Abstract
Pulmonary hypertension (PH) is a serious complication of chronic obstructive pulmonary disease (COPD), and there is no effective pharmacological treatment for COPD-associated PH. We evaluated the effect of udenafil, a phosphodiesterase-5 (PDE-5) inhibitor, on the exercise capacity of patients with severe COPD. Patients with severe and very severe COPD (forced expiratory volume in one second (FEV1) <50% of predicted) received udenafil (50 mg daily) for 8 weeks. A 6-min walk test (6MWT), lung function test, Doppler echocardiography, and Saint George's Respiratory Questionnaire (SGRQ) were completed before and after therapy. The primary outcome was a change in the 6-min walk distance (6MWD). Thirty-eight patients were screened for eligibility, and 23 completed the study. After 8 weeks of udenafil treatment, the mean 6MWD increased from 315 to 348 m (p = 0.02), and median PASP decreased from 36 to 30 mmHg (p = 0.02). There were no changes in the SGRQ score, Borg dyspnea score, or pulmonary function parameters. The PDE-5 inhibitor udenafil improved exercise capacity and decreased pulmonary artery pressure in patients with severe COPD. However, due to the small sample size, uncontrolled design and high dropout rate, the efficacy of udenafil in severe COPD needs to be confirmed in a large-scale randomized controlled study. This study was registered at ClinicalTrials.gov (number: NCT01364181).
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease whose prevalence is increasing. Patients with COPD suffer from chronic respiratory symptoms and have increased morbidity and mortality (Citation1). Pulmonary hypertension (PH) is a serious complication of COPD and is associated with shorter survival time, more frequent exacerbation, and increased use of health resources (Citation2). The prevalence of PH, as defined by a mean pulmonary artery pressure (mPAP) > 20 mmHg, is about 35% in patients with severe COPD (Citation2). There is no effective pharmacological treatment for COPD-associated PH. Only the long-term oxygen therapy is helpful in a subset of patients.
Alveolar hypoxemia is a main cause of elevated pulmonary vascular resistance, as it leads to changes in the balance between released endothelium-derived mediators with vasodilator (nitric oxide) and vasoconstrictive properties (endothelin). Sildenafil, a phosphodiesterase-5 (PDE-5) inhibitor, stabilizes the second messenger nitric oxide, reduces pulmonary vascular resistance, increases exercise tolerance, and increases survival in patients with pulmonary arterial hypertension (Citation3). Thus, it is plausible that a PDE-5 inhibitor may also have a beneficial effect on PH secondary to COPD. However, PDE-5 inhibitors entail a risk for worsening arterial oxygenation due to the inhibition of hypoxic pulmonary vasoconstriction in COPD. A recent study showed that sildenafil improved pulmonary hemodynamics at rest and during exercise, but impaired arterial oxygenation at rest (Citation4).
This study was conducted to evaluate the effect of udenafil, a PDE-5 inhibitor, on the exercise capacity of patients with severe COPD.
Methods
Patients
Patients with severe COPD who showed a post-bronchodilator forced expiratory volume in 1 second (FEV1) of less than 50% of the predicted value were asked to participate in the study. Exclusion criteria were acute exacerbation within 4 weeks of study entry, coronary heart disease, and a history of adverse events with PDE-5 inhibitors. The study was approved by the Ethics Review Committee of Seoul National University Bundang Hospital (B-0904/073-001) and conducted in compliance with the Declaration of Helsinki. This study was registered at ClinicalTrials.gov (number: NCT01364181).
Study design
This was a prospective, single-arm study performed in a tertiary teaching hospital from March 2010 to March 2011. The results of baseline forced spirometry, oxygen saturation, Borg dyspnea score, transthoracic Doppler echocardiography, N-terminal pro-B-type natriuretic peptide (NT-proBNP) level, and Saint George's Respiratory Questionnaire (SGRQ) were collected, and a 6-min walk test (6MWT) was performed. Patients received 50 mg of udenafil once a day for 8 weeks. After 1 week of treatment, patients were checked for adverse events, oxygen saturation, and Borg dyspnea score. After 8 weeks, the previous examinations were repeated. In this study, PH was defined as pulmonary artery systolic pressure (PASP) ≥ 45 mmHg (Citation5).
