Abstract
The aim of this study was to determine if components of the COPD Assessment Test (CAT), a validated health status impairment instrument, had additional utility in identifying patients at risk for COPD in whom spirometry testing is appropriate. This study was part of the Canadian Obstructive Lung Disease prevalence study. Consenting participants ≥ 40 years of age were identified by random digit dialing. Smoking history, 8-item CAT scores, and post-bronchodilator spirometry were recorded for each. Stepwise logistic regression analysis was used to identify variables related to the presence of airway obstruction and a final logistic model was developed which best predicted COPD in this sample. Of the 801 individuals approached, 532 were included: 51 (9.6%) had COPD, the majority (92%) of whom fit GOLD I or II severity criteria. Items that correlated significantly with a COPD diagnosis included the CAT total score (p = 0.01) and its breathlessness (p < 0.0001) and phlegm (p = 0.001) components. The final logistic model included: age (<55 or ≥55 years), smoking status (current, former, never) and the CAT breathlessness score (ordinal scale 0–5). The area under the receiver-operating characteristic curve for this model was 0.77, sensitivity was 77.6%, specificity was 64.9% and the positive likelihood ratio was 2.21. In summary, the triad of smoking history, age at least 55 years and the presence of exertional breathlessness were key elements of a simple model which had reliable measurement properties when tested in a random population. This may help identify patients at risk for COPD for whom spirometry testing is recommended.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. The onset of symptoms is insidious: as many as 50% of patients are not diagnosed until they have reached advanced stages of the disease (Citation1,2). Earlier diagnosis of COPD is desirable given the availability of effective management interventions which may favourably alter the natural history of this disease (Citation3,4). General population screening of all smokers for COPD has not been shown to be cost-effective (Citation5) and most clinical practice guidelines currently recommend targeted case finding using spirometry in the primary care setting (Citation6,7,Citation8). However, successful identification of COPD in its earlier stages remains an elusive goal.
It is currently unknown if those factors that predict COPD in a targeted population sample are the same as those in a general randomly sampled population. Targeted screening of patients at risk for COPD remains widely under-utilized (Citation1,Citation3). Possible explanations include: inadequate access to resources, limited time, low index of suspicion for COPD and uncertainty about efficacy of available treatment options. Therefore, there is a need to develop a simple tool that is easy to apply and that can reliably identify those individuals with a high probability of having COPD. Such tools could be used to identify individuals at risk in the general population (e.g., as part of public awareness campaigns) or in targeted populations attending family practice clinics.
Previous studies have examined a number of tools designed to identify those at risk for COPD (Citation9–16). These questionnaires have incorporated different combinations of baseline demographics, smoking status and respiratory symptoms (Citation9–11). Age, smoking and various combinations of shortness of breath, wheeze and cough have been shown to be reasonably good predictors of the presence of objective airway obstruction (Citation99–Citation13). These studies were conducted in non-random population samples of mainly symptomatic patients with a smoking history attending family practice or specialized pulmonary centers. COPD diagnosis in these studies was often made using pre-bronchodilator spirometry, making it difficult to classify COPD severity by GOLD criteria. Moreover, diagnostic accuracy of COPD was not rigorously confirmed in all patients as studies relied exclusively on fixed FEV1/FVC ratio criterion (<70%) which is known to overestimate diagnosis in the elderly (Citation17). None of the instruments developed to-date are broadly utilized for COPD screening purposes in clinical practice.
The COPD Assessment Test (CAT) was recently developed as a simple tool to evaluate health status impairment in COPD (available online at www.catestonline.org/english/index.htm) (Citation18,19). The CAT consists of 8 items with 6-point ordinal scales (scored 0–5) of severity for each item. This instrument is easy to self-administer and has been extensively evaluated in patients of varying disease severity in several European countries (Citation19,20). The CAT has been shown to have good internal validity, acceptable test-retest properties and is responsive to interventions such as pulmonary rehabilitation (Citation19,Citation21). CAT total score also correlated well with validated health-status instruments such as the St. George's Respiratory Questionnaire (Citation20,21). Because the CAT evaluates perceived severity of many of the pertinent symptoms predictive of COPD (e.g. shortness of breath, cough, and phlegm) (Citation9–13), and has clearly been shown to be quite abnormal even in mild COPD (Citation20), we postulated that it could be successfully used to identify subjects in the general population with undiagnosed COPD who should be selected for spirometry.
