Abstract
Background: Fibrinogen is a marker of systemic inflammation and may represent an important biomarker for the progression of chronic obstructive pulmonary disease (COPD). Methods: We used baseline data from the Third National Health and Nutrition Examination Survey (NHANES III) and follow-up mortality data to determine the relation between fibrinogen levels and COPD and to examine how fibrinogen levels at baseline affected long-term outcomes. Elevated fibrinogen was defined as the upper 10% of the fibrinogen level distribution Results: Our study sample included 8,507 subjects, including 245 with Stage 3 or 4 COPD and 826 with Stage 2 COPD. Then, 3,290 of the 8,507 subjects died during the follow-up period. The mean fibrinogen level was 303.6 g/dL and 10% of the sample had levels higher than 403.0 mg/dL. Subjects with Stage 3 or 4 COPD were more likely to have a fibrinogen level > 403.0 mg/dL (odds ratio 3.4, 95% confidence interval [CI], 2.1, 5.6) than were people with normal lung function, after adjusting for covariates. An elevated fibrinogen level increased the risk of mortality (hazards ratio [HR] 1.36, 95% CI 1.13, 1.63) in the entire study sample and in subjects with Stage 3 or 4 (HR 2.11, 95% CI 1.27, 3.50) or Stage 2 (HR 1.45, 95% CI 1.08, 1.96) COPD. Conclusion: In the nationally representative NHANES III data, impaired lung function is a correlate of fibrinogen levels and the presence of higher fibrinogen levels increases the risk of mortality both in the overall population and among subjects with COPD.
Keywords :
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is an important cause of morbidity and mortality worldwide (Citation1). In the United States, COPD is now the third leading cause of death, surpassing stroke (Citation2). Understanding the natural history of COPD has been important in the field of pulmonary medicine, dating back to the work of Burrows (Citation3), and Fletcher and Peto (Citation4,5). Over subsequent years, researchers have championed different hypotheses about COPD development, including the “British” hypothesis stating that the presence of cough and sputum was the key factor (Citation6) and the “Dutch” hypothesis stating that the presence of increased airways responsiveness was the major factor (Citation7).
The relation between COPD and other diseases and the effect that these other diseases have on COPD has become increasingly important in recent years (Citation8,9). Epidemiological data indicates that many COPD deaths result from cardiovascular complications (Citation10) and cardiovascular events are increased in COPD patients (Citation11). Although this relation has been, traditionally, thought to be related to the shared risk factor of smoking, recent work has suggested that systemic inflammatory processes may be important in both of these processes.
Fibrinogen is an acute phase reactant protein predominantly derived from the liver that is a marker of systemic inflammation (Citation12). Fibrinogen levels are increased in both cardiovascular disease (CVD) and COPD (Citation13,14). Fibrinogen may be a tool for stratifying COPD patients in clinical trials by identifying populations at higher risk for poor outcome such as frequent exacerbations (Citation13) or hospitalization (Citation15).
Literature exists linking fibrinogen and CVD (Citation16), fibrinogen and COPD (Citation17), and fibrinogen and COPD outcomes (Citation15). This paper examines correlates of fibrinogen levels in a nationally representative cohort of U.S. adults, with a special focus on the presence of COPD at baseline, and determines the effect of elevated fibrinogen levels on long term mortality in this population controlling for multiple concurring risks, including CVD and COPD at baseline.
Methods
The Third National Health and Nutrition Examination Survey (NHANES III) was conducted from 1988 to 1994 by the National Center for Health Statistics of the Centers for Disease Control and Prevention, Atlanta, GA. In this study a stratified multistage clustered probability design was used to select a sample of the U.S. population. Study participants completed extensive questionnaires in the household and a comprehensive physical examination, including pulmonary function testing, either in the household or at a specially equipped mobile examination center. A total of 81 sites were included in the final sample. The study was approved by the National Center for Health Statistics’ Institutional Review Board (No approval number provided). A follow-up of the original NHANES III cohort, linking study subjects to the National Death Index, determined vital status in December 2006.