Data analysis
The primary outcome was a change in the 6-min walk distance (6MWD). Secondary outcomes were changes in lung function, SGRQ score, Borg dyspnea index, oxygen saturation, and PASP measured by transthoracic Doppler echocardiography. A sample size of 20 was calculated to discriminate 50 meters of difference in the 6MWD with 90% power for p < 0.05. Factoring in a 20% dropout rate, a sample size of 25 patients was determined. Changes in parameters were analyzed by the Wilcoxon Signed Rank test. Analyses were performed using SPSS 18.0 software (SPSS; Chicago, IL, USA).
Results
Baseline characteristics of study patients
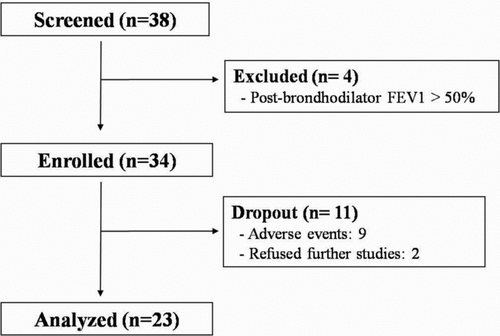
A total of 38 patients were screened for eligibility. Four patients were excluded due to a post-bronchodilator FEV1 > 50% of the predicted value. Of the 34 patients who were included, nine dropped out owing to adverse events, and two patients refused further examinations. Thus, outcome measurements were available for 23 patients (). All patients except one female patient had a history of smoking (). The median predicted FEV1 was 35%. Four patients (17%) were receiving long-term oxygen therapy. The median PASP measured by Doppler echocardiography was 36 mmHg. Four patients (17.4%) exhibited PH, which was defined as PASP ≥45 mmHg.
Table 1. Baseline characteristics of the study patients
Effect of udenafil
After 8 weeks of udenafil treatment, the mean 6MWD increased significantly, from 315 to 348 m (p = 0.02, ), and the median PASP decreased from 36 to 30 mmHg (p = 0.02). However, there was no change in the SGRQ score, the Borg dyspnea score, or pulmonary function after 8 weeks of udenafil treatment. Oxygen saturation at resting state and after the 6MWT did not decrease after udenafil treatment.
Table 2. Change of parameters after 8 weeks of udenafil treatment*
Baseline oxygen saturation was lower in patients whose 6MWD increased by more than 50 m after udenafil treatment (improvers) compared with non-improvers (91% vs. 96%, p = 0.04, ). Other baseline parameters, including PASP, did not differ between improvers and non-improvers.
Table 3. Differences in baseline parameters between improvers (increased 6MWD) and nonimprovers (decreased 6MWD) after 8 weeks of udenafil treatment
Adverse events
Of 34 patients treated with udenafil, nine patients (26.4%) dropped out due to adverse events. Three patients complained of worsening dyspnea; other adverse events are described in . These events were generally mild or moderate in severity and none were associated with clinical sequelae. However, patients with adverse events refused further treatment. Of the 23 patients who completed the study, only one presented with a mild adverse event (mild flushing). The patients who dropped out had lower baseline PASP than the patients who completed the study ().
Table 4. Adverse events in dropout patients
Table 5. Comparisons between patients with or without adverse events
Discussion
Despite the fact that COPD-associated PH is a major cause of shorter survival time and increased use of health resources, there is no effective medical treatment for PH in COPD. Several drugs, including prostacyclin analogues and endothelin-1 receptor antagonists, were developed for the treatment of pulmonary arterial hypertension and have been tried to treat COPD-associated PH. However, endothelin-1 receptor antagonists showed deleterious effects on gas exchange in a randomized controlled trial in patients with severe COPD (Citation6).