We therefore designed the current observational study to determine if the CAT total score had predictive accuracy as a case-finding tool for detection of airway obstruction in the general population. In particular, we wished to identify which combination of the 8 items from the CAT questionnaire could best identify risk of airway obstruction that was not fully reversible. Diagnosis of COPD was based on spirometry using both GOLD (post-bronchodilator FEV1/FVC< 70%) (Citation7) as well as lower limit of normal criteria for this ratio (Citation22). Finally, we sought to develop a model where dominant respiratory symptoms extracted from CAT, in conjunction with other known predictors of COPD (e.g., smoking history and age), could be combined to reliably identify those suitable for diagnostic spirometry.
Methods
This study is a subset of the Canadian Obstructive Lung Disease (COLD) Study, which is a Canada-wide study looking at the population prevalence of COPD. The study was approved by the local research ethics board (DMED-1240-09). Subjects were identified by random digit dialling (land line), and those over 40 years of age were invited to participate. Institutionalized individuals and anyone unable to provide informed consent were excluded. Those recruited were requested to participate in a lung health study, which involved one visit to the laboratory. After providing consent, a questionnaire was administered to collect demographic information, medical and exposure history, after which they completed the modified Medical Research Council (MRC) dyspnea scale (Citation23). Pre- and post-bronchodilator spirometry was performed as per ATS guidelines (Citation24) using a hand-held EasyOne™ spirometer (ndd Medical Technologies; Andover, MA, USA). Tests were reviewed for acceptability and reproducibility and poor tracings were rejected and the subject retested; if they could not be retested, their data was excluded. Those identified by random digit dialing who did not consent to the study were asked several short questions to ensure that the study population was not biased in any way.
This study reports the data from subjects recruited at the Kingston, Ontario, site of the COLD study where testing was conducted between December 2009 and December 2010. These subjects were all administered the CAT questionnaire. Each of the 8 components of the CAT questionnaire are scored on a scale of 0 to 5 for a total possible score ranging from 0 to 40, with the highest scores representing the greatest impact on health status. The 8 questions relate to: cough, phlegm, chest tightness, breathlessness on exertion, activity limitation, impact of lung condition on confidence in leaving home, impact of the lung condition on sleep and energy levels.
The diagnosis of COPD was based on spirometry using both GOLD criteria (post-bronchodilator FEV1/FVC < 70%) (Citation7) as well as lower limit of normal (post-bronchodilator FEV1/FVC < LLN) based on NHANES III (Citation22). To be classified as COPD, subjects also had to have either a smoking history of at least 10 pack-years or at least 10 years of exposure to other inhaled substances (i.e., second hand smoke, biomass), and no physician diagnosis of asthma or other lung disease.
Statistical analysis
Baseline demographic and physiologic data of all participants were assessed. They were then divided into two groups; those with a diagnosis of COPD and those without. Mean and standard deviations for continuous variables and proportions for categorical data were then calculated. Statistically significant differences between the two groups were then compared by using Student's t-test (or their non-parametric equivalence - Wilcoxon), and the Chi-square test as appropriate. The association of each component of the CAT score as well as the CAT total score to COPD was further evaluated with different probability cutpoints to assess its screening capability.
Further analyses were undertaken using COPD diagnosis as a dichotomous outcome. A stepwise logistic regression analysis was then applied to select the variables that were significantly related to COPD (with probability of COPD <0.10). The linearity assumption for logistic regression was assessed by categorizing each continuous variable into categorical variables, and by a likelihood ratio test. As a result of the stepwise procedure, the final logistic model was developed which only included variables found to be significant in predicting COPD. To assure the consistency of the final selected model, alternative variable selection, including both backward and forward stepwise elimination procedures with different selection criteria were applied to the same data set.
