effects than others. Overall, these findings confirm the importance of the relationships between colonisation, bronchial and systemic inflammation, and identify species-specific mechanisms that could be targeted for management in COPD patients.
Median total cell counts in colonised and no colonised COPD patients were similar in our study (9 [4–22]/ml × 106 vs. 13 [4–23]/ml ×106), and associated with prevalence of sputum neutrophilia higher than 65%, a value considered as the upper limit of normality (Citation24, Citation35), a finding also reported by Barnajee et al. (Citation5). Neutrophilic inflammation has been related to colonisation in studies that have used bronchoalveolar lavage to sample peripheral lung secretions of stable COPD patients (Citation6–8), a difference that may be related to specific characteristics of the cellular compartments targeted by bronchoalveolar lavage.
This last explanation is supported by studies that have found a higher proportion of neutrophils in sputum and bronchial aspirates recovered from COPD patients, in comparison with bronchoalveolar lavage fluid (Citation36), suggesting that the effect of colonisation on the neutrophilic response in the bronchial mucosa is overwhelmed by the magnitude of inflammation associated with COPD. Colonised COPD patients enrolled in the present study showed a mild eosinophilic inflammatory response in sputum, with percentages of patients with sputum eosinophilia over 3% slightly higher in colonised than in no colonised patients, with values similar to figures reported for stable COPD (Citation35, Citation37–41). Our findings suggest that the effect of colonisation on the cellular inflammatory response in bronchial secretions in stable COPD patients may be minimized by the magnitude of the local inflammation associated with the disease.
Increased levels of IL-1β and IL-8 in sputum were found to be associated with bronchial colonisation in our stable COPD patients with moderate disease. This finding supports the capability of PPMs to induce bronchial inflammation, as previously reported in severe patients (Citation7, 8, Citation42). IL-8 is a well-known neutrophil chemo-attractant and activator (Citation43), and the relationship between bronchial colonisation and the level of this cytokine in the sputum supernatant has been previously reported in both cell models (Citation44) and COPD patients (Citation45, 46), with a dose-response relationship to bacterial load in several studies (Citation5, Citation10). IL-1 is a family of cytokines that comprises 11 proteins that have demonstrated a central role in a number of inflammatory diseases (Citation47). IL-1 is a non-specific mediator (Citation48) and elevated levels of IL-1β have also been reported as a response to the presence of PPMs in cell cultures and sputum samples from COPD patients (Citation3).
COPD exacerbations are known to involve high levels of blood CRP (Citation49, 50) and fibrinogen (Citation51), and it is also widely accepted that COPD patients show a systemic inflammation pattern during their periods of stability. Gan et al. (Citation4), in a systematic review, reported significantly raised levels of CRP in stable COPD patients when compared with healthy controls, and in these patients elevated CRP has been a predictor of COPD hospitalisation and death (Citation52). Man et al. (Citation34), studying the large cohort of COPD patients enrolled in the Lung Health Study, observed that patients attaining CRP values over 7.06 mg/l, are at risk for the appearance of cardiovascular events and death, when compared with COPD patients with low CRP concentrations.
In our study CRP levels found in no colonised patients (median 3.5 [1.7–5.4] mg/l) were similar to the values observed in other studies of stable COPD patients (Citation50). However, a much higher level (6.5 [2.5–8.5] mg/l) was observed in colonised patients, over one third of whom reached values exceeding 7.06 mg/l. The association continued to be statistically significant after the adjustment for covariates (adjusted OR 2.57, 95% CI 1.07–6.18), FEV1% being the only other measure also demonstrating a statistically significant inverse relationship with CRP elevation.
These findings support the hypothesis of a direct effect of bronchial colonisation on systemic inflammation. The elevation in blood CRP concentration observed when PPMs colonise the bronchial mucosa would be a risk factor that would remain undetected unless sputum cultures are performed during periods of stability.
Colonisation by H. influenzae was associated in our study with higher cumulative smoking, in spite of similar current smoking prevalence and lung function. This PPM was also associated with a clearly higher bronchial inflammatory response, as shown by much higher levels of IL-1β and IL-8, and a dose-response relationship was demonstrated between Haemophilus influenzae load and IL-8 levels in sputum. Thus, colonising H. influenzae may have species-specific effects identifiable through higher cytokine levels in bronchial secretions. In patients who had sputum cultures positive for this PPMs we observed an impaired health-related quality of life as measured by the activity and impact domains of St. George's Respiratory Questionnaire, a relationship that did not reach statistical significance when other PPMs are the colonisers. These associations were consistent with previous findings reporting significantly worse health status in colonised stable COPD patients with sputum cultures positive for H. influenzae (Citation5).