This analysis was limited to subjects aged 40 years and older (fibrinogen levels were not obtained on subjects younger than 40 years old) who completed the baseline survey and had their vital status determined at the end of follow-up.
Fibrinogen levels
Fibrinogen levels in the NHANES III survey were measured using thrombin clotting times of diluted plasma, as has been described previously(Citation18).
Baseline lung function
Predicted values from NHANES III (developed using only asymptomatic, lifelong nonsmoking subjects with at least two acceptable maneuvers) were used in the analysis(Citation19). We used age, sex, height and race/ethnicity to determine the predicted values. We used the values for “white” subjects to classify those of “other race”. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has developed criteria to aid both the diagnosis and epidemiologic study of COPD (Citation20).
The study participants were classified into a modified “GOLD Stage”, using the pre-bronchodilator lung function (post-bronchodilator values were not obtained in this survey), into categories based on a modification of COPD classification criteria: Stage 3 or 4 (FEV1/FVC <0.70 and FEV1<50% predicted), Stage 2 (FEV1/FVC <0.70 and FEV1 > = 50 to <80% predicted), Stage 1 (FEV1/FVC <0.70 and FEV1 > = 80% predicted), Restricted (FEV1/FVC >70% and FVC <80% predicted, Symptoms only (presence of chronic respiratory symptoms in the absence of any lung function abnormality), and no lung disease.
Definitions
Data included in this analysis are age, sex, race/ethnicity, body mass index (BMI), smoking status, modified GOLD Stage (defined above), diabetes mellitus, cardiovascular disease, educational level, and poverty income ratio. Age was classified at baseline and was categorized for use in tables (40–49, 50–59, 60–69, 70–79, and 80 and older), and was used as a continuous variable in the regression analyses.
Race/ethnicity was classified as white, black, Mexican-American, or other (typically Asian, American Indian, or mixed race). BMI was classified at baseline and was categorized into 5 categories (missing, < 18.5, 18.5–24, 25–29, and > = 30 kg/m2). We defined subjects as being current smokers, former smokers, or never smokers based on their responses to series of questions. One had to have smoked more than 100 cigarettes to qualify as a former or current smoker. A person was considered as having diabetes mellitus either if they reported physician diagnosed diabetes or reported treatment for diabetes or had a fasting blood glucose of higher than 126 g/L.
A person was considered as having cardiovascular disease if they reported a physician diagnosis of a heart attack, coronary artery disease, congestive heart failure, or stroke. Education status was stratified into three levels (< 12 years, 12 years, and > 12 years). Poverty income ratio was extracted from the database and stratified into four categories, < 1 (very poor), 1 to < 2 (poor), 2 or higher (not poor), and “unknown” for those not reporting income.
Mortality
Death from any cause was ascertained by the end of 2006 and the date was used to determine months of follow-up from the baseline examination.
Statistical analysis
Data analysis was completed using statistical software (Statistical Analysis Software, version 9.2; SAS Institute; Cary, NC and SUDAAN version 10.1; RTI, Research Triangle Park, NC, and SPSS 17, Somers, NY). NHANES III weights were used in all of the analyses.
We determined the correlates of fibrinogen levels in linear models using the SUDAAN procedure REGRESS in models controlling for age, sex, race/ethnicity, BMI, smoking status, modified GOLD stage, diabetes mellitus, cardiovascular disease, educational level, and poverty income ratio. These were replicated examining fibrinogen levels in the top decile (> 403.0 mg/dL) using the SUDAAN procedure RLOGIST, controlling for the same factors. Our primary outcome of interest in the survival models was mortality by 2006. Cox proportional hazard regression models were developed using the SUDAAN procedure SURVIVAL to account for differential follow-up in cohort participants.
