Abstract
Rationale: Pulmonary hypertension with exercise is common in chronic obstructive pulmonary disease (COPD) and may contribute to exercise limitation in this disease. We aimed to determine the effects of treatment with sildenafil on exercise capacity in patients with COPD and emphysema. Methods: We performed a randomized, double-blind, placebo-controlled 2-period crossover trial of sildenafil thrice daily in ten adults with COPD and emphysema on CT scan without pulmonary hypertension. We randomized study participants to 4 weeks of sildenafil (or placebo) followed by a 1-week washout and then 4 weeks of placebo (or sildenafil). The 2 primary outcomes were the 6-minute walk distance and oxygen consumption at peak exercise. Results: Sildenafil had no effect on 6-minute walk distance (placebo-corrected difference = -7.8 m, 95% confidence interval, -23.2 to 7.5 m, p = 0.35) or oxygen consumption at peak exercise (placebo-corrected difference = -0.1 ml/kg/min, 95% confidence interval -2.1 to 1.8 ml/kg/min, p = 0.89). Sildenafil increased the alveolar-arterial oxygen gradient (p = 0.02), worsened symptoms (p = 0.04), and decreased quality-of-life (p = 0.03). Adverse events were more frequent while receiving sildenafil (p = 0.005). Conclusions: Routine sildenafil administration did not have a beneficial effect on exercise capacity in patients with COPD and emphysema without pulmonary hypertension. Sildenafil significantly worsened gas exchange at rest and quality of life. (clinicaltrials.gov NCT00104637).
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by dyspnea and exercise limitation, most commonly attributed to airflow obstruction, hyperinflation, gas exchange abnormalities, and inspiratory muscle weakness (Citation1). Pulmonary vascular disease and right ventricular (RV) changes are also well-documented in COPD, even in the absence of systemic hypoxemia (Citation2). Although modest in severity, pulmonary hypertension with exercise is present in many individuals with COPD (Citation3). Not surprisingly, the inability to increase stroke volume with exercise, which has been attributed to increased RV afterload, accounts to some degree for the cardiac limitation in these patients (Citation4).
While supplemental oxygen may blunt the progressive increase in pulmonary artery pressures in COPD (Citation5, 6), trials of other pulmonary vasodilator therapy for COPD have been largely unsuccessful (Citation7–9). A major limitation of this approach has been worsened ventilation-perfusion matching with resultant arterial hypoxemia and (in some cases) worsened exercise performance and lower health-related quality of life (Citation8). Sildenafil inhibits phosphodiesterase 5 (PDE5), allowing increases in cGMP leading to vascular smooth muscle dilation, and has been touted to cause less ventilation-perfusion inequality in interstitial lung disease (Citation10). Sildenafil may also have beneficial effects on the diseased right ventricle, in which PDE5 is upregulated (Citation11). Although acutely dosed sildenafil studies in COPD may decrease pulmonary vascular resistance (Citation12, 13), there has been no double-blind, placebo-controlled randomized clinical trial (RCT) of sildenafil for patients with COPD.
Because exertional pulmonary hypertension is common in COPD (Citation3), we aimed to show the feasibility of studying sildenafil in patients with COPD and emphysema without resting pulmonary hypertension to get estimates of efficacy and safety of routine sildenafil administration. We hypothesized that exercise capacity would be greater in subjects after treatment with 4 weeks of sildenafil compared to placebo.
Methods
Ethics statement
The protocol was approved by the Columbia University Medical Center (CUMC) Institutional Review Board and the medical monitor. All participants provided written informed consent. The manuscript was written by the authors, and the decision to submit the manuscript for publication was made solely by the authors.
Study design
This was a single-center, randomized double-blind, placebo-controlled, two-period crossover study to determine the efficacy and safety of sildenafil in patients with COPD and emphysema on a CT scan. The protocol called for the recruitment of 14 subjects (anticipating 10 completers). The first subject was randomized in February 2005 and a total of 10 subjects were randomized by November 2008, when the study was terminated because of slow enrollment.
