Abstract
Background: Although guidelines recommend monitoring symptoms in patients with chronic obstructive pulmonary disease (COPD), there is limited information on the longitudinal changes in patient-reported dyspnea (PRD) related to activities of daily living. The hypothesis was that PRD scores on the modified Medical Research Council (mMRC) scale, the self-administered computerized (SAC) transition dyspnea index (TDI), and the University of California San Diego Shortness of Breath questionnaire (UCSD SOBQ) would demonstrate progression over two years. Methods: Observational cohort study of symptomatic patients with stable COPD evaluated every 6 months for 2 years. Patients rated the impact of activities of daily living on dyspnea using three patient-reported instruments presented in random order, and then performed post-bronchodilator (pBD) spirometry. Results: Seventy patients (37 female/33 male; age: 66 ± 9 years; and pBD forced expiratory volume in one second [(FEV1): 51 ± 16% predicted] participated. Using fixed effects regression modeling, there was significant worsening in the PRD scores with the SAC TDI (–0.9 ± 2.7; p = 0.03) and UCSD SOBQ (+5.7 ± 18.3; p = 0.001), but not with the mMRC scale (p = 0.52). Both pBD FEV1 (p = 0.19) and pBD forced vital capacity (p = 0.65) were unchanged. Conclusions: Multidimensional instruments (SAC TDI and UCSD SOBQ) demonstrated the frequently observed decline in PRD experienced by patients with COPD. The progression in PRD occurred despite stable lung function. Monitoring PRD provides unique clinical information and should be considered along with measuring lung function to assess patient status over time.
Introduction
Guidelines for chronic obstructive pulmonary disease (COPD) recommend that symptoms be monitored to assess the efficacy of therapy (Citation1–6). In clinical practice, physicians typically query patients to assess qualitative improvement or decline in breathlessness compared with previous visits. However, in clinical trials it is important to quantify the impact of physical activities on the severity of breathlessness using a validated instrument (Citation7–9). Various dyspnea instruments have been used in randomized controlled trials investigating the efficacy of pharmacological therapies for up to one year in patients with COPD (Citation10–13). Of interest, patient-reported dyspnea (PRD) was not included as an outcome measure in the 3-year TORCH and 4-year UPLIFT studies (Citation14, 15). As a result, there is limited information available on the longitudinal changes of breathlessness relative to daily physical activities over an extended period.
To our knowledge, only three studies have described the longitudinal changes in PRD for two or more years. In 1995, we observed a small, but significant deterioration in the severity of dyspnea in a cohort of patients (n = 110 at baseline; n = 76 at 2 years) with symptomatic COPD (Citation16). For the group, the interviewer-administered transition dyspnea index (TDI) total score declined by –0.7 ± 2.9 units (p = 0.04) in patients receiving standard treatment for their COPD (Citation16).
In a randomized controlled trial examining pulmonary rehabilitation, Ries and colleagues (Citation17) reported no significant change in the scores for the University of California San Diego Shortness of Breath questionnaire (UCSD SOBQ) in patients (n = 62 at baseline; n = 31 at two years) receiving education as a control group. In 2006, Oga and colleagues (Citation18) reported small, but significant changes in scores (i.e., worse breathlessness) on the Medical Research Council (MRC) scale (+0.14 ± 0.02/year) and the dyspnea component of the Chronic Respiratory Questionnaire (–0.10 ± 0.02/year) in male patients (n = 137 at baseline; n = 45 at 5 years) with COPD.
These limited and inconsistent results prompted the present study to compare longitudinal changes in dyspnea using three widely used PRD questionnaires in patients with symptomatic COPD. We selected instruments that quantify the impact or burden of breathlessness on activities of daily living according to recommendations that dyspnea be patient-reported, multidimensional where possible, and adhere to standardized methodology (Citation8). These included the modified (m) MRC scale (19), the self-administered computerized (SAC) version of the TDI (Citation20, 21), and the UCSD SOBQ (Citation22). The mMRC scale relates dyspnea to physical tasks, such as walking and dressing, whereas the SAC TDI and the UCSD SOBQ are multidimensional. The hypothesis of this study was that scores on all 3 instruments would demonstrate significant worsening in dyspnea over 2 years.
Methods
This observational cohort study included an initial visit (first patient, first visit: January 2007) and follow-up visits every 6 months for 2 years (last patient, last visit: October 2010). Each visit took place at the same time of day for individual patients. After patients rated their dyspnea on the three instruments which were presented in randomized order, they performed post-bronchodilator spirometry. Changes in maintenance therapy for COPD were permissible throughout the study as directed by each patient's physician.