The fit of the final model was assessed by the Hosmer-Lemeshow goodness of fit test (Citation25). With varying cutpoints for the predicted probability in the model, a receiver-operating characteristic (ROC) curve was constructed. An optimum cut-off point for predicting COPD was sought. The area under the ROC curve (AUC) was calculated to measure the ability of the logistic model to discriminate high-risk subjects from low-risk subjects. Internal validation using bootstrapping method was then applied (Citation26) using 1000 bootstrap samples (sample with replacement) generated from the original data set, of the same size as the original data set. Each bootstrap sample was used as a training sample (to fit the logistic model), and the original data set and the bootstrap sample were used as test samples. The difference between the AUC estimated from using the original data as the test sample and the AUC estimated using the bootstrap sample was considered as a measure of optimism. This difference was subtracted from the original AUC to estimate the internally validated AUC (called optimism-corrected AUC). All statistical analyses were performed with the SAS statistical software version 9.2 (SAS Institute, Cary, NC).
Results
Of the 801 subjects called after recruitment from random-digit dialing, 552 subjects consented to come to the laboratory for testing. Eighteen of these subjects were retested at a second visit to obtain satisfactory spirometry and 20 subjects were not included due to incomplete or unsatisfactory spirometry (n = 13 refused post-bronchodilator testing; n = 2 experienced an adverse event during pre-bronchodilator testing (fainting, coughing); n = 3 could not achieve reproducible results; n = 2 equipment malfunction). Of the 532 subjects with satisfactory data, 51 (9.6%) had COPD by both LLN and GOLD criteria as well as the required inhalant exposure: 5.1% met GOLD stage I criteria, 3.8% stage II, 0.6% stage III and 0.2% stage IV. The use of GOLD criteria alone resulted in a COPD prevalence of 13.9%. Those diagnosed by using GOLD criteria who did not meet LLN criteria were all in stage I (n = 22) or II (n = 1). Baseline demographics for the whole group as well as those with and without COPD are presented in . Those with COPD were significantly older, had a greater smoking history, had a lower FEV1 and FEV1/FVC, and had more cough, phlegm, breathlessness and chest tightness than those without.
Table 1. Subject characteristics
Other respiratory diseases found in the group included 22 subjects with a clear diagnosis of asthma and 1 subject with pulmonary sarcoidosis. Although 7 of these subjects had post-bronchodilator spirometry that fit GOLD and LLN criteria for COPD, they were all non-smokers and were included in the non-COPD group for analysis.
A series of stepwise regression analyses revealed that age at least 55 years (p<0.0005) and both current and former smoking (p<0.0001) were significant for predicting COPD. The CAT total score (p = 0.01) and its breathlessness (p<0.0001) and phlegm (p = 0.001) components were all significantly related to a COPD diagnosis. The breathlessness item was strongest in the logistic model and displaced the association with the CAT total score. The sleep component did not relate to COPD diagnosis on its own (p = 0.31) but became significantly (p = 0.008) correlated with COPD when included in the model; however, because this unexplainable negative correlation lacked a clear biological rationale, it was excluded from the final model.
Phlegm added little to the final model (likelihood ratio test p = 0.4) and was also excluded. The best final model thus included age group (<55 or ≥55 years), smoking status (current, former, never) and breathlessness (0–5), the odds ratios for these components are presented in . presents the ROC curve for the final model. The AUC for the model was 0.772. Sensitivity was 77.6% and specificity 64.9% with a positive likelihood ratio of 2.21 (with cutpoint 0.097). This gives us a positive predictive value of 18.6% and a negative predictive value of 96.5%. The Hosmer-Lemeshow Goodness-of-fit was 0.777 indicating a good model. Internal validation performed with this sample resulted in an optimism corrected AUC estimate of 0.759 (95% CI 0.691-0.831), suggesting a well-validated model.
Figure 1. ROC curve for the final model which included age, smoking status and the CAT breathlessness score. The area under the curve for the model is 0.772.