Study participants
We included former smokers with a clinical diagnosis of COPD, post-bronchodilator FEV1/FVC ratio < 0.70 and FEV1 < 80% of predicted, and pulmonary emphysema (assessed qualitatively on CT scan) who met the criteria in . We specifically excluded those with known pulmonary hypertension by right heart catheterization or an elevated estimated right ventricular systolic pressure by resting echocardiography. Participants were recruited from general medicine and pulmonary clinics at CUMC. This study was registered at clinicaltrials.gov before the initiation of enrollment (NCT00104637)
Table 1. Inclusion/exclusion criteria
Study procedures
This study was designed as a crossover study with two 4-week periods comparing sildenafil 75 mg thrice daily to placebo. The CUMC Research Pharmacy created a computer-generated randomization list with 1:1 assignment to sildenafil followed by placebo or placebo followed by sildenafil in blocks of 4. The research pharmacy assembled sequentially numbered bottles (labeled “Period 1” and “Period 2”) containing placebo or sildenafil tablets which were identical in color and taste. All investigators, subjects, and personnel were blinded to treatment sequence. Study drug was administered at a dose of 25 mg thrice daily for 14 days followed by 75 mg thrice daily for 14 days during each period. In response to the results of an RCT of sildenafil in pulmonary arterial hypertension and on advice of the medical monitor after reviewing adverse events, we revised the protocol to maintain study participants on the 25 mg dose thrice daily in July 2007 (after 5 participants had enrolled).
Subjects were evaluated at baseline and after each of the four-week treatment periods; there was a one-week washout between treatment periods. Participants maintained a diary of study medication use and recorded any new medications taken during the study period. At each visit, a pill count was performed and the diary reviewed to assess compliance.
Outcome assessments
Details of the outcome assessments are available in the Online Supplement. The primary outcomes were: 1) 6-minute walk distance (6MWD) and 2) peak oxygen consumption (VO2peak) during cycle ergometry. Secondary outcomes included oxygen saturation after 6-minute walk and at peak exercise, Borg dyspnea score after the 6-minute walk, work performed during exercise, oxygen pulse at peak exercise, spirometry, diffusing capacity of carbon monoxide (DLCO), arterial blood gas measurements at rest and alveolar-arterial oxygen (A-a) gradient, symptoms, and health-related quality of life. As most participants were unable to reach the anaerobic threshold, ventilatory efficiency (VE/VCO2 slope at anaerobic threshold) could not be measured reliably and was not included as an endpoint. We used a standardized list of known side effects of sildenafil to assess adverse events.
Statistical analysis
Linear mixed effects modeling with an unstructured covariance matrix was used to assess differences between sildenafil and placebo. Models included fixed effects for drug, period, and sequence. Subject was included as a random effect. Least squares means and standard errors and treatment coefficients (“placebo-corrected difference”) from the fully adjusted models are reported. There were no significant carryover effects. There was a significant period effect for spirometry (FEV1 and FVC); we only used data from Period 1 to analyze these two endpoints.
With α = 0.05 and β = 0.20, the study could detect a one standard deviation difference in exercise capacity with 10 subjects. To account for possible drop-outs, we planned to recruit 14 subjects. There was no adjustment planned for multiple comparisons.
The primary analysis proceeded according to the intent-to-treat principle. All randomized participants were analyzed in their originally assigned group whether or not they discontinued study drug. P-values < 0.05 were considered significant. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). There were no interim analyses or stopping rules planned a priori for the trial.
Results
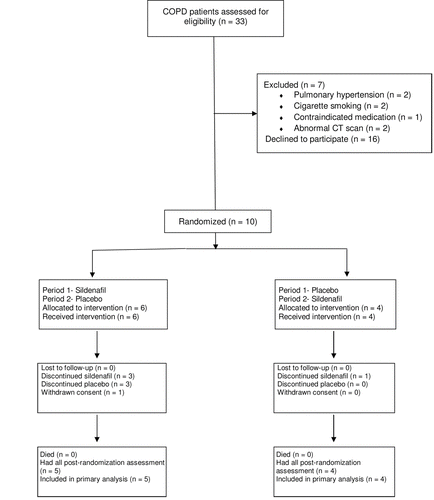
We screened 33 COPD patients between February 2005 and November 2008 (). Ten subjects were eligible and randomized, and 9 completed all study assessments. One subject withdrew consent during Period 1 because of blurry vision and headache.
Participant characteristics are shown in . The mean age was 66 ± 4 years and 80% were male. The mean FEV1 was 38 ± 17% predicted, and DLCO was 36 ± 15 ml/min/Torr. At baseline, the mean 6MWD was 458 ± 69 meters, and VO2peak was 14.1 ± 4.0 ml/min/kg.
Table 2. Participant characteristics
All subjects received the study treatments in the assigned order. The study drug dose was reduced from 75 mg to 25 mg in 2 subjects due to exertional dyspnea and headache. Study drug was discontinued in 4 subjects because of blurry vision (n = 2), dyspnea (n = 1), and abdominal pain (n = 1). All study drug dose reductions and discontinuations occurred during treatment with sildenafil (rather than placebo) and without breaking the blind.