Subjects
Seventy patients were recruited from the outpatient clinics at Dartmouth-Hitchcock Medical Center, Lebanon, NH. The diagnosis of COPD was based on standard criteria (Citation2). Other inclusion criteria were: age > 50 years; self-reported breathlessness with daily activities; the ability to read and understand English; and clinically stable disease. Exclusion criteria were clinically significant co-morbid disease (e.g., symptomatic coronary artery disease, poorly controlled diabetes mellitus, or any functional impairment) and current substance abuse.
Procedures
The Committee for Protection of Human Subjects at Dartmouth College approved the study, and each patient provided written informed consent.
Modified MRC Scale
The patient read the 5-point mMRC scale presented on a piece of paper and circled the grade (0 –Citation4) that most closely matched his/her breathlessness (Citation19). Higher scores represent more breathlessness.
SAC versions of the BDI/TDI
The SAC versions were presented on a desktop computer. For the BDI (initial visit), the patient selected grades for each of the three components, which were summed to obtain a total score (0 to 12) (20, 21). Lower scores represent more breathlessness. For the TDI (four follow-up visits), the patient reported changes in breathlessness from baseline for each component by adjusting the length of a bar along a bidirectional visual analog scale (Citation20, 21). The three scores were summed and divided by two to obtain a total score (–9 to + 9). A negative score indicates deterioration, whereas a positive score indicates improvement.
University of California San Diego Shortness-of-Breath questionnaire (UCSD SOBQ)
Patients circled a number on a 6-point scale to rate the severity of their breathlessness for each of 24 items (Citation22). The scores were summed to obtain a total score (0 to 120). Higher scores represent more breathlessness.
Lung Function
At each visit the patient performed spirometry using standard equipment (Collins model CPL; Braintree, MA) twenty minutes after inhalation of 2 puffs (180 μg) of albuterol via metered-dose inhaler. Predicted values for spirometry were taken from Morris et al. (Citation23).
Data Analysis
Data are presented as mean ± SD. Values for post-bronchodilator FEV1% predicted were used to grade the stage of COPD (Citation2). Changes in dyspnea scores and lung function over two years were assessed using fixed effects regression modeling (Citation24). Unpaired-t testing was used to investigate any differences in baseline values for those who completed all sessions (n = 48) compared to those who did not complete the final visit (n = 22). Values are presented as mean ± standard deviations. Differences are described by p-values and confidence intervals (CI). Pearson product-moment correlation coefficients were calculated to examine the relationship between changes in selected outcomes.
Results
Seventy patients were tested at the initial visit. There were 37 females and 33 males; age was 66 ± 9 years. Forty-one patients had stage II disease, 20 patients had stage III disease, and 9 patients had stage IV disease based on GOLD criteria (2). At visit 1, maintenance medications used to treat COPD included: muscarinic antagonists = 59; long-acting beta-agonists = 48; inhaled corticosteroids = 48; and theophylline = 8.
At one year, 18 patients had withdrawn from the study; these included six females and 12 males. At two years, one additional male patient withdrew from the study, while three male patients had died. The main reason given by patients for withdrawal was inconvenience due to travel distance/time for follow-up visits. Forty-eight patients completed all visits. Group data at baseline, 1 year, and 2 years are shown in
. There were no statistical differences (p > 0.05) for baseline variables between patients who completed all visits (n = 48) and those patients who withdrew or died (n = 22).
Table 1. Initial and longitudinal data at one and two years
There was a significant decrease in the SAC TDI scores () and a significant increase in the UCSD SOBQ scores () that represent worsening of dyspnea over 2 years in our patients. In contrast, there was no change in the mMRC scale during this period (p = 0.52). Changes in post-bronchodilator FEV1 (p = 0.19) and FVC (p = 0.65)% predicted were not consistent over time. The modest numerical declines in the absolute values for FEV1 and FVC appear related to the greater number of withdrawals and deaths of male participants.
Figure 1. Mean and standard error values for the total scores of the self-administered computerized version of the transition dyspnea index (SAC TDI) at six month intervals in patients with COPD. The SAC TDI represents changes in dyspnea scores compared with the Baseline Dyspnea Index at the initial or baseline visit. Using fixed effects regression modeling, there was a significant decline in the SAC TDI scores over 2-years (p = 0.03). The time in years and the number of participants at each visit are shown at the bottom of the figure.