Time of follow-up was used as the underlying time metric. Censoring occurred at the date of death certificate or date the participant was last known to be alive. Plots of the log-log survival curves for each covariate were produced to evaluate the proportional hazards assumptions. We determined the interaction between modified GOLD Stage and elevated fibrinogen levels as predictors of mortality in models adjusted for covariates as noted above. We also analyzed the data stratified by modified GOLD Stage to determine whether elevated fibrinogen levels predicted mortality in subjects at each disease stage.
Results
The NHANES III adult cohort consisted of 20,050 individuals. We excluded 8,602 who were under the age of 40, 2,098 who did not have fibrinogen levels obtained, 839 who did not have pulmonary function testing done, and 4 who did not have mortality data. The studied cohort consisted of 8,507 subjects representing an estimated 83.8 million U.S. adults aged 40 and older during 1988–1994. By the end of 2006, 3,209 subjects, representing an estimated 23.4 million (27.9%, weighted percentage) of the original cohort, had died.
The distribution of age, sex, race/ethnicity, BMI, smoking status, modified GOLD stage, diabetes mellitus, cardiovascular disease, educational level, and poverty income ratio is displayed in , including the actual numbers of studied subjects and the weighted percentage. This Table also reports the mean fibrinogen levels, the proportion of subjects with fibrinogen levels > 403.0 mg/dL, and the proportion of subjects who had died by the end of the follow-up period.
Table 1. Demographic characteristics, fibrinogen levels, and mortality of subjects included in analysis
Tables and report the correlates of fibrinogen levels () and the correlates of elevated fibrinogen levels (). There was considerable overlap between these two analyses; for example, in both age, current smoking, and the presence of diabetes or cardiovascular disease were significant correlates of fibrinogen. The presence of severe or very severe COPD was one of the strongest predictors of fibrinogen levels, with a mean increase of 43.7 mg/dL (standard error 9.70 mg/dL) in the linear regression models and an odds ratio (OR) for elevated fibrinogen of 3.10 (95% confidence interval (CI) 1.99, 4.81).
Table 2. Correlates of fibrinogen levels from linear regression models
Table 3. Correlates of Fibrinogen levels > 403 mg/dL (top decile) from logistic regression models
In Cox proportional hazard models adjusted for all covariates, fibrinogen predicted a higher risk of death both as a categorical variable (Level > = 403 mg/dL, Model A) or a continuous one (, Model B). An elevated fibrinogen level increased the risk of mortality in all modified GOLD subgroups (), although this comparison only reached statistical significance in those with normal lung function. In models stratified by GOLD Stage () both the categorical and continuous measures of elevated fibrinogen predicted higher mortality among both Stage 2 and Stage 3 or 4 COPD.
Figure 1. Interaction between elevated fibrinogen levels and modified GOLD stage, in Cox proportional hazard models predicting mortality and adjusted for age, sex, race-ethnicity, body mass index, smoking, diabetes, cardiovascular disease, education level, and poverty income ratio. The box represents the point estimate and the vertical line represents the 5% and 95% confidence interval.
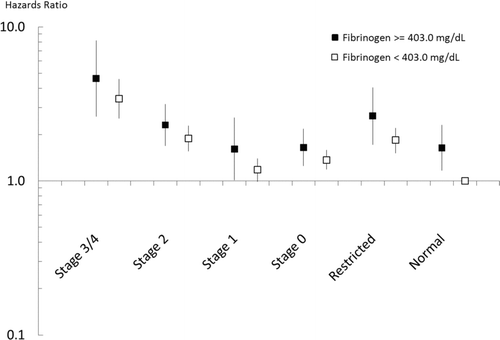
Table 4. Results of Cox Proportional Hazards Models for mortality at up to 18 years of follow-up.