Sildenafil had no significant effect on 6MWD at four weeks (placebo-corrected difference = –7.8 m, 95% confidence interval (CI), –23.2 to 7.5 m, p = 0.35; ). Least-squares mean 6MWD was 458.2 meters during the sildenafil period and 466.1 meters during the placebo period. Similarly, sildenafil had no effect on VO2peak at 4 weeks (placebo-corrected difference = –0.1 ml/min/kg, 95% CI -2.1 to 1.8 ml/min/kg, p = 0.89). The least-squares mean VO2peak after sildenafil was 13.7 ml/min/kg and after placebo was 13.8 ml/kg/min. There were no significant effects of sildenafil on other measures of exercise performance, such as work performed, estimated dead space fraction at peak exercise, oxygen pulse, or oxygen saturation at peak exercise.
Sildenafil significantly increased the A-a gradient at rest (sildenafil, 25.9 mm Hg vs. placebo, 23.0 mm Hg, p = 0.02) and may have lowered arterial PaO2 (). There were no significant differences in PaCO2, spirometric measures, or DLCO between sildenafil or placebo.
Quality-of-life was worse during the sildenafil period than during the placebo period (). The physical component of the SF-36 was 38 after sildenafil vs. 42 after placebo (p = 0.03). The mastery component of the CRQ was 5.0 after sildenafil vs. 5.7 after placebo (p = 0.052). (Lower scores on the SF-36 and CRQ indicate worse quality-of-life). The St. George's Respiratory Questionnaire symptom score was 40 after sildenafil vs. 30 after placebo (p = 0.04), consistent with increased symptoms while using sildenafil.
Table 3. Least-squares means (95% confidence interval) after sildenafil and placebo for physiologic measures
shows the adverse events during the sildenafil and placebo periods. Adverse events were more common during the sildenafil period (9 participants had at least 1 adverse event) than during the placebo period (1 participant) (McNemar's p-value = 0.005). The most common events were dyspnea and blurry vision.
Table 4. Least-squares means (95% confidence interval) after sildenafil and placebo for dyspnea and quality-of-life measures
Discussion
To our knowledge, this is the first randomized, double-blind, placebo-controlled clinical trial of sildenafil in patients with COPD. We showed that standing-dose sildenafil did not improve 6MWD or VO2peak among adults with COPD and emphysema without pulmonary hypertension at rest but did impair gas exchange. Sildenafil led to worsening in quality of life by accepted measures and was associated with frequent adverse events necessitating dose reductions and even discontinuation.
Table 5. Adverse events
Limitation in RV stroke volume reduces exercise capacity in COPD (Citation4). Therefore, one goal of COPD treatment has been to decrease RV afterload without worsening arterial hypoxemia due to worsened ventilation-perfusion matching, a common effect of non-specific pulmonary vasodilators. One RCT of inhaled nitric oxide (iNO) approached this goal (Citation14). That trial randomized 40 COPD patients to either pulsed iNO plus supplemental oxygen or supplemental oxygen alone. iNO reduced mean pulmonary artery pressure and pulmonary vascular resistance and increased resting cardiac output. VO2peak tended to increase in the iNO group, but this was not statistically significant. iNO improved patient-reported physical functioning and decreased PaCO2, but did not change resting PaO2. These data suggest that the ability to decrease RV afterload while optimizing ventilation-perfusion matching could result in clinical benefit.
As iNO has been difficult to deliver chronically on an outpatient basis, sildenafil has been suggested to be a potential alternative therapy since it increases NO-produced cGMP. Sildenafil leads to decreased pulmonary vascular resistance and improved outcomes in pulmonary arterial hypertension (Citation15, 16) and preserves (or even improves) ventilation-perfusion matching in interstitial lung disease (Citation10, Citation17).
Two studies have examined the acute effects of single doses of sildenafil on hemodynamics and gas exchange at rest and with exercise in COPD. Holverda et al. studied the effect of sildenafil 50 mg (or placebo) on resting and exertional hemodynamics and on exercise parameters in patients with COPD (Citation12). Sildenafil significantly reduced mean pulmonary artery pressure during submaximal exercise, but did not affect pulmonary vascular resistance, stroke volume, or cardiac output during exercise. Sildenafil had no effect on peak workload or VO2peak, O2 pulse, VE/VCO2 slope, PaO2, or PaCO2. Blanco et al. studied the effects of acutely administered sildenafil on 20 COPD patients with pulmonary hypertension (either resting or with exercise) (Citation13).