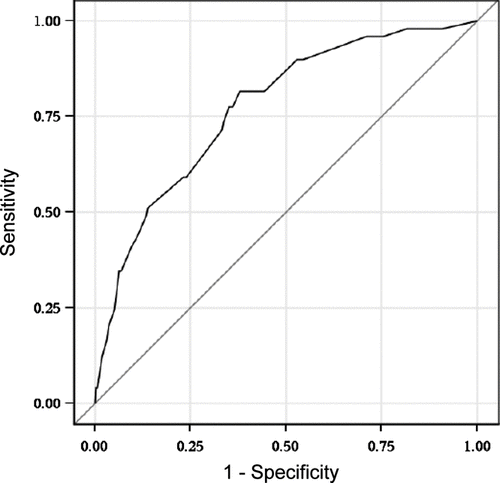
Table 2. Odds ratio estimates for components of the final model that related to COPD
The results of the final model are presented in in the form of a decision tree to aid in clinical decision making. The data for this figure can be found in . Non-smokers had a low probability of COPD regardless of other results and, based on this scale, would only be chosen for screening if their age was ≥55 years and their breathlessness score was ≥4. Former smokers all deserved screening if they were 55 or older. Current smokers would need screening either if they were at least 55 or if they were under 55 but had a breathlessness score of 1 or greater.
Figure 2. Decision tree based on the final logistic regression model. Where the outcome of the model had a low probability of COPD, we recommend no further screening. Where the model determines an increased probability of COPD, we recommend going on to confirmatory testing with spirometry.
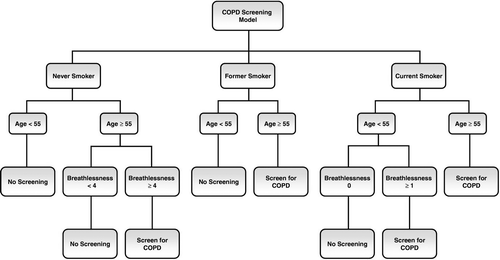
Table 3. Diagnosis of COPD based on components of the final model and a logistic probability of 0.097
Alternate models
The use of GOLD diagnostic criteria alone resulted in a similar, but less statistically significant, model than that using the combined GOLD and LLN criteria. Age and smoking status appeared in this model but a third age group was defined since age ≥70 yrs showed greater risk of COPD than age 55–69 yrs. Again, the CAT breathlessness item (p = 0.08) was the only variable worthy of including in the model. The sensitivity for the model using GOLD criteria alone was 76% and specificity was 67%.
The MRC dyspnea scale was also significantly (p = 0.01) associated with COPD: the score was dichotomized into 0 vs. >0 since higher scores did not detect more COPD cases. However, when the MRC dyspnea scale was included as a variable within the stepwise selection procedure, the same final logistic regression model resulted, i.e., MRC was not as predictive of COPD (likelihood ratio test p = 0.62) as the CAT breathlessness component.
Discussion
The findings of this study are as follows: 1) the CAT total score was predictive of the presence of airway obstruction in our random population sample but was displaced by its breathlessness component in the final regression model; 2) of the 8 items listed in the CAT, activity-related breathlessness emerged as the predominant symptom predictive of the presence of COPD and; 3) a simple model which incorporated age, smoking status and perceived intensity of activity-related breathlessness demonstrated good sensitivity and acceptable specificity as a screening tool for the diagnosis of COPD in the general population.
By using both post-bronchodilator GOLD and LLN criteria (rather than GOLD criteria alone) for the diagnosis of COPD, a diagnosis was confirmed in almost 10% (51/532) of this population sample which is consistent with prevalence rates reported in recent epidemiological studies (Citation27). The majority (92%) of these met GOLD stage I or II severity criteria.
The CAT instrument has been carefully validated as a useful measure of health status impairment in patients with COPD. Remarkably, recent non-randomized, cross-sectional studies in large COPD population samples in primary care have shown significant health status impairment (an average CAT total score of ∼16 units out of a possible 40) even in mild COPD (Citation20). In fact, CAT scores were similar in GOLD I and II subgroups. Therefore, it seemed reasonable to postulate that individual CAT total scores may have additional utility in identifying patients with undiagnosed COPD for whom spirometry testing would be appropriate.
However, the CAT total score in our random population was not as useful as its breathlessness component in predicting the presence of airway obstruction. The average CAT score for the group fitting COPD criteria in the current study was 9.2, which was significantly higher (by a mean difference of 2.3 units) than the score in the majority without airway obstruction. Lower CAT scores in our patients with mild airway obstruction compared with those reported in previous studies (Citation20) may reflect the fact that higher scores are more likely encountered in patients with established COPD, some of whom visit their family physician with troublesome or worsening symptoms (e.g., acute exacerbation) than in randomly selected samples such as ours.
