Abstract
COPD is a heterogeneous disorder whose assessment is going to be increasingly multidimensional. Grading systems such as BODE (Body-Mass Index, Obstruction, Dyspnea, Exercise), mBODE (BODE modified in grading of walked distance), ADO (Age, Dyspnea, Obstruction) are proposed to assess COPD severity and outcome. Computed tomography (CT) is deemed to reflect COPD lung pathologic changes. We studied the relationship of multidimensional grading systems (MGS) with clinically determined COPD phenotypes and CT lung density. Seventy-two patients underwent clinical and chest x-ray evaluation, pulmonary function tests (PFT), 6-minute walking test (6MWT) to derive: predominant COPD clinical phenotype, BODE, mBODE, ADO. Inspiratory and expiratory CT was performed to calculate mean lung attenuation (MLA), relative area with density below-950 HU at inspiration (RAI-950), and below -910 HU at expiration (RAE-910). MGS, PFT, and CT data were compared between bronchial versus emphysematous COPD phenotype. MGS were correlated with CT data. The prediction of CT density by means of MGS was investigated by direct and stepwise multivariate regression. MGS did not differ in clinically determined COPD phenotypes. BODE was more closely related and better predicted CT findings than mBODE and ADO; the better predictive model was obtained for CT expiratory data; stepwise regression models of CT data did not include 6MWT distance; the dyspnea score MRC was included only to predict RA-950 and RA-910 which quantify emphysema extent. BODE reflect COPD severity better than other MGS, but not its clinical heterogeneity. 6MWT does not significantly increase BODE predictivity of CT lung density changes.
Keywords: :
Introduction
There is increasing evidence that chronic obstructive pulmonary disease (COPD), one of the worldwide leading causes of death and disability, is a systemic disorder (Citation1,2). Many factors, besides airflow obstruction, interact to give different clinical expressions of the disease among different individuals (Citation3–8). Although the diagnosis of COPD is made by spirometry, several multidimensional grading systems (MGS) have been recently developed by combining information derived from respiratory, perceptive, and systemic involvement in COPD (Citation9–10).
Among these indexes BODE (Body-Mass Index, Airflow Obstruction, Dyspnea, Exercise Capacity), mBODE (BODE modified by a different grading of the distance walked in 6 min), and ADO (Age, Dyspnea, Airflow Obstruction) have been recently demonstrated to stratify patients with COPD according not only to prognostic outcomes but also according to disease severity (Citation9–10). In particular BODE has been considered as a staging tool, as a surrogate marker of disease modification, and as a tool to reflect disease progression; it contributed to the acceptance that severity and prognostic assessment in patients with COPD should go beyond airflow obstruction (Citation10).
The reduction in FEV1 is the integral result of a spectrum of different pathological conditions, unified under the acronym COPD, which may cause different clinical manifestations or phenotypes of the disease itself. Several attempts to phenotype COPD have been recently performed (Citation4, Citation6) to ascertain whether patients affected by predominant chronic bronchitis or by predominant emphysema could have different clinical presentation and, possibly, different prognosis and response to therapy. It is presently not know whether the different clinical phenotypic expressions of COPD could be reflected by different severity degrees of MGS.
Accurate in vivo information about lung pathologic changes and disease progression are provided by quantitative chest computed tomography (CT)(Citation11–12). Few studies have previously investigated the relationship between quantitative CT data and COPD symptoms and signs (Citation4, Citation12–16), and only one evaluated the relationship between BODE and quantitative CT data (Citation17). The purpose of this study was to describe the distribution of BODE, mBODE and ADO, their univariate linear relationship with COPD clinical phenotypic presentation and with quantitative inspiratory and expiratory CT density parameters. Furthermore, we evaluated whether the lung pathologic changes of COPD, as reflected by CT density parameters, could be independently predicted by the multidimensional grading systems considered and by their items entered stepwise in multivariate regression analysis.
Methods
Patients
Seventy-two outpatients with COPD (male/female 53/19) were consecutively enrolled and completed the study. The hospital ethical committee approved the study and written informed consent was obtained from all patients.
Clinical and functional assessment
All patients underwent full clinical, functional and chest radiographic evaluation, according to a standardized protocol. Pulmonary function testing was performed by a constant volume body plethysmograph (V6200 Autobox DL, Vmax 22 spirometer-gas analyzer, Sensor Medics, Yorba Linda, CA, USA). FEV1, FEF25–75, FRC, and DLCO were expressed as percentage of the predicted values according to ATS standards (Citation18). The Six-minute walk test (6MWT) was performed according to the ATS guideline (Citation19) and BODE, mBODE and ADO were calculated.