Patients were randomized to receive a single dose of 20 or 40 mg of sildenafil. Sildenafil reduced total pulmonary resistance and mean pulmonary artery and pulmonary capillary wedge pressures and increased stroke volume and cardiac output with exercise. Sildenafil increased ventilation-perfusion mismatch and, similar to our study, decreased PaO2 at rest (-6 mm Hg). Sildenafil had no significant effect on oxygen delivery either at rest or with exercise.
One non-randomized, unblinded study enrolled six COPD patients and treated them with sildenafil 50 mg twice daily for three months (Citation18). Participants demonstrated decreased mean pulmonary artery pressure and pulmonary vascular resistance and increased 6MWD. Rietema et al. studied a cohort of 15 patients with moderate to severe COPD who were assessed with cardiac MRI (at rest and with exercise), exercise testing, and right heart catheterization before and after 12 weeks of sildenafil 50 mg thrice daily (Citation19). There were no changes in maximum workload, VO2peak, oxygen pulse, or VE/VCO2 slope at anaerobic threshold after treatment with sildenafil. Additionally, there were no sildenafil-associated changes in stroke volume, 6MWD, or gas exchange at rest or with exercise.
We found that adverse events were frequent during blinded treatment with high-dose thrice daily sildenafil. These events may have contributed to the sildenafil-induced reductions in quality-of-life. Our effect estimates were consistent with previously reported minimal clinical important differences for the SF-36 (Citation20), CRQ (Citation21), and SGRQ (Citation22), suggesting that the impact of sildenafil on quality of life and symptoms was not only statistically but also clinically significant. These findings suggest that chronic, multi-dose, high-dosage sildenafil may make adults with COPD and emphysema without evidence of pulmonary hypertension at rest feel worse.
There are several possible explanations for our results. Most likely, chronically administered sildenafil neither improves ventilation-perfusion matching (as shown acutely by Blanco et al.) (Citation13) nor increases RV stroke volume (Citation19) in patients with COPD, resulting in no significant effect (or even trend) in terms of dyspnea or exercise capacity. It is possible that our treatment period was not of sufficient duration to see beneficial effects; however, the significant adverse effects and worsened quality of life even in our short-term study make it unlikely that a longer treatment period would change the conclusions. We initially used a high dose of sildenafil to prevent a negative result attributable to underdosing.
However, after considering the results of a Phase III study of sildenafil for pulmonary arterial hypertension, which showed efficacy of a lower dose, and the multiple side effects in our trial, we lowered the dose to one similar to that approved by the FDA for pulmonary arterial hypertension. Last, it is possible that our sample size was insufficient to detect the effects of sildenafil. However, we powered the study using clinically important effect sizes and employed a crossover study design (which is particularly efficient in comparing each subject's treatment period to his or her own placebo period), which requires fewer subjects than similarly-powered parallel trials. Our results did not suggest any signal of efficacy in any endpoint but did show worsening in gas exchange, quality of life, and adverse events making insufficient power an unlikely explanation for the results.
Our study had several limitations. First and foremost, since we did not perform exercise echocardiography or right heart catheterization in our study, it is unclear which (if any) of the study participants had exercise-induced pulmonary hypertension. Without this information, it is impossible to know which subjects might have theoretically benefited from sildenafil. If none of our study participants had exercise-induced pulmonary hypertension, then our study may simply have demonstrated the known phenomena of PDE5 inhibitors causing V/Q mismatching, a finding, which is not novel. If future studies attempt to address the question of whether sildenafil improves exercise-induced pulmonary hypertension in COPD, some assessment of pulmonary artery pressure during exercise should be performed.
Second, the study was terminated due to enrollment that was more difficult than anticipated. Four subjects prematurely discontinued the study drug (all during the sildenafil period) due to adverse events. Nevertheless, all but one participant completed all assessments. While this could bias efficacy measures toward the null, the significant negative effects on gas exchange and quality of life suggest that we could still gauge the clinical impact of the study drug.
Dropout may certainly have contributed to our limited power to draw statistically significant conclusions.
Third, we used relatively short treatment periods to prevent drop-out, which significantly compromises the value of a cross-over study, and successfully minimized the occurrence of such events.
Fourth, sildenafil dosing in our study (25 mg TID) was unconventional. The use of a higher than normal dose (20 mg TID) may explain some or even all of the adverse events in our study. It is not known whether sildenafil or other PDE-5 inhibitors used for erectile dysfunction would have similar effects in patients with COPD.