PDE-5 inhibitors have also been used to treat pulmonary arterial hypertension (Citation3). PDE-5 inhibitors induce vasodilation of the pulmonary artery by increasing the nitric oxide concentration via stabilization of cyclic guanosine monophosphate. Thus, it is possible that a PDE-5 inhibitor may reduce PAP in patients with severe COPD. However, there is a concern that treatment with a PDE-5 inhibitor could worsen hypoxemia owing to hypoxic vasoconstriction inhibition in COPD patients (Citation7). Despite the potential beneficial effect of a PDE-5 inhibitor on COPD-associated PH, its effect on the clinical outcome of COPD patients has not been well evaluated. Few studies have reported the effect of a PDE-5 inhibitor on PH in COPD patients (Citation8–10).
Holverda et al. reported that the PDE-5 inhibitor sildenafil attenuated the exercise-induced increase in mPAP (Citation8). Recently, one study evaluated the changes in spirometric properties, hemodynamic parameters, and gas exchange 1 h after the administration of 20–40 mg of sildenafil in COPD-associated PH (Citation4). In that study, sildenafil reduced the exercise-induced increase in mPAP and slightly increased forced vital capacity and FEV1. However, these previous studies evaluated only the acute effects of sildenafil, and not its long-term clinical effects.
We evaluated the impact of the PDE-5 inhibitor udenafil on the clinical parameters of COPD, including exercise tolerance, quality of life, and pulmonary function. Our study confirmed that udenafil improved exercise tolerance and decreased PASP in patients with severe and very severe COPD. However, in a study evaluating stroke volume and exercise capacity of 15 COPD patients after 12 weeks of sildenafil treatment, neither stroke volume nor exercise capacity was improved (Citation9). The patient group in that study was moderate to very severe COPD patients (GOLD stage II-IV), whereas our study included only severe COPD patients (FEV1 <50% of predicted, GOLD stage III-IV). This difference between the two studies may account for the different findings regarding exercise tolerance after treatment with a PDE-5 inhibitor.
It should be noted that in the present study, the difference in the 6-min walk distance was less than 50 meters, which is generally accepted as a clinically significant difference (Citation11). One previous study found a within-person change of 35 meters for 6MWD to be an important effect (Citation12), and another recent study showed that 25 meters was the minimal important difference for 6MWD in COPD (Citation13). Thus, the change in 6MWD in our study is clinically meaningful. Although udenafil improved exercise capacity, the dyspnea index and quality of life score did not improve. Similarly, Rietema et al. (Citation9) found no improvement in the Borg dyspnea index after 12 weeks of sildenafil treatment. Further study is needed to evaluate the impact of PDE-5 inhibitors on dyspnea and quality of life of COPD.
Interestingly, patients whose 6MWD increased by more than 50 meters with udenafil treatment had lower baseline oxygen saturation than patients whose 6MWD did not increase. This suggests that patients with severe COPD and impaired gas exchange may benefit more from a PDE-5 inhibitor. Careful selection of candidates who would benefit from a PDE-5 inhibitor may be important.
Oxygen saturation at rest and after exercise did not decrease after udenafil treatment. This result is consistent with the findings of Holverda et al., who reported no change in oxygen saturation after long-term treatment with sildenafil (Citation8). Although sildenafil impaired arterial oxygenation at rest in a study by Blanco et al., oxygen delivery to the tissues did not change after sildenafil administration (Citation4). Despite concerns regarding deteriorating hypoxemia after PDE-5 inhibitor treatment in COPD-associated PH, PDE-5 inhibitors appear to have little effect on arterial oxygenation.
Udenafil, which has a long half-life of 12 h, is typically administered at a dosage of 50–200 mg once per day for treatment of erectile dysfunction. We administered 50 mg of udenafil once per day. Sildenafil has been given at a dosage of 25–100 mg for erectile dysfunction and at 25 mg three times a day for pulmonary arterial hypertension. The dose of udenafil in our study was lower than the usual dosage for erectile dysfunction. Thus, a higher dose of udenafil could possibly be used in the treatment of COPD-associated PH, but a phase II study using a different dosage would be required.