The selection of patients used in the PAC-COPD study places limits on the extrapolation of the results, which cannot be applied to more severe patients because participants in this study had to have been hospitalised only once for a COPD exacerbation. This approach, however, determines that bronchial colonisation, the main variable considered in the present study, was not modified by recurrent admissions, which introduce in-hospital strains into the colonising flora. Thus, this apparent limitation in fact increases the strength of conclusions based on the observed relationships for COPD outpatients.
Additionally, we have not performed genomic analysis of the sputum samples not growing PPMs, and we cannot rule out under diagnosis of bronchial colonisation at loads below the detection limit of the sputum culture. Such colonisation should be considered as unusual, however, because when this approach has been used undiagnosed bronchial colonisation has only been identified in one tenth of the culture-negative sputum samples (Citation6).
In conclusion, the present study confirms the relationship between bronchial colonisation and both local and systemic inflammation in stable COPD patients. Local inflammation is identifiable in colonised patients through high levels of IL-1β, IL-6, IL-8 and, to a lesser degree, through systemic inflammation indicated by raised blood CRP levels. The magnitude of systemic inflammation, however, is great enough to have significant effects on the course of disease. Moreover, a species-specific effect on bronchial inflammation patterns was observed, suggesting that the impact of colonisation by H. influenzae may be expected to exceed that of other PPMs. If these findings are confirmed by further research, there would be a theoretical basis for targeting bronchial colonisation with specific management approaches in COPD patients that would have a potential impact on prognosis.
Declaration of Interest
The authors report no conflicts of interest. The authors are responsible for the content and the writing of this paper.
Acknowledgments
We thank Mary Ellen Kerans for her assistance with the English expression in versions of the manuscript. The PAC-COPD Study is funded by grants from Fondo de Investigación Sanitaria (FIS PI060684), Fundació Catalana de Pneumologia, Fondo de Investigación Sanitaria (FIS PI020541); Agència d'Avaluació de Tecnologia i Recerca Mèdiques (AATRM035/20/02); Spanish Society of Pneumology and Thoracic Surgery (SEPAR 2002/137); Red RESPIRA (RTIC C03/11); Red RCESP (RTIC C03/09); Fondo de Investigación Sanitaria (PI052486); Fondo de Investigación Sanitaria (PI052302); Fundació La Marató de TV3 (num.041110); and Novartis Farmacèutica, Spain. CIBERESP and Ciber de EnfermedadesRespiratorias-CibeRes is an initiative of Instituto de Salud CarlosIII. Judith Garcia-Aymerich has a researcher contract from the Instituto de Salud Carlos III (CP05/00118), Ministry of Health, Spain.
References
- Rosell A, Monsó E, Soler N, Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med 2005; 165:891–897.
- Wilkinson TM, Hurst JR, Perera WR, Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006; 129:317–324.
- Marin A, Monsó E, Garcia-Nuñez M, Variability and effects of bronchial colonisation in patients with moderate COPD. Eur Respir J 2010; 35(2):295–302.
- Gan WQ, Man SFP, Senthilselvan A, Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–580.
- Barnajee D, Khair OA, Honeybourne D. Impact of sputum bacteria on airway inflammation and health status in clinical sable COPD. Eur Respir J 2004; 23:685–691.
- Chin CL, Manzel LJ, Lehman EE, Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers. Am J Respir Crit Care Med 2005; 172:85–91.
- Soler N, Ewig S, Torres A, Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 1999; 14:1015–1022.
- Sethi S, Maloney J, Grove L, Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173: 991–998.
- Wilkinson T, Patel I, Wilks M, Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167:1090–1095.
- Hill A, Campbell EJ, Hill SL, Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med 2000; 109:288–295.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2010). http://www.goldcopd.org (accessed 08 June 2011).
- Celli BR, MacNee W, ATS/ERS Task Force.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946.
- Burrows B, Bloom JW, Traver GA, The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. N Engl J Med 1987; 317:1309–1314.
- Mahler DA, Harver A. A factor analysis of dyspnea ratings, respiratory muscle strength, and lung function in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1992; 145:467–470.
- Wegner RE, Jorres RA, Kirsten DK, Factor analysis of exercise capacity, dyspnoea ratings and lung function in patients with severe COPD. Eur Respir J 1994;7:725–729.
- Fuchs-Climent D, Le Gallais D, Varray A, Factor analysis of quality of life dyspnea, and physiologic variables in patients with chronic obstructive pulmonary disease before and after rehabilitation. Am J Phys Med Rehabil 2001; 80:113–120.
- Weatherall M, Shirtcliffe P, Travers J, Use of cluster analysis to define COPD phenotypes. Eur Respir J 2010; 36:472–474.