Table 5. Results of Cox Proportional Hazards Models for mortality at up to 18 years of follow-up, stratified by GOLD Stage and adjusted for age, sex, race-ethnicity, body mass index, smoking status, diabetes mellitus, cardiovascular disease, education level, and poverty income ratio
Discussion
In this nationally representative data set of a U.S. population we found that fibrinogen levels were related to the presence of spirometrically determined obstructive lung disease and that these levels predicted long term mortality in this cohort. Higher fibrinogen levels predicted mortality when evaluated as both a continuous and categorical variable. This effect was seen both in the overall population and among the subset of subjects with Stage 2 and Stage 3 or 4 COPD at baseline.
Fibrinogen is a major plasma protein coagulation factor that is linked to adverse health events when levels are either low or high. Low levels have been linked to an increased risk of bleeding due to impaired hemostasis whereas high levels have been linked to an increased risk of cardiovascular events (Citation21). Variability in fibrinogen levels are thought to be related to both inherited and acquired factors (Citation22), and the presence of COPD is a well-established predictor of elevated fibrinogen levels (Citation23,24). In addition, patients with COPD are at increased risk for thrombotic events, such as venous thromboembolism (Citation25) and acute cardiac events (Citation11).
This analysis demonstrated that fibrinogen levels in this cohort were significantly related to a number of factors, including age, sex, smoking status, race/ethnicity, and the presence of chronic diseases such as cardiovascular disease, diabetes mellitus, and COPD. As fibrinogen levels were only determined at baseline we are unable to infer whether the elevated fibrinogen levels preceded or followed the development of these chronic diseases.
Our analysis demonstrated a dose-response effect for COPD, with more advanced GOLD stages 3 or 4 of the disease exhibiting a greater elevation in fibrinogen (43.71 mg/dL, p < 0.0001) than GOLD stage 2 or GOLD stage 1 disease (19.79 mg/dL, p < 0.0001 and 3.04 mg/dL, p = 0.4731 respectively), relative to people with normal lung function. The elevation among those with Stage 3 or 4 COPD was higher than that seen with diabetes (16.78 mg/dL) or CVD (11.96 mg/dL) at baseline. We also demonstrated that people with a restrictive spirometric impairment also had elevated fibrinogen levels (17.19 mg/dL, p = 0.0011), suggesting this group may be a subtype of COPD or have other characteristics that precludes their inclusion as “normal” subjects in epidemiologic and clinical studies (Citation17,Citation26).
While we demonstrated an effect of elevated fibrinogen on mortality in the overall cohort (), this effect was diminished in the analysis that examined the interaction between lung function impairment and elevated fibrinogen levels (). In the stratified analysis; however, the risk of elevated fibrinogen for mortality was highest among those with Stage 3 or 4 COPD at baseline. The finding of elevated fibrinogen and higher mortality, however, raises the intriguing possibility that fibrinogen might serve as both a biomarker of disease activity in COPD and a potential target for therapeutic intervention (Citation27).
This analysis has certain limitations. As noted above, fibrinogen was assessed at a single point in time, so one cannot determine the temporal association between fibrinogen levels and any of our markers of disease. The strict classification of COPD using GOLD criteria requires the use of a post-bronchodilator FEV1, which was not available in this study (Citation20). Thus, some subjects with “asthma” may have been considered as “COPD” in this analysis. In addition, not all subjects were able to complete pulmonary function testing, potentially biasing our sample towards a healthier population. We did not have other indicators of disease activity for COPD, such as validated measures of exacerbations or imaging data.
In summary, we have demonstrated in this nationally representative cohort that fibrinogen levels are increased in subjects with COPD and other chronic diseases and that elevated fibrinogen levels predict a higher risk of mortality. Fibrinogen is a marker for the systemic component of COPD and may provide an opportunity for improved targeting of interventions to patients with evidence of systemic inflammation.
