Abstract
Background: Chronic obstructive pulmonary disease (COPD) is associated with impaired exercise tolerance, but it has not been established to what extent cardiac autonomic function impacts on exercise capacity. Objective: To evaluate whether there is an association between airflow limitation and cardiac autonomic function and whether cardiac autonomic function plays a role in exercise intolerance and daily physical activity (PA) in patients with COPD. Methods: Univariate and multivariate analyses were performed to evaluate the association between both 6-minute walking test (6MWT) and PA (steps per day) and pulmonary function, cardiac autonomic function (HR at rest, HRR and heart rate variability, HRV) in patients with COPD. Results: In 154 COPD patients (87 females, mean [SD]: age 62.5 [10.7] years, FEV1%predicted (43.0 [19.2]%), mean HR at rest was elevated (86.4 [16.4] beats/min) and HRV was reduced (33.69 [28.96] ms) compared to published control data. There was a significant correlation between FEV1 and HR at rest (r = -0.32, p < 0.001), between HR at rest and 6MWD (r = -0.26, p = 0.001) and between HR at rest and PA (r = -0.29, p = 0.010). No correlation was found between HRV and 6MWD (r = 0.089, p = 0.262) and PA (r = 0.075, p = 0.322). In multivariate analysis both HR and FEV1 were independent predictors of exercise capacity in patients with COPD. Conclusions: In patients with COPD the degree of airflow limitation is associated with HR at rest. The degree of airflow limitation and cardiac autonomic function, as quantified by HR at rest, are independently associated with exercise capacity in patients with COPD.
Introduction
Expiratory flow limitation in patients with chronic obstructive pulmonary disease (COPD) results from progressive airway inflammation causing parenchymal destruction, mucosal oedema, airway remodelling, mucoid impaction and increased cholinergic airway smooth muscle tone (Citation1). Advanced COPD is associated with reduced exercise tolerance and daily physical activity (PA) resulting in impaired health-related quality of life (Citation2), high health care use (Citation3), and increased mortality (Citation4). It has been recognized that COPD is a systemic disease, which has been shown to negatively affect the cardiovascular and cardiac autonomic system (Citation5). Patients with COPD have functional alterations of cardiac autonomic modulation as reflected in elevated HR at rest, reduced baroreflex sensitivity and reduced heart rate variability (HRV) (5–10). Elevated HR at rest and reduced HRV possibly play a role in the morbidity and mortality of these patients (6–9, 11).
The mechanisms of exercise intolerance in patients with COPD are complex and multifaceted (12–18). Although it has been demonstrated that the main pathophysiological mechanism involved in the alteration of exercise capacity in patients with COPD is impaired lung function, the role of cardiac autonomic dysfunction on exercise tolerance is mostly unknown (18–20). Investigating the cardiac autonomic function and its contribution to exercise intolerance may be important in understanding the pathophysiology of exercise limitation and reduced daily PA in patients with COPD.
Cardiopulmonary exercise testing has been classically performed on a cycle-ergometer or motorised treadmill using a rapid ramped incremental protocol to the limit of tolerance (Citation21). However, previous studies have indicated that maximal distance walked during a 6-minute walk test (6MWT) in patients with COPD is closely correlated with daily PA as measured by accelerometry (Citation22). Because most activities of daily living are performed in a non-incremental fashion and at submaximal level of exertion, the 6MWT may reflect the functional exercise level for daily physical activities accurately (Citation23). Therefore, in order to evaluate contributing factors to exercise limitation in daily living, we used both the 6MWT and accelerometry in the current study.
The objectives of this study were to determine whether there is an association between disease severity and cardiac autonomic function and whether cardiac autonomic function plays a significant role in exercise intolerance and daily physical activity in patients with COPD.
Material and methods
Study design
This was a cross-sectional observational study in patients with COPD (GOLD-classification I-IV).