- Burgel PR, Paillaseul JL, Caillaud D, Clinical phenoptypes: a novel approach using principal component and cluster analyses. Eur Respir J 2010; 36:531–539.
- Garcia-Aymerich J, Gomez FP, Benet M, Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax 2011; 66: 430–437.
- Balcells E, Antó JM, Gea J, Characteristics of patients admitted for the first time for COPD exacerbation. Respir Med 2009; 103:1293–302.
- Garcia-Aymerich J, Gómez FP, Antó JM; en nombre del Grupo Investigador del Estudio PAC-COPD. Phenotypic characterization and course of chronic obstructive pulmonary disease in the PAC-COPD Study: design and methods. Arch Bronconeumol 2009; 45(1):4–11.
- Fujimoto K, Kubo K, Yamamoto H, Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysema. Chest 1999; 115:697–702.
- Pin I, Gibson PG, Kolendowicz R, Use induced sputum cell counts to investigate airway inflammation in asthma. Thorax 1992;47:25–29.
- Pizzichini E, Pizzichini MM, Efthimiadis A, Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med 1996; 154:308–317.
- Sutherland ER, Pak J, Langmack EL, Safety of sputum induction in moderate-to-severe chronic obstructive pulmonary disease. Respir Med 2002; 96:482–6.
- Efthimiadis A, Jayaram L, Weston S, Induced sputum: time from expectoration to processing. Eur Respir J 2002; 19:706–708.
- Aaron SD, Angel JB, Lunau M, Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:349–355.
- Pye A, Stockley RA, Hill SL. Simple method for quantifying viable bacterial numbers in sputum. J Clin Pathol 1995; 48:719–724.
- Balows A, Hausler WJ, Herrmann KL, Manual of Clinical Microbiology, 5th Ed. Washington DC, American Society of Microbiology, 1991.
- Cabello H, Torres A, Celis R, Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J 1997; 10:1137–1144.
- Sethi S, Sethi R, Eschberger K, Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:356–361.
- Middleton AM, Dowling RB, Mitchell JL, Haemophilus parainfluenzae infection of respiratory mucosa. Respir Med 2003; 97:375–381.
- Sethi S, Muscarella K, Evans N, Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 2000; 118:1557–1565.
- Man SFP, Connett JE, Anthonisen NR, C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 2006; 61:849–853.
- Bathoorn E, Liesker JJW, Postma D, Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J COPD 2009; 4:101–109.
- Hloz O, Kips J, Magnussen H. Update on sputum methodology. Eur Respir J 2000; 16:355–359.
- Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. Am J Respir Crit Care Med 1997; 155:449–453.
- Lacoste JY, Bousquet J, Chanez P, Eosinophilic and neutrophilic inflammation in asthma, chronic bronchitis, and chronic obstructive pulmonary disease. J Allergy Clin Immunol 1993; 92:537–548.
- Balzano G, Stefanelli F, Iorio C, Eosinophilic inflammation in stable chronic obstructive pulmonary disease. Relationship with neutrophils and airway function. Am J Respir Crit Care Med 1999; 160:1486–1492.
- Snoeck-Stroband JB, Lapperre TS, Gosman MM, Chronic bronchitis sub-phenotype within COPD: inflammation in sputum and biopsies. Eur Respir J 2008 Jan; 31:70–77.
- Bhowmik A, Seemugal TAR, Sapsford RJ, Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000; 55:114–120.
- Keatings VM, Collins PD, Scott DM, Differences in interleukin-8 and tumor necrosis factor-alfa in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996; 153:530–534.
- Richman-Eisenstat JB, Jorens PG, Hebert CA, Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol 1993; 264:L413–418.
- Berenson CS, Murphy TF, Wrona CT, Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines. Infection and Immunity 2005; 73:2728–2735.
- Bresser P, Out TA, VanAlphen L, Airway inflammation in nonobstuctive and obstructive chronic bronchitis with Haemophilus influenzae airway infection. Am J Respir Crit Care Med 2000; 162:947–952.
- Zhang M, Li Q, Zhang XY, Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infect Dis 2010; 29:1487–1493.
- Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal 2010 Jan 19; 3 (105): cm2. Review.
- Miravitlles M, Marín A, Monsó, Colour of sputum is a marker of bacterial colonisation in chronic obstructive pulmonary disease. Respir Res 2010; 11:58.
- Hurst JR, Donaldson GC, Perera WR, Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:867–874.
- Stolz D, Christ-Crain M, Morgenthaler NG, Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest 2007; 131:1058–1067.
- Wedzicha JA, Seemungal TA, MacCallum PK, Acute exacerbations of chronic obstructive disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost 2000; 84:210–215.
- Dahl M, Vetsbo J, Lange P, C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175:250–255.