Each patient was assigned a predominantly bronchial or predominantly emphysematous clinical phenotype, by using a multivariate model available on line (www.clipcopd.com). Briefly, the previously published predictive model is derived from the analysis of 38 clinical, functional, and chest radiographic variables collected from 322 COPD patients and is based on the linear combination of the coefficients attributed by multivariate analysis to the characteristics of sputum (occasional, chronic, or purulent), to the presence or absence of two physical signs (adventitious breath sounds, chest hyperresonance), to the value of FEV1/VC (% predicted), and to the presence or absence of four plain chest film radiographic findings (increased lung volume, reduced lung density, increased vascular markings, bronchial wall thickening)(Citation6). After linearly combining the coefficients of the above variables, the patients were considered affected by a predominant bronchial phenotype of COPD if their score was equal or lower than 0.56 and affected by a predominant emphysematous phenotype if their score was higher than 0.56(Citation6).
Imaging assessment
CT examinations were performed on a 16 rows of detectors scanner (Sensation16, Siemens, Erlangen, Germany) using a detector configuration of 16 × 0.75 mm, 140 kVp, 150 effective mAs, tube rotation time 500 ms and a pitch of 1.25. A single spiral acquisition was obtained from the apex to the base during one breath-hold at end inspiration and repeated at end expiration. The acquisition time was 8–12 seconds. Thick (5 mm) slices with smooth filter (B31S) and 5 mm reconstruction increment for whole-lung optimized densitometry were obtained (Citation20).The system was calibrated on a phantom and on air daily (Citation21).
A software program automatically determined the boundaries of each lung (Citation22) and calculated the mean lung attenuation and the relative lung area with density values below -950 HU in inspiratory scans (MLAinsp and RAI-950, respectively); mean lung attenuation and the relative lung area with density values below -910 HU (MLAexp and RAE-910, respectively) were computed in expiratory scans (Citation23,24).
Statistical analysis
Baseline characteristics are expressed as mean ± standard deviation (SD). The distributions of BODE, mBODE and ADO were evaluated by descriptive statistical analysis. Continuous variables and categorical data in the two subsets of patients obtained by considering each patient as belonging to the predominantly bronchial or the predominantly emphysematous clinical phenotypes of COPD were compared by unpaired t-test and χ2-test. Pearson's correlation coefficients were used to assess the relationship of the multidimensional grading systems with CT data.
The ANOVA and Welch tests were used to verify the hypothesis that the mean of the densitometric values could be different for BODE, mBODE and ADO. Multivariate linear regression models were developed using lung density parameters as the dependent variable and the multidimensional grading systems and each of their singular items as the independent ones. The analyses were performed with SPSS version 18.1 (SPSS, IBM corporation, Somers, NY, USA). P-values lower than 0.05 were considered as statistically significant.
Results
Patients characteristics and multidimensional grading system distribution
Anthropometric, pulmonary function and CT data, 6MWT results and multidimensional grading systems data of the 72 patients are reported in . The data have a wide range of variation that is representative of the wide spectrum of COPD clinical presentations. shows the distribution of BODE, mBODE and ADO in the study set. BODE and mBODE frequencies distribution are bimodal. In particular, the absolute modal value for BODE is 5 (14 cases, 19.4%) with a second peak for BODE = 1 (11 cases, 16.7%).
Figure 1. Distribution of multidimensional grading systems BODE, mBODE and ADO in the cohort of 72 patients with COPD.
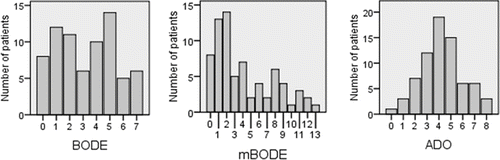
Table 1. Anthropometric, PFT, CT and 6MWT data, multidimensional grading indexes of 72 COPD patients (males/females 53/19)
Also mBODE frequency distribution has a bimodal shape with an absolute modal value at mBODE = 2 (14 cases, 19.4%) and a second peak at mBODE = 8 (6 cases, 8.3%). On the other hand, ADO frequencies distribution shows a gaussian shape. Interpolating the frequencies histogram we obtain a mean and a modal value of ADO = 4 (19 cases, 26.4%).