Subjects
Patients with COPD referred to the Department of Respiratory Medicine, Ruhrlandklinik, University of Duisburg-Essen (Germany) between November 2008 and July 2009 and to the Pulmonary Division, University Hospital of Zurich, Switzerland between November 2009 and April 2011 were considered for participation in the study. The inclusion criteria for patients were: male/female subjects aged 40–75 years, confirmed COPD according to GOLD-guidelines (Citation24). The exclusion criteria were: acute or recent (within last 6 weeks) exacerbation of COPD according to GOLD-guidelines (Citation24), patients on long-term oral corticosteroids or morphine medication, mental or physical disability precluding informed consent or compliance with the protocol. The study was approved by the Research Ethics Committee of the Medical Faculty, University of Essen-Duisburg (Germany) (Number 07–3524) and Zurich (Switzerland) (Number EK-1734) and written informed consent was obtained from all patients.
Pulmonary function measurements
Spirometry, body plethysmography and diffusion capacity measurements were performed according to the American Thoracic Society (ATS) and the European Respiratory Society (ERS) guidelines with a commercially available system (Body 500™, ZAN, Oberthulba, Germany) (Citation25, 26). Spirometry was performed post bronchodilatation followed by the exercise test (6MWT).
Six-minute walk test
All patients performed the 6MWT following pulmonary function testing with broncholysis. After a recovery period of 5 minutes, 6MWT was determined based on the American Thoracic Society (ATS) protocol (Citation23, Citation27). Oxygen saturation and pulse rate were recorded using a finger-adapted pulse oximeter (Nexus-10™, TMS International BV, Netherlands). Additionally, scored sensations of breathlessness and leg effort were assessed using a modified Borg scale (Citation28). The 6MWT was performed on a 30-meter corridor by a trained investigator with specific experience using a standardized encouragement strategy.
Physical activity, accelerometry
A multisensor accelerometer (SenseWear Pro™ armband; BodyMedia, Inc., Pittsburgh, PA, USA) worn on the upper right arm was used in a subset of 64 patients recruited at the Pulmonary Division, University Hospital of Zurich, Switzerland. The device estimates energy expenditure (EE) using measurements from a biaxial accelerometer and sensors that record the number of steps (Citation30). The patients were instructed to wear the accelerometer continuously during 7 consecutive days, except when taking a bath or shower.
Cardiac autonomic modulation
Cardiac autonomic function was assessed by using the standard time- and frequency domain measures of HRV from 5-minutes of the R-R interval recordings in the ECG, while the patient was seated. Heart rate variability was obtained via 3-channel ECG recording (Nexus-10™ medical device class IIa, TMS International BV, Netherlands) as recommended in international guidelines (Citation10). An automatic filter excluded RR sequence differing by more than 30% from the previous interval to eliminate artifacts (Citation10). Time domain measures of HRV were calculated from the RR interval tachograms. The following time domain measures of HRV were calculated: mean RR (NN mean) in milliseconds, the standard deviation of RR intervals (SDNN) in milliseconds and the root mean square successive difference of RR intervals (rMSSD) in milliseconds.
The following standard frequency domain measures of HRV were computed: high-frequency spectral power (HF power, the density of the beat-to-beat oscillation in the R-R interval of HRV in the high-frequency band; HF = 0.15 - 0.4 Hz), low-frequency spectral power (LF power, the density of the beat-to-beat oscillation in the R-R interval of HRV in the low-frequency band; LF = 0.04 - 0.15 Hz), the ratio of LF to HF power (LF/HF ratio). Heart rate reserve (HRR) was calculated as describes previously (Citation10, Citation27).