Conclusions
In summary, we have shown that 4 weeks of sildenafil did not affect exercise capacity compared to placebo, but did impair gas exchange, increase adverse events, and reduce quality-of-life compared to placebo in adults with moderate-to-severe COPD and emphysema without evidence of pulmonary hypertension at rest. Our results do not support the routine clinical use of sildenafil for treatment of COPD with emphysema who lack pulmonary hypertension at rest. Future studies should focus on the effects of sildenafil used for traditional indications in these patients.
Declaration of Interest
Supported by Pfizer through the Viagra Research Grants Program (Grant #1022). Dr. Lederer has served on Advisory Boards for Gilead; has served as a consultant for Gilead and Intermune; has received grant funding from Gilead, Boehringer-Ingelheim, and NIH. Dr. Thomashow has served on Advisory Boards for GSK, Talecris, Intermune, Forrest, and Boehringer-Ingelheim. Dr. Schluger has served on an Outcome Adjudication Committee for a clinical trials sponosored by Pfizer. Dr. Bartels has a pending grant application to study the effects of budesonide/formoterol fumarate dihydrate on exercise from AstraZeneca. Dr. Kawut has served on Advisory Boards for Bayer, Gilead, Pfizer, NIH, ALA; has served as a consultant for Gilead and Novartis; has received grant funding from Actelion, Gilead, United Therapeutics, Lung Rx, Merck, Pfizer, Bayer, and NIH; and has received payment for lecturing from Gilead and Actelion. Ms. Jellen and Ms. Brogan have no competing interests.
Acknowledgments
We thank R. Graham Barr, MD, DrPH for serving as the medical monitor for this study, and Liyi Cen, PhD for assistance with statistical analyses.
References
- O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, Gelb AF, Mahler DA, Webb KA. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc 2007 May; 4(2):145–168.
- Vonk-Noordegraaf A, Marcus JT, Holverda S, Roseboom B, Postmus PE. Early changes of cardiac structure and function in COPD patients with mild hypoxemia. Chest 2005 Jun; 127(6):1898–1903.
- Kessler R, Faller M, Weitzenblum E, Chaouat A, Aykut A, Ducolone A, Ehrhart M, Oswald-Mammosser M. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med 2001 Jul 15; 164(2):219–224.
- Holverda S, Rietema H, Westerhof N, Marcus JT, Gan CT, Postmus PE, Vonk-Noordegraaf A. Stroke volume increase to exercise in chronic obstructive pulmonary disease is limited by increased pulmonary artery pressure. Heart 2009 Feb; 95(2):137–141.
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980 Sep; 93(3):391–398.
- Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981 Mar 28; 1(8222):681–686.
- Vestri R, Philip-Joet F, Surpas P, Arnaud A, Saadjian A. One-year clinical study on nifedipine in the treatment of pulmonary hypertension in chronic obstructive lung disease. Respiration 1988; 54(2):139–144.
- Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, Tamm M. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008 Sep; 32(3):619–628.
- Kalra L, Bone MF. Effect of nifedipine on physiologic shunting and oxygenation in chronic obstructive pulmonary disease. Am J Med 1993 Apr; 94(4):419–423.
- Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002 Sep 21; 360(9337):895–900.
- Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007 Jul 17; 116(3):238–248.
- Holverda S, Rietema H, Bogaard HJ, Westerhof N, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Acute effects of sildenafil on exercise pulmonary hemodynamics and capacity in patients with COPD. Pulm Pharmacol Ther 2008; 21(3):558–564.
- Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, Roca J, Barbera JA. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010 Feb 1; 181(3):270–278.
- Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P, Germann P, Block LH. Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 2003 Apr; 58(4):289–293.
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil Use in Pulmonary Arterial Hypertension Study G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005 Nov 17; 353(20):2148–2157.
- Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB, Group PS. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008 Oct 21; 149(8):521–530.
- Idiopathic Pulmonary Fibrosis Clinical Research, Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 2010 Aug 12; 363(7):620–628.
- Alp S, Skrygan M, Schmidt WE, Bastian A. Sildenafil improves hemodynamic parameters in COPD—An investigation of six patients. Pulm Pharmacol Ther 2006; 19(6):386–390.
- Rietema H, Holverda S, Bogaard HJ, Marcus JT, Smit HJ, Westerhof N, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Sildenafil treatment in COPD does not affect stroke volume or exercise capacity. Eur Respir J 2008 Apr; 31(4):759–764.
- Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res 2005 Apr; 40(2):577–591.
- Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 1996 Nov; 49(11):1215–1219.
- Jones PW. Quality of life, symptoms and pulmonary function in asthma: Long-term treatment with nedocromil sodium examined in a controlled multicentre trial. Nedocromil Sodium Quality of Life Study Group. Eur Respir J 1994 Jan; 7(1):55–62.