Comparisons of data in patients subdivided according to predominant clinical phenotype
compares the multidimensional grading systems and their predictors, pulmonary function tests, 6MWT results, including distance walked in meters, Borg dyspnoea scale and SpO2 at the end of exercise, and CT data between patients classified as being affected by a predominant bronchial or a predominant emphysematous clinical phenotype of COPD.
Table 2. Antropometric, PFT and CT data, 6MWT distance and multidimensional grading systems according to COPD predominant phenotype
None of the multidimensional grading systems nor the predictor MRC and 6MWT distance differ significantly between the two subgroups. Conversely, BMI, functional, and CT density data show significant differences compatible with the different characteristics of the two clinical phenotypes of COPD.
Relationship of multidimensional grading systems with CT quantitative data
reports the results of the univariate regression analysis between the multiparametric indexes and the CT densitometric parameters. CT data acquired in the expiratory phase showed closer relationships with the multiparametric indexes than those obtained in the inspiratory phase. shows the distribution of the mean values of the four CT density parameters at each score value of BODE, mBODE, and ADO. The mean values of the CT density parameters showed a regular monotone up-slope or down-slope distribution with respect to each of the BODE score values. More irregular distributions were observed in the relationship between CT parameters and mBODE and to a much greater extent with ADO. The results have been also confirmed by ANOVA (p<0.001). As shown in and ADO index had the weaker relationships with CT densitometric parameters with respect to the other indexes considered.
Figure 2. Point by point score value of each multidimensional grading systems (x-axis) plotted against mean densitometric values at each score point (y-axis).
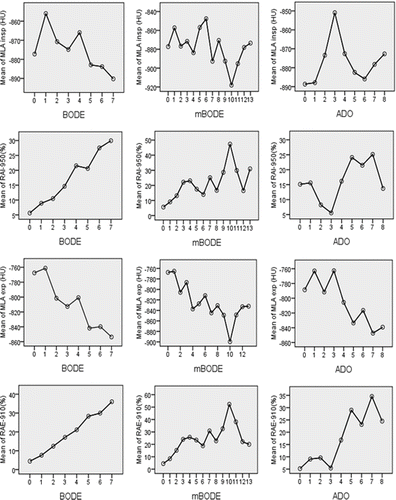
Table 3. Univariate linear regression analysis of multidimensional grading systems with inspiratory-expiratory quantitative CT lung density
Prediction of CT lung density data by multivariate regression analysis
shows the results of the multivariate regression analysis of densitometric data with the predictors included into multidimensional grading systems BODE and ADO (mBODE has not been considered as it includes the same predictors of BODE).
Table 4. Multivariate regression analysis of BODE and ADO with inspiratory-expiratory quantitative lung CT densitometric parameters
Expiratory CT densitometric parameters are better predicted than the inspiratory ones either when all the items of each multidimensional grading system are included (direct method) or when some of them are excluded (stepwise method). Noticeably, the stepwise process excluded from the multivariate predictive models the 6MWT for the BODE index and age for the ADO index. The item MRC entered in the stepwise analysis only in the prediction of RAI-950(%) and RAE-910(%), which are expression of the extent of the emphysematous changes in inspiratory and expiratory CT scans, respectively. In general, the predictive models derived by the BODE index or its items showed a greater accuracy in predicting all CT densitometric parameters with respect to the ADO index.
Discussion
This observational study moved from recently acquired convincing evidence that such multidimensional grading system as BODE, mBODE and ADO can predict not only prognosis but can also stratify COPD severity (Citation9,10). We investigated the relationship of these multidimensional grading systems with phenotypes of COPD clinical presentation and with CT determined inspiratory and expiratory lung density recently proposed to describe daylife impairment in COPD. In general, we found that BODE, mBODE and ADO are linearly related with inspiratory and expiratory CT density changes.
Among the three grading systems BODE predicted CT lung density changes better than mBODE and ADO. Its predictive value was higher for CT expiratory density measurements; noticeably, no significant difference was observed between the values attained by the multidimensional grading systems in the two subgroups of patients classified as being affected by a predominantly bronchial or a predominantly emphysematous COPD phenotype.
BODE is a validated summary index of several clinical predictors of COPD which are related not only to prognosis and mortality rate but also to the severity of the disease and its progression (Citation9, Citation25). BODE has also been proposed to evaluate the impairment in daily activities and the response to rehabilitative or surgical therapies (Citation9, Citation26) Recently, BODE has been updated (modified BODE, mBODE) to assign a greater weight to the item 6MWT distance that, among the various items, is considered the strongest predictor of mortality (Citation10). Furthermore, a simplified risk index including age, dyspnoea and airflow obstruction (ADO) has been developed to increase the applicability of COPD grading systems outside of the specialized respiratory medicine settings (Citation10).