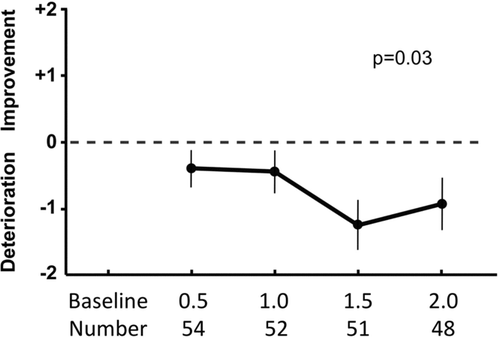
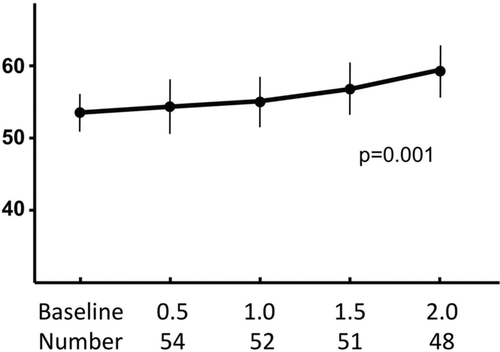
Figure 2. Mean and standard error values for the total scores of the University of California San Diego Shortness of Breath questionnaire (UCSD SOBQ) at baseline and at 6-month intervals in patients with COPD. Using fixed effects regression modeling, there was a significant increase (worse dyspnea) in the UCSD SOBQ scores over 2-years (p = 0.001). The time in years and the number of participants at each visit are shown at the bottom of the figure.
At 2 years, the correlation coefficient between the SAC TDI scores and changes in UCSD SOBQ scores was –0.31 (p = 0.009). There were no significant correlations among changes in dyspnea scores (SAC TDI and UCSD SOBQ) and changes in lung function (FEV1 and FVC) over 2 years.
Discussion
The results of this 2-year longitudinal study in a heterogenous group of symptomatic patients with COPD and no clinical evidence of co-morbidity at baseline demonstrate:
1) modest but significant deterioration in PRD as measured with two multidimensional instruments –SAC TDI and UCSD SOBQ; 2) no change in PRD on the unidimensional mMRC scale; and 3) lung function was generally stable over the 2 years.
Baseline data in groups who completed all visits (n = 48) and those patients who withdrew or died (n = 22) were similar. Our longitudinal analyses included all available data for each patient until the point at which the participant was no longer available for study. For example, data from a patient who died at 22 months after entering the study were analyzed at time periods 0, 6, 12, and 18 months. Fixed effect regression modeling was used as our previous findings showed that changes in dyspnea (using the interviewer-administered TDI) were correlated with independent variables, such as lung function and components of general health (Citation16).
Based on our current understanding of the different domains of dyspnea, the mMRC, SAC TDI, and UCSD SOBQ quantify the impact of activities of daily living and functional capacity on PRD (Citation25). The three questionnaires were selected because they have been used widely in clinical trials examining various interventions in patients with COPD (Citation10, Citation26–31). Scores with both multidimensional dyspnea instruments, the SAC TDI and the UCSD SOBQ, demonstrated deterioration in the severity of dyspnea over two years in our patients. The SAC TDI score (–0.9 ± 2.7 units) at 2-years was close to our previous change of –0.7 ± 2.9 units observed using the interviewer-administered TDI in a longitudinal study of patients with COPD who had similar baseline characteristics (Citation16). The mean value for the SAC TDI (–0.9) approximates the one unit change that represents the minimal clinically important difference (MCID) (Citation32, 33).
The change in the UCSD SOBQ (+5.7 ± 18.3 units) in our patients was statistically significant and the mean value exceeds the MCID of 5 units (Citation34). The UCSD SOBQ was developed and has been used primarily in exercise and pulmonary rehabilitation trials (Citation17, Citation35–37) with use in one pharmaceutical study (Citation38).
There was no significant change in the mMRC scale over 2 years in our patients. These findings contrast with the observations of Oga and colleagues (Citation18) who reported that scores on the MRC scale increased significantly (0.14 ± 0.02 units/year) in 45 male patients with COPD over 5-years. These discordant findings may be due to differences in baseline patient characteristics, concomitant medications, and/or study durations. In general, the MRC scale is considered an excellent discriminative instrument (i.e., it differentiates between people who have less dyspnea and those that have more dyspnea) (Citation7). The mMRC is one of four components of the multidimensional BODE index (Citation27) and has been incorporated as a metric to help guide treatment for symptomatic patients with COPD (Citation6). However, the mMRC scale is a poor evaluative instrument (i.e., how dyspnea changes in response to therapy) because it relates exclusively to performance of physical tasks, and it five grades are quite broad (Citation20, Citation39).