Data analysis
Baseline anthropometric characteristics, pulmonary function data and cardiac autonomic function data were analysed to define the study population. Descriptive data for continuous variables are expressed as mean ± standard deviation (SD). A statistical software package was used for all calculations (SPSS for Windows, version 11.0, SPSS Inc., Chicago, IL, USA). Univariate (Pearson's correlation) and multivariate analyses were performed to evaluate the association between FEV1, DLCO and the 6MWD and the average number of steps per day. Variables with high intercorrelations (FEV1/ IVC, TLC, RV/ TLC and NNmean) were removed from the analysis. A p- value of < 0.05 was considered to indicate statistical significance.
Results
Patients’ characteristics
One hundred and fifty-four patients with COPD (GOLD-class I-IV) agreed to take part and were included in the study. Anthropometrical characteristics, pulmonary function, respiratory symptoms and exercise capacity data of the 154 patients (87 females) with COPD are presented in . COPD was mild (GOLD I) in 5.2% of the patients, moderate (GOLD II) in 30.5% of the patients, severe (GOLD III) in 31.2% of the patients, and very severe (GOLD IV) in 33.1% of the patients. Mean BMI was normal with 24.9 ± 6.1 kg/m2 and the patients had a reduced exercise capacity (6MWD).
Table 1. Anthropometrics, pulmonary function, respiratory symptoms, cardio-pulmonary exercise capacity, cardiac autonomic function and physical activity
Determinants of exercise capacity (6MWD) and physical activity (steps per day)
establishes the correlations between exercise capacity (6MWD), physical activity (steps per day) and parameters reflecting cardiac autonomic function and lung function. There was a significant positive correlation between exercise capacity (6MWD), physical activity (steps per day) and FEV1, FEV1/ IVC, DLCO and NNmean. A significant negative correlation was found between exercise capacity (6MWD) and physical activity (steps per day) and TLC, RV/ TLC and HR at rest (bpm) (). HRR only correlates with physical activity (steps per day). The HRV-variables SDNN, rMSSD, HFpower, LFpower did not significantly correlate with 6MWD and steps per day.
Table 2. Pearson correlation of dependent variables of 6mwd and steps per day
A multiple linear regression analysis including variables with significant correlations in the univariate analysis was performed to determine predictors of 6MWD (). Both FEV1 and HR at rest were found to be significantly and independently associated with 6MWD. 53.3% of the variance in 6MWD could be explained by the model (r2 = 0.53, p < 0.001).
Table 3. Multiple regression analysis: Analysis of predictors for 6MWD
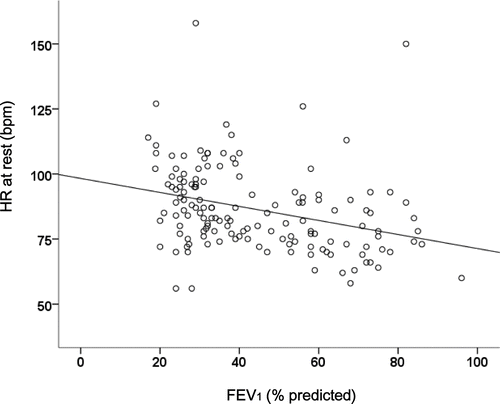
We found a significant negative correlation between FEV1 (%predicted) and HR at rest (r = -0.32, p < 0.001) as presented in . In addition, we found that HR at rest was significantly correlated with TLC (r = 0.24, p = 0.004) and RV/TLC (r = 0.29, p < 0.001). We also found a significant negative correlation between FEV1 (%predicted) and heart rate reserve during the 6-minute walking test (r = -0.29, p = 0.006).
Discussion
In this study we found that heart rate at rest and FEV1 were independently associated with 6MWD. Cardiac autonomic dysfunction, as quantified by HR at rest, was increased with advanced airflow limitation and disease severity, in patients with COPD.
Physical inactivity in daily life and exercise intolerance are prominent features in patients with COPD (1–4). Objectively measuring PA using accelerometry seems to be the most accurate field-based estimate of PA and has become more important recently (Citation29). Correlations between exercise capacity, PA and pulmonary function underline the role of the imbalance between ventilatory load and capacity in these patients (Citation30). This imbalance directly impacts on exercise tolerance and daily physical activity. In addition, airway obstruction, diffusion capacity and lung hyperinflation seems to be important factors influencing exercise limitation in COPD.