It is intriguing that perceived dyspnea intensity (ranked 0–5) on climbing one flight of stairs or walking up a hill was the only symptom that was predictive of airway obstruction in the logistic regression model. Yawn et al. (Citation10) similarly found dyspnea to be the most important predictor when compared with the other symptoms such as cough and phlegm production. However, van Schayk et al. (Citation14) reported cough as a significant predictor but not dyspnea (assessed on a dichotomous, yes/no scale); these investigators used pre-bronchodilator FEV1 < 80% predicted as their definition of COPD, which makes comparisons with the current study difficult. In another study by Price et al. (Citation15), dyspnea, as measured by a series of dichotomous questions and the MRC dyspnea scale, was excluded from their final predictive model as they did not find it to be significant; however, this model was subsequently found not to be a useful discriminating tool on external validation (Citation16).
The importance of exertional dyspnea as a dominant symptom predictive of airway obstruction (that is not fully reversible) is supported by recent physiological studies which have demonstrated consistent abnormalities in small airway function and in dynamic ventilatory mechanics during exercise, even in GOLD stage I COPD (Citation28–30). Clearly, some individuals with minor spirometric abnormalities can have lung hyperinflation and ventilation/perfusion abnormalities which contribute to exertional dyspnea and activity limitation when formally tested (Citation31). The presence of dyspnea in smokers should not be ignored, even in those with preserved spirometry (previously known as GOLD stage 0 “at risk”), since this symptom has been shown to predict subsequent decline in lung function (Citation32). Thus, it seems reasonable that middle-aged smokers with persistent exertional dyspnea such as those identified here may benefit from additional investigations and merit careful follow-up, regardless of initial spirometry results.
Of interest, the MRC dyspnea questionnaire, a category scale which is often used to evaluate dyspnea and activity restriction in COPD, was not as sensitive as the CAT breathlessness score as a predictor for detection of airway obstruction in our study population. This may be because its magnitude of task categories are broad and not sufficiently discriminative, particularly in those with milder airway obstruction. Consistent with a previous study (Citation11), perceived magnitude of activity limitation at home was also not predictive of COPD diagnosis in the regression model. This is not surprising as patients who experience dyspnea may modify the pace or intensity of daily activities so as to avoid symptom provocation –a well recognized behavioural adaptation in early COPD (Citation33,34).
Although symptoms of mucus hyper-secretion such as cough and sputum productivity were weakly predictive of the presence of airway obstruction, these symptoms did not add to the final regression model and were not included. Their presence in smokers is suggestive of chronic bronchitis which can exist in the absence of airway obstruction, at least as measured by spirometry. It remains to be determined whether such patients are susceptible to subsequent decline in pulmonary function with time or whether they have a propensity to develop infective exacerbations.
The negative correlation of sleep disturbance to the diagnosis of COPD was unexpected. We reasoned that the inclusion of this variable without a clear biological rationale was problematic and therefore excluded it from the final model. The association may be due to the wording of the anchors, “I sleep soundly” and “I don't sleep soundly because of my lung condition”, which may be variably interpreted by the respondent and may require further refinement depending on the population being tested. Such an association was not reported during development or validation of the CAT questionnaire in a COPD population and further study is required to examine the inter-relationships between airway obstruction, sleep quality and the presence of respiratory symptoms in the general population (Citation18–20).
Contributing factors for our prediction model were in agreement with those used in previous screening tools such as the COPD Population Screener Questionnaire (COPD-PS) and the Lung Function Questionnaire (LFQ) (Citation9,10,Citation12,13). Previous studies have mainly used target COPD population samples (Citation9,10,Citation14–16) or were retrospective in design (Citation12). In many studies, post-bronchodilator testing for COPD diagnosis was not conducted and stringent quality assurance for spirometry according to ATS guidelines was not always in place (10,11,13). Despite these differences, the key predictors of COPD remain remarkably constant. Our model is the first to be developed in a random population sample and to use a more rigorous definition of COPD, which included both LLN and GOLD spirometric criteria in conjunction with inhalational exposure history.