The overall mean changes in the SAC TDI and the UCSD SOBQ are consistent with the clinical observation that many patients with COPD report that their breathlessness with physical activities progresses over time. However, the large standard deviations for these scores throughout the study indicate considerable variability in individual responses as has been observed in previous longitudinal studies (Citation16–18).
An accelerated decline in FEV1 has been considered a classic feature of the natural history of patients with COPD for decades (Citation40). However, our patients with COPD exhibited stable post-bronchodilator FEV1 and FVC values over 2-years. Our results are similar to those observed in a 2-year study in which lung function tests were performed without control for bronchodilator use on the day of testing (Citation16). More recent longitudinal studies have shown that the rate of change in FEV1 is highly variable among patients with COPD (Citation41, 42). For example, Casanova and colleagues (Citation41) reported that only 18% of 1,198 patients with COPD had a significant FEV1 slope decline over a median duration of 64 months, although testing was not controlled for bronchodilator use on study visits. Vestbo and colleagues (Citation42) also reported considerable individual variability for changes in post-bronchodilator FEV1 over 3 years among 2,163 patients in the COPD ECLIPSE cohort study. Although smoking status, emphysema phenotype, and bronchodilator reversibility are factors associated with increased rates of decline in FEV1, the use of inhaled long-acting medications may alter the progression of airflow obstruction (Citation43, 44).
There are some limitations of our study. First, the number of patients was modest. However, complete data were available in 48 patients throughout our study, whereas 31 and 45 patients completed testing in two other longitudinal studies examining PRD as an outcome measure (Citation17, 18). Second, our drop-out rate was 31% at 2 years. This number compares favorably with the drop-out rates of 31% and 50% in other 2-year longitudinal studies (Citation16, 17) and of 67% at 5-years (Citation18). Third, we did not include or consider treatment effect in our analysis. This approach is supported by Vestbo and colleagues (Citation42) who noted that the effects of treatment are likely to be confounded by biases that are characteristic of observational pharmacoepidemiologic studies. Fourth, we did not collect information during the study on the number of exacerbations experienced by our patients with COPD. However, no patient withdrew from the study because of an exacerbation, and all patients were stable clinically at each study visit. Finally, we did not include a comparison group of patients who had breathlessness with daily activities due to a different chronic respiratory disease, such as interstitial lung disease.
What are the clinical implications of our study? Our results illustrate that progression of COPD may be defined not only by the traditional decline in FEV1, but also by the change in PRD observed in our study. Any worsening in dyspnea is quite important to the individual patient with COPD. When assessing patients who complain of an increase in breathing difficulty, health care providers should not only consider possible deterioration in lung function, but also whether co-morbidities (anemia and heart disease), weight gain, deconditioning, and/or psychological problems (anxiety and depression) may be contributing to dyspnea. Our findings raise the question whether PRD should be measured routinely in clinical practice in order to quantify this important outcome. For example, the SAC BDI/TDI, and UCSD SOBQ quantify the impact or burden of dyspnea on activities of daily living, whereas the sensory-perceptual experience (intensity) and affective distress (unpleasantnesss) are other important domains of dyspnea measurement (Citation25).
Conclusions
Our results demonstrate that scores on the multidimensional SAC TDI and UCSD SOBQ reflect worsening of dyspnea related to daily activities as frequently reported by patients with COPD. In contrast, the unidimensional mMRC scale did not show a change. Progression in PRD in our patients occurred despite stable lung function. Various COPD guidelines as well as expert opinion recommend that symptoms be monitored, and that decisions on therapy should be guided by the severity of symptoms (Citation3, 4, Citation6, Citation9, Citation45). Both the SAC TDI and the UCSD SOBQ have been shown to be responsive to various interventions (Citation17, Citation20, Citation46, 47). Although the mMRC scale is excellent for discriminative purposes, the five broad grades limit its ability to detect change (Citation20, Citation39). These collective data suggest that it is reasonable to quantify changes in PRD with a multidimensional instrument, such as the BDI/TDI or the UCSD SOBQ. This information complements the more routine measurement of lung function to assess the clinical status of a patient.
Declaration of Interest
This work was supported by the National Institutes of Health Grant R44 HL076888-02, SBIR; Principal investigator: John C. Baird, Ph.D. Donald A. Mahler, Joseph Ward, Laurie A. Waterman, have no actual or potential conflict of interest. John C. Baird was the Scientific Director for Psychological Applications, LLC, which owns the copyright of the self-administered computerized versions of the baseline and transition dyspnea indexes. Professor Baird died on June 8, 2011.