Interestingly, we found that HR at rest was independently associated with 6MWD. This observation emphasizes that, beside pulmonary limitation, cardiac autonomic dysfunction plays a predominant role in limiting exercise capacity in patients with COPD (Citation5). Patients with hyperinflation are known to have impaired left ventricular diastolic filling pattern and right-heart dysfunction. This effect may have an independent impact on stroke volume, cardiac output and therefore HR at rest (Citation32, 33). Accordingly, we found a significant correlation between HR at rest and lung hyperinflation in the current study.
Cardiac autonomic dysfunction in patients with COPD
The presence of cardiac autonomic dysfunction in COPD is associated with worse prognosis (5–9). We found that, compared to previously published reference values, the mean HR at rest was elevated and mean HRV was reduced in patients with COPD (Citation34, 35). These finding underline predominantly sympathetic tone at rest in the patients of the current study (Citation10, Citation34, Citation35). Predominant sympathetic tone at rest may have negative effects on cachexia and skeletal muscle dysfunction in COPD (Citation36) and elevated HR at rest may also be associated with increased cardiovascular morbidity and mortality (Citation9, Citation37). Although HRV was previously described to be independently associated with health related quality of live (HRQL) (Citation38), we have found that HR rather than HRV may influence exercise tolerance in patients with COPD.
Mean HR at rest was correlated to the degree of airflow obstruction. This suggests that with advanced airflow limitation and disease severity, sympathetic tone is increased in patients with COPD. All patients received the same dose of salbutamol as part of the lung function testing before the measurement of HR at rest was performed, controlling the impact of β2-agonist use.
Limitations
The number of subjects is small given the variance in data. We did not investigate potential exercise limiting factors such as hemodynamic factors, respiratory and locomotor muscle dysfunction and psychological factors (motivation, anxiety and depression). Neither did we control for the use of long-acting β2-agonists, which may have a small effect on HR at rest. The design of this study does not allow establishing a causal relationship between the severity of airflow limitation and cardiac autonomic dysfunction. Future studies are needed to assess the impact of pulmonary rehabilitation programs on cardiac autonomic function in patients with COPD.
Conclusions
Our findings emphasize that, beside pulmonary dysfunction, cardiac autonomic dysfunction, as quantified by HR at rest, plays a predominant role in limiting exercise tolerance in patients with COPD. Furthermore, cardiac autonomic dysfunction, as quantified by HR at rest, is increased with advanced airflow limitation and disease severity. Treatment aimed to restore the sympathovagal balance towards a reduction of resting sympathetic activity may contribute to improved exercise tolerance in COPD.
Declaration of interest
None of the authors has a conflict of interest. The authors are responsible for the writing and the content of this paper.
References
- O’Donell DE, Lavenesiana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev 2006; 100:61–67 and 1993; 148:1351–1357.
- Simpson K, Killian K, McCartney N, Randomized controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax 1992; 47:70–75.
- Decramer M, Gosselink R, Troosters T, Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J 1997; 10:417–423.
- Garcia-Aymerich J, Lange P, Benet M, Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61:772–778.
- Van Gestel AJR, Steier J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J Thorac Dis 2010; 2:215–222.
- Heindl S, Lehnert M, Criée CP. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med 2001; 164:597–601.
- Volterrani M, Scalvini S, Mazzuero G. Decreased heart rate variability in patients with chronic obstructive pulmonary disease. Chest 1994; 106:1432–1437.
- Stewart AG, Waterhouse JC, Howard P. Cardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary disease. Eur Respir J 1991; 4:1207–1214.
- Bilmann, GE, Schwartz, PJ, Stone, HL. Baro-receptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation 1982; 66:874–880.