Our results do not support the hypothesis that mBODE and ADO grading systems could provide a better scoring and staging assessment of patients with COPD because their relationship with CT lung density changes, an objective tool to measure the extent of lung involvement in the disease, was not as strong as that of BODE. As it is shown in as the CT density alterations increase there is no parallel increase in mBODE and ADO, while there is an almost linear relationship between BODE and CT density parameters such as RAI-950 and RAE-910.
The observation that ADO had, among the three grading systems above mentioned, the weaker correlations with the variables considered in this study and that it did not add significance in the prediction of CT lung density changes, support the notion that ADO was developed to predict the risk of death by means of statistical reasoning poorly related to clinical data (Citation27). In this line of evidence, our results show that ADO index is driven more by age than by the domains of COPD. In fact, age is an aleatory variable that normalizes the distribution of the index itself among the study population, as clearly shown in . In epidemiological studies the variable age is always considered the most important determinant of survival independent of the disease diagnosis and, for this reason, it has a limited value in the stratification of any specific disease process (Citation28). Age cannot be improved while rehabilitative and pharmacological interventions can possibly improve exercise capacity and lung function.
The multidimensional grading systems evaluated in this study were not related to the clinically determined COPD phenotypes. Accordingly, the items MRC, 6MWT, and age included in BODE, mBODE, and ADO did not significantly differ between the two clinically identified COPD phenotypes, while a significantly reduced BMI and more impaired lung function prevailed in the predominantly emphysematous COPD phenotype together with CT findings compatible with more markedly reduced lung density. These results, besides showing that there is no link between clinical phenotype and severity of COPD, confirm previous observations that a subset of COPD patients with an emphysematous phenotype have muscle wasting and weight loss as expressed by a low BMI (Citation4,Citation6,Citation29).
Multivariate analysis showed that in this cohort the variability of CT determined inspiratory and expiratory lung density can be independently predicted by BODE better than by ADO index; the stepwise procedure, employed to obtain a comparable predictive value with the least number of variables, excluded 6MWT distance among the predictors included in BODE and age among those included in ADO. The predictor MRC, which describes one of the most disabling condition in the disease, entered the stepwise regression model only for the prediction of CT parameters related to the percentage of lung area with markedly reduced lung density values in inspiration (RAI-950) and expiration (RAE-910), which reflect the extent of emphysematous destructive changes.
This confirms to some extent the results of a previous article where it has been shown that dyspnoea perception was related to DLCO reduction, considering this functional parameter as indicative of alveolar surface reduction (Citation13). Furthermore this result is also in keeping with those of Han and coworkers who showed that BODE index predicts mortality risk suggesting that patients with emphysema detected at CT may have a more severe disease, an accelerated death, and that they might be identified by BODE index (Citation25).
6MWT is a widely used measure of exercise capacity in patients with COPD. The results of this study do not confirm a recent paper that found an inverse relationship between the distance walked and the extent of CT low attenuation areas measured during inspiration (Citation30). Our data show that 6MWT walking distance is, among the items included in the BODE grading system, the only one that is never selected in the stepwise regression analysis to predict CT density data. Reduction in the distance walked can be ascribed, besides lung parenchymal destruction, also to other common comorbidities of COPD affecting the heart and the musculoskeletal system.
Conversely, BMI is selected in the stepwise multivariate process for the prediction of all the CT density parameters included in this study. This could indicate that the reduction of BMI, often associated to muscle wasting and weakness is a comprehensive indicator of disease severity and the strongest “systemic” predictor of lung damage in COPD. This result is in keeping with previous data showing a relationship of muscle composition and strength with mortality in COPD (Citation26, Citation31).
The major limitation of this study is the reduced size of the cohort of patients considered. However, all patients had been examined with the same pulmonary function test equipment and by means of the same CT scanner. It is known that despite a daily air and phantom calibration there is a very low reproducibility of CT density data when different scanners are employed (Citation32). Moreover, the study cohort could be considered to represent the wide spectrum of clinical presentations and disease severity, as shown by the gender partitioning of patients, the functional and CT density findings, and the multidimensional grading systems distribution.