The best predictive regression model had three components: age (≥55 years), history of previous or active smoking (regardless of smoking duration) and presence of activity related breathlessness. The numerical cut-off for presence of breathlessness varied according to the baseline risk derived from smoking status and age. Based on the results of our model, we recommend no further screening if the individual has a low probability of COPD but we recommend confirmatory testing by spirometry if the probability of COPD is higher than our cutpoint (, ).
On initial testing, our simple 3-item model is comparable or superior to existing case-finding tools and showed good AUC of 0.77, sensitivity of 77% and an excellent negative predictive value of 96.5%. All of these are desirable attributes in a population screening tool. The low positive predictive value (18.6%) and specificity (69.4%) in large part reflect our use of a random population sample and will likely improve as the model is tested in the primary care setting.
Strengths and limitations
A strength of the current study is that it was undertaken in a random population sample, thus avoiding selection bias inherent in sampling patients who present to their physician's office with specific respiratory complaints. A relatively small subsample (n = 51) had airway obstruction meeting diagnostic criteria for COPD and we therefore had a limited range of CAT scores. Thus, we were unable to assess our models’ discriminatory validity across GOLD severity groups, sexes or age groups.
However, the sample was sufficiently large to successfully test internal validity of the predictive model and sets the stage for a second study to examine its predictive power in a new random population and convenience samples in family practice. Finally, as we have taken components out of a validated scale developed to evaluate symptoms in COPD and repurposed them, the new tool needs further study and external validation in the target family practice population prior to any consideration of dissemination and implementation.
Conclusion
Although the CAT total score was predictive of a diagnosis of COPD, at least in the general population, its breathlessness component was a stronger correlate and displaced the total score in our final logistic regression model. This suggests that careful assessment of activity-related dyspnea is an important component of the clinical evaluation of individuals who are at risk for COPD. The triad of smoking status, age at least 55 years and the presence of exertional dyspnea are key elements of a simple model which has reliable measurement properties when tested in a random population. Extensive further validation of this screening tool for COPD is required before it can be recommended for use in the target primary care setting or, indeed, in public awareness campaigns and Lung Health advocacy initiatives in the general population.
Conflicts of interest
NR, YML, KAW, JAG, NA and RR and have no conflicts of interest to report. WCT has received unrestricted educational grants for the Canadian Obstructive Lung Disease (COLD) study operations from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim and Pfizer; and participated in speaking activities in workshops funded by Boehringer Ingelheim, GSK and Telacris. JB has received research funding, via the Research Institute of the McGill University Health Centre, from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Nycomed and Pfizer; and has served on speakers, consultation panels and advisory boards for the above listed pharmaceutical companies. DEO has received research funding, via Queen's University, from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Nycomed and Pfizer; and has served on speakers bureaus, consultation panels and advisory boards for the above listed pharmaceutical companies.
References
- Yawn B, Mannino D, Littlejohn T, Ruoff G, Emmett A, Raphiou I, Crater G. Prevalence of COPD among symptomatic patients in a primary care setting. Curr Med Res Opin 2009; 25(11):2671–2677.
- Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000; 160(11):1683–1689.
- Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008; 63(5):402–407.
- Mapel DW, Robinson SB, Dastani HB, Shah H, Phillips AL, Lydick E. The direct medical costs of undiagnosed chronic obstructive pulmonary disease. Value Health 2008; 11(4):628–636.
- U.S. Preventive Services Task Force. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008; 148(7):529–534.
- O'Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk DD, Balter M, Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease –2007 update. Can Respir J 2007; 14(Suppl B):5B–32B.
- Rabe KF, Hurd S, Anzueto A, Barnes PL, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555.
- Celli BR, MacNee W; on behalf of the ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946.
- Freeman D, Nordyke RJ, Isonaka S, Nonikov DV, Maroni JM, Price D, Halbert RJ. Questions for COPD diagnostic screening in a primary care setting. Respir Med 2005; 99(10):1311–1318.