References
- van den Bemt L, Schermer T, van Weel C. Rational monitoring of COPD: where do current clinical guidelines stand? Eur Respir J 2007; 29(6):1078–1081.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555.
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946.
- O'Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk DD, Balter M, Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease Û 2007 update. Can Respir J 2007; 14 Suppl B:5B–32B.
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155(3):179–191.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Revised 2011. At: www.goldcopd.com [accessed 1 March 2012].
- Mahler DA. Measurement of Dyspnea: Clinical Ratings. In: Mahler DA, O'Donnell DE, eds. Dyspnea: Mechanisms, Measurement, and Management. 2nd ed. Boca Raton, FL: Taylor & Francis; 2005: 147–165.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31(2):416–469.
- Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J 2006; 27(4):822–832.
- Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19(2):217–224.
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J 2003; 22(6):912–919.
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21(1):74–81.
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010; 65(6):473–479.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356(8):775–789.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359(15):1543–1554.
- Mahler DA, Tomlinson D, Olmstead EM, Tosteson AN, O'Connor GT. Changes in dyspnea, health status, and lung function in chronic airway disease. Am J Respir Crit Care Med 1995; 151(1):61–65.
- Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995; 122(11):823–832.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Longitudinal deteriorations in patient reported outcomes in patients with COPD. Respir Med 2007;101(1):146–153.
- Brooks SM. Task group on surveillance for respiratory hazards in the occupational setting. ATS News. 1982; 8:12–16.
- Mahler DA, Waterman LA, Ward J, McCusker C, ZuWallack R, Baird JC. Validity and responsiveness of the self-administered computerized versions of the baseline and transition dyspnea indexes. Chest 2007; 132(4):1283–1290.
- Mahler DA, Ward J, Fierro-Carrion G, Waterman L, Lentine TF, Mejia-Alfaro R, Development of self-administered versions of the modified baseline and transition dyspnea indexes in COPD. J COPD 2004; 1:165–172.
- Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998; 113(3):619–624.
- Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 1971; 103(1):57–67.
- Diggle PJ, Heagerty P, Liang K, Zeger SL, eds. Analysis of Longitudinal Data. 2nd ed. Oxford University Press New York; 2002.
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med 2012; 185(4):435–452.
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166(8):1084–1091.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012.
- Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010; 182(2):155–162.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374(9691):695–703.
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest 2003; 124(3):834–843.
- Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J 2005; 26(4):630–636.
- Witek TJ, Jr, Mahler DA. Meaningful effect size and patterns of response of the transition dyspnea index. J Clin Epidemiol 2003; 56(3):248–255.
- Mahler DA, Witek TJ, Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD 2005; 2(1):99–103.
- Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD 2005;2(1):105–110.
- Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med 2003; 67(6):880–888.
- California Pulmonary Rehabilitation Collaborative Group. Effects of pulmonary rehabilitation on dyspnea quality o flife and health care costs in California. J Cardiopulm Rehab 2004; 24:52–62.
- Stulbarg MS, Carrieri-Kohlman V, Demir-Deviren S, Nguyen HQ, Adams L, Tsang AH, Exercise training improves outcomes of a dyspnea self-management program. J Cardiopulm Rehabil 2002; 22(2):109–121.
- Criner GJ, Sharafkhaneh A, Player R, Conoscenti CS, Johnson P, Keyser MT, Efficacy of tiotropium inhalation powder in african-american patients with chronic obstructive pulmonary disease. COPD 2008; 5(1):35–41.
- de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest 2002; 121(4):1092–1098.
- Fletcher C, Peto R. Natural history of chronic respiratory tract obstruction. Bull Int Union Tuberc 1978; 53(2):79–87.
- Casanova C, de Torres JP, Aguirre-Jaime A, Pinto-Plata V, Marin JM, Cordoba E, The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med 2011; 184(9):1015–1021.
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011; 365(13):1184–1192.
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008; 178(4):332–338.
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP, Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374(9696):1171–1178.
- Barnes PJ, Chowdhury B, Kharitonov SA, Magnussen H, Page CP, Postma D, Pulmonary biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 174(1):6–14.
- Tashkin DP, Littner M, Andrews CP, Tomlinson L, Rinehart M, Denis-Mize K. Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trial. Respir Med 2008; 102(4):479–487.
- Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006; 354(25):2655–2666.