- American Heart Association. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996; 17:354–381.
- Stein PK, Nelson P, Rottman JN, Howard D, Heart rate variability reflects severity of COPD in PiZ α1-antitrypsin deficiency. Chest 1998; 113:327–333.
- Bauerle O, Chrusch CA, Younes M. Mechanisms by which COPD affects exercise tolerance. Am J Respir Crit Care Med 1998; 157:57–68.
- West JB, Wagner PD, Neder JA, The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles vs. lower limb muscle dysfunction vs. dynamic hyperinflation. J Appl Physiol 2008; 105:758–762.
- Killian KJ, Leblanc P, Martin DH, Exercise capacity and ventilatory, circulatory and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis 1992; 146:935–940.
- Muriari C, Ghezzo H, Milic-Emili J, Gauthier H. Exercise limitation in obstructive lung disease. Chest 1998; 114:965–968.
- American Thoracic Society and European Respiratory Society. Statements on skeletal muscle dysfunction in COPD. Am J Respir Crit Care Med 1999; 159 (suppl): 1029–1356 and Eur Respir J 2000; 15: 807–815.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153:976–980.
- Neder JA, Jones PW, Nery LE, Whipp BJ. Determinants of the exercise endurance capacity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 162:497–504.
- O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation: the role of lung hyperinflation. Am Rev Respir Dis 1993; 148:1351–1357.
- O’Donnell DE, Bertley JC, Chau LKL, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation. Am J Respir Crit Care Med 1997; 155:109–115.
- Varray A, Pre´faut C. Exercise training in patients with respiratory disease: procedures and results. Eur Respir Rev 1995; 5:51–58.
- Pitta F, Troosters T, Spruit MA, Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171(9):972–977.
- American Thoracic Society Statement. Guidelines for the six-minute walk test. Am J Respir Care Med 2002; 166:111–117.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Chron Respir Dis 2010; 7(3):131–133.
- Miller MR, Hankinson J, Brusasco V. Burgos F, et al. Series “ATS/ERS Task Force: Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Miller MR, Crapo R, Hankinson J, General considerations for lung function testing. Eur Respir J 2005; 26:153–161.
- American Thoracic Society/American College of Chest Physicians. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med 2003; 167:211–277.
- Kroidl RF, Schwarz S, Lehnigk B. Kursbuch Spiroergometrie. Georg Thieme Verlag 2007; 3.
- Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity—a cross-sectional study. Am J Respir Crit Care Med 2008; 177:743–751.
- Patel SA, Benzo RP, Slivka WA, Sciurba FC. Activity monitoring and energy expenditure in COPD patients: a validation study. COPD 2007; 4:107–112.
- Jolley CJ, Moxham J. A physiological model of patient-reported breathlessness during daily activities in COPD. Eur Respir Rev 2009; 18:66–79.
- Watz H, Waschki B, Meyer T, Kretschmar G, Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest 2010; 138(1):32–38.
- Barr RG, Bluemke DA, Ahmed FS, Percent Emphysema, Airflow Obstruction, and Impaired Left Ventricular Filling. N Engl J Med 2010; 362:217–227.
- Kuo TB, Lin T, Yang CC, Effect of aging on gender differences in neural control of heart rate. Am J Physiol 1999; 277:233–239.
- Sinnreich R, Kark JD, Friedlander Y, Five minute recordings of heart rate variability for population studies: repeatability and age–sex characteristics. Heart 1998; 80:156–162.
- Andreas S, Anker SD, Scanlon PD, Neurohumoral activation as a link to systemic manifestations of chronic lung disease. Chest. 2005; 128:3618–3624.
- Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133:14–20.
- van Gestel AJR, Kohler M, Steier J, Teschler S, Cardiac autonomic dysfunction and health-related quality of life in patients with chronic obstructive pulmonary disease. Respirology 2011; 12:1440–1843.