- Yawn BP, Mapel DW, Mannino DM, Martinez FJ, Donohue JF, Hanania NA, Kosinski M, Rendas-Baum R, Mintz M, Samuels S, Dalal AA. Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J COPD 2010; 5:1–10.
- Hanania NA, Mannino DM, Yawn BP, Mapel DW, Martinez FJ, Donohue JF, Kosinski M, Rendas-Baum R, Mintz M, Samuels S, Jhingran P, Dalal AA. Predicting risk of airflow obstruction in primary care: Validation of the lung function questionnaire (LFQ). Respir Med 2010; 104(8):1160–1170.
- Calverley PMA, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D. Development of a population-based screening questionnaire for COPD. COPD 2005; 2(2):225–232.
- Martinez FJ, Raczek AE, Seifer FD, Conoscenti CS, Curtice TG, D'Eletto T, Cote C, Hawkins C, Phillips AL. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS). COPD 2008; 5(2):85–95.
- van Schayck CP, Loozen JM, Wagena E, Akkermans RP, Wesseling GJ. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. Br Med J 2002; 324(7350):1370.
- Price DB, Tinkelman DG, Halbert RJ, Nordyke RJ, Isonaka S, Nonikov D, Juniper EF, Freeman D, Hausen T, Levy ML, Østrem A, van der Molen T, van Schayck CP. Symptom-based questionnaire for identifying COPD in smokers. Respir 2006; 73(3):285–295.
- Kotz D, Nelemans P, van Schayck CP, Wesseling GJ. External validation of a COPD diagnostic questionnaire. Eur Respir J 2008; 31(2):298–303.
- Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, Jensen RL, Falaschetti E, Schouten JP, Hankinson JL, Stocks J, Quanjer PH. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008; 63(12):1046–1051.
- Jones P, Harding G, Wiklund I, Berry P, Kline Leidy N. Improving the process and outcome of care in COPD: development of a standardised assessment tool. Prim Care Respir J 2009; 18(3):208–215.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34(3):648–654.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, Perez T, Soler Cataluña JJ, van der Molen T, Adamek L, Banik N. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011; 38(1):29–35.
- Dodd JW, Hogg L, Nolan J, Jefford H, Grant A, Lord VM, Falzon C, Garrod R, Lee C, Polkey MI, Jones PW, Man WDC, Hopkinson NS. The COPD Assessment Test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011; 66(5):425–429.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159(1):179–187.
- American Thoracic Society (ATS); Task Group on Screening for Respiratory Disease in Occupational Settings. Surveillance for respiratory hazards in the occupational setting. Am Rev Respir Dis 1982; 126:952–956.
- Miller MR, Hankinson J, Brusasco V, et al.; on behalf of the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York: Wiley; 2000.
- Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med 2000; 19(4):453–473.
- Buist AS, McBurnie MA, Vollmer WM, et al.; on behalf of the BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370(9589):741–750.
- Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177(6):622–629.
- O'Donnell DE, Laveneziana P, Ora J, Webb KA, Lam Y-M, Ofir D. Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax 2009; 64(3):216–223.
- Guenette JA, Jensen D, Webb KA, Ofir D, Raghavan N, O'Donnell DE. Sex differences in exertional dyspnea in patients with mild COPD: Physiological mechanisms. Respir Physiol Neurobiol 2011; 177(3):218–227.
- Deesomchok A, Webb KA, Forkert L, Lam YM, Ofir D, Jensen D, O'Donnell DE. Lung hyperinflation and its reversibility in patients with airway obstruction of varying severity. COPD 2010; 7(6):428–437.
- Stavem K, Sandvik I, Eriksson J. Can global lung initiative for chronic obstructive lung disease stage 0 provide prognostic information on long term mortality in men? Chest 2006; 130(2):318–325.
- Montes de Oca M, Tálamo C, Halbert RJ, Perez-Padilla R, Lopez MV, Muiño A, Jardim JR, Valdivia G, Pertuzé J, Moreno D, Menezes AM; PLATINO Team. Health status perception and airflow obstruction in five Latin American cities: the PLATINO study. Respir Med 2009; 103(9):1376–1382.
- Pitta F, Troosters T, Spruit MA, Decramer M, Gosselink R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2005; 86(10):1979–1985.