Conclusions
Multidimensional grading systems have been developed to evaluate patients with COPD more comprehensively than with the simple spirometry and to better describe the impact of treatment on the outcome, while lung CT scanning has provided a useful tool to stage and phenotype the disease. Although this study shows that none of the grading systems evaluated could reflect the clinical heterogeneity of the disease, BODE does reflect, better than the other grading systems tested, the severity of the disease itself as described by CT scanning densitometry. Furthermore it appears that addition to FEV1 of the items BMI and MRC could substantially reflect the complexity of such a systemic disease, either considering the extension of lung damage or the impairment of daily performances and quality of life. 6MWT, the clinically most difficult item to be obtained in the BODE grading system, does not significantly add to the evaluation of lung parenchymal density changes quantitatively determined by CT scanning.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agustí AG, Noguera A, Sauleda J, Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21:347–360.
- Schols AM. Nutrition in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2000; 6:110–115.
- Berry CE, Wise RA. Mortality in COPD: causes, risk factor and prevention. COPD 2010; 7: 375–382.
- Ogawa E, Nakano Y, Ohara T, Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax 2010; 64:20–25.
- Pistolesi M. Beyond airflow limitation: another look at COPD. Thorax 2009; 64:2–4.
- Pistolesi M, Camiciottoli G, Paoletti M, Identification of a predominant COPD phenotype in clinical practice. Respir Med 2008; 102:367–376.
- Lomas DA, Silverman EK. The genetics of chronic obstructive pulmonary disease. Respir Res 2001; 2:20–26.
- Agusti A, Calverley PM, Celli B, Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11:122.
- Celli BR, Cote CG, Marin JM, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–12.
- Puhan MA, Garcia-Aymerich J, Frey M, Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009; 374:704–11.
- Madani A, Van Muylem A, de Maertelaer V, Pulmonary emphysema: size distribution of emphysematous spaces on multidetector CT images. Comparison with macroscopic and microscopic morphometry. Radiology 2008; 248:1036–41.
- Matsuoka S, Kurihara Y, Yagihashi K, Morphological progression of emphysema on thin-section CT: Analysis of longitudinal change in the number and size of low-attenuation clusters. J Comput Assist Tomogr 2006; 30:669–74.
- Camiciottoli G, Bartolucci M, Maluccio NM, Spirometrically gated high-resolution CT findings in COPD: lung attenuation vs lung function and dyspnea severity. Chest 2006; 129: 558–64.
- Giuntini C, Camiciottoli G, Maluccio NM, Chronic effort dyspnea explained by lung function tests and by HRCT and CRX radiographic patterns in COPD: a post-hoc analysis in 51 patients. COPD 2007; 4:169–76.
- Patel BD, Coxson HO, Pillai SG, Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir CritCare Med 2008; 178:500–5.
- Grydeland TB, Dirksen A, Coxson HO, Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med 2010; 181:353–9.
- Diaz AA, Valim C, Yamashiro T, Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest 2010; 138:880–7.
- Standardization of Spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:1107–36.
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–7.
- Stoel BC, Stolk J. Optimization and standardization of lung densitometry in the assessment of pulmonary emphysema. Invest Radiol 2004; 39:681–8.
- Parr DG, Stockley RA. Standardization of CT densitometry. Radiology 2004; 230:887–92.
- Kalender WA, Fichte H, Bautz W, Semiautomatic evaluation procedures for quantitative CT of the lung. J Comput Assist Tomogr 1991; 15:248–55.
- Gevenois PA, de Maertelaer V, De Vuyst P, Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995; 152:653–7.
- Gevenois PA, De Vuyst P, de Maertelaer V, Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996; 154:187–92.
- Han MK, Bartholmai B, Liu Lx Clinical significance of radiologic characterizations in COPD. COPD 2009; 6:459–67.
- Imfeld S, Bloch KE, Weder W, The BODE index after lung volume reduction surgery correlates with survival. Chest 2006; 129:873–8.
- Celli BR, Marin JM, Cote CG, Prognostic assessment of patients with COPD. Lancet 2009; 374:1885.
- van den Bemt L, Smeele I, van Weel C. Prognostic assessment of patients with COPD. Lancet 2009; 374:1886.
- Decramer M, De Benedetto F, Del Ponte A, Systemic effects of COPD. Respir Med 2005; 99 (Suppl B):3–10.
- Diaz AA, Bartholmai B, San Josè Estèpar R, Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med 2010; 104:1145–51.
- Schols AM, Slangen J, Volovics L, Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1791–7.
- Kemerink GJ, Lamers RJ, Thelissen GR, Scanner conformity in CT densitometry of the lung. Radiology 1995; 197:749–52.