Declaration of Interest Statement
David M. Mannino has received research funding from GlaxoSmithKline, Novartis, Boehringer-Ingelhiem, and Pfizer and has worked as a consultant or advisor for GlaxoSmithKline, Novartis, Boehringer-Ingelhiem, Astra-Zeneca and Pfizer. This project was funded by a research grant from GlaxoSmithKline Research and Development. Deepa Valvi declares no conflicts of interest. Hana Mullerova and Ruth Tal-Singer are employees of GlaxoSmithKline.
Acknowledgments
The authors would also like to thank Ms. Susan Mittenzwei and Ms. Rebecca Copeland for their assistance in this project. Funding for the analysis was provided by GlaxoSmithKline. The authors alone are responsible for the content and writing of the paper.
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–773.
- Minino AM, Xu J, Kochanek KD. Death in the United States, 2008. National Vital Statistics Reports 2010; 59:1–72.
- Burrows B, Strauss RH, Niden AH. Chronic Obstructive Lung Disease .3. Interrelationships of Pulmonary Function Data. Amer Rev Respir Dis 1965; 91:861–868.
- Fletcher C, Peto R, Tinker CM, Speizer FE. The natural history of chronic bronchitis and emphysema. Oxford University Press, Oxford, UK, 1976.
- Peto R, Speizer FE, Cochrane AL The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation. Am Rev Respir Dis 1983; 128:491–500.
- Anthonisen NR. The British hypothesis revisited. Euro Respir J 2004; 23:657–658.
- Vestbo J, Prescott E. Update on the “Dutch hypothesis” for chronic respiratory disease. Thorax 1998; 53 Suppl 2:S15–S19.
- Sin DD, Man SF, Marciniuk DD, Can inhaled fluticasone alone or in combination with salmeterol reduce systemic inflammation in chronic obstructive pulmonary disease? Study protocol for a randomized controlled trial (NCT00120978). BMC Pulm Med 2006; 6:3.
- Decramer M, Rennard S, Troosters T COPD as a lung disease with systemic consequences–clinical impact, mechanisms, and potential for early intervention. COPD 2008; 5:235–256.
- Mannino DM, Doherty DE, Buist AS. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006; 100:115–122.
- Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax 2008; 63:599–605.
- Redman CM, Xia H. Fibrinogen biosynthesis. Assembly, intracellular degradation, and association with lipid synthesis and secretion. Ann NY Acad Sci 2001; 936:480–495.
- Wedzicha JA, Seemungal TA, MacCallum PK, Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost 2000; 84:210–215.
- Engstrom G, Lind P, Hedblad B, Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation 2002; 106:2555–2560.
- Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:1008–1011.
- Ford ES, Giles WH. Serum C-reactive protein and fibrinogen concentrations and self- reported angina pectoris and myocardial infarction: findings from National Health and Nutrition Examination Survey III. J Clin Epidemiol 2000; 53:95–102.
- Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med 2003; 114:758–762.
- Gunter EW, Lewis B, Koncikowski S. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, 1996.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–187.
- Rodriguez-Roisin R, Rabe KF, Anzueto A, Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2008 Update). www.goldcopd.org. 2009. 4–26–2011.
- Lowe GD, Rumley A, Mackie IJ. Plasma fibrinogen. Ann Clin Biochem 2004; 41:430–440.
- De Maat MP, Verschuur M. Fibrinogen heterogeneity: inherited and noninherited. Curr Opin Hematol 2005; 12:377– 383.
- Engstrom G, Segelstorm N, Ekberg-Aronsson M, Plasma markers of inflammation and incidence of hospitalisations for COPD: results from a population-based cohort study. Thorax 2009; 64:211–215.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107:1514–1519.
- Gunen H, Gulbas G, In E, Yetkin O, Hacievliyagil SS. Venous thromboemboli and exacerbations of COPD. Eur Respir J 2010; 35:1243–1248.
- Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest 2009; 135:419–424.
- Lomas DA, Miller BE, Willitis L, Keene O, Barnacle H, Barnes NC, Inhibition of p38 MAP kinase reduces plasma fibrinogen in COPD. J Clin Pharmacol. In press.