Although udenafil improved exercise capacity, nine of 34 patients refused further study due to adverse events, including aggravation of dyspnea. However, these events were generally mild or moderate in severity and none were associated with clinical sequelae. These nine patients showed low initial PASP compared with the patients who completed the study. Thus, it is expected that COPD patients with elevated PASP may have less adverse events from a PDE-5 inhibitor, than patients with low PASP.
There were several limitations in this study. First, this was a small-sized single-arm study, and the results should be confirmed in a large-scale randomized study. Second, right heart catheterization was not performed, and PASP was measured by echocardiography. It is known that the estimation of systolic PAP by echocardiography is frequently inaccurate (Citation5). However, echocardiographic estimation of PAP is noninvasive and correlates with right heart catheterization (Citation14, 15), and echocardiography has replaced right heart catheterization in a National Institutes of Health–sponsored clinical trial (Citation16). Third, blood gas analysis was not performed, and the effect of udenafil on gas exchange could not be analyzed. Instead, we measured oxygen saturation at rest and immediately after the 6MWT, and we monitored the change of arterial oxygenation.
The present study showed improvement of exercise capacity and PAP with a PDE-5 inhibitor in patients with severe COPD. Moreover, oxygen saturation in both the resting state and immediately after 6MWT did not change after udenafil treatment. Some COPD patients with PH may benefit from a PDE-5 inhibitor. However, due to the small sample size, uncontrolled design and high dropout rate, the efficacy of udenafil in severe COPD needs to be confirmed in a large-scale randomized controlled study.
Acknowledgments
Declaration of Interests
All authors have no financial or other potential conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper. Funding by Dong-A PharmTech Co., Ltd.
Role of funding source
This study was an investigator-initiated trial, which was supported by our sponsor. The authors played an important role in the study design, data collection, data analysis, and writing the report. The sponsor provided cost of the study but did not participate in the study design and data analysis.
References
- GOLD. Global strategy for the diagnosis and prevention of COPD. www.goldcopd.com.
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008 Nov; 32(5):1371–85.
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005 Nov 17; 353(20):2148–57.
- Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, Roca J, Barbera JA. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010 Feb 1; 181(3):270–8.
- Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003 Mar 1; 167(5):735–40.
- Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, Tamm M. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008 Sep; 32(3):619–28.
- Barbera JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet 1996 Feb 17; 347(8999):436–40.
- Holverda S, Rietema H, Bogaard HJ, Westerhof N, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Acute effects of sildenafil on exercise pulmonary hemodynamics and capacity in patients with COPD. Pulm Pharmacol Ther 2008; 21(3):558–64.
- Rietema H, Holverda S, Bogaard HJ, Marcus JT, Smit HJ, Westerhof N, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Sildenafil treatment in COPD does not affect stroke volume or exercise capacity. Eur Respir J 2008 Apr; 31(4):759–64.
- Chapman TH, Wilde M, Sheth A, Madden BP. Sildenafil therapy in secondary pulmonary hypertension: Is there benefit in prolonged use? Vascul Pharmacol 2009 Aug-Sep; 51(2–3):90–5.
- Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997 Apr; 155(4):1278–82.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 2008 Sep; 32(3):637–43.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010 Feb; 91(2):221–5.
- Selimovic N, Rundqvist B, Bergh CH, Andersson B, Petersson S, Johansson L, Bech-Hanssen O. Assessment of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2007 Sep; 26(9):927–34.
- Hecht SR, Berger M, Berdoff RL, Van Tosh A, Stimola JM. Use of continuous-wave Doppler ultrasound to evaluate and manage primary pulmonary hypertension. Chest 1986 Nov; 90(5):781–3.
- http://clinicaltrials.gov/ct2/show/NCT00495638. Updated March 5, 2011.