Abstract
COPD is a leading chronic disease, increasing globally. Given this condition's irreversible and progressive nature, health-related quality of life (HRQOL) is increasingly a primary end-point in COPD management. We evaluated several HRQOL tools with a primary goals of (1) investigating how the generic Assessment Quality of Life (AQOL) functions compared to the Medical Outcomes Study 36-item Short Form Health Survey (SF36) and the St. Georges Respiratory Questionnaire (SGRQ); and (2) considering the extent to which clinical disease severity, as measured by the BODE index, predicts variation in HRQOL reports. Methods: 134 consecutive patients entering a pulmonary rehabilitation program were recruited. Participants completed two generic measures of HRQOL (SF36 and AQOL) and one disease specific measure (SGRQ). The clinical severity of COPD was assessed using a composite global COPD severity score, BODE. Results: Significant associations were demonstrated between AQOL and both the SF36 (r = .68) and SGRQ (r = –.60). BODE significantly predicted AQOL scores (R = –.31); mMRC (R = –.36) and 6MWD (R = .39) were stronger contributors to these predictions than were FEV1 or BMI. Conclusions: This study establishes convergent validity between AQOL, and the SF36 and SGRQ in patients with COPD. For future studies wishing to examine HRQOL from a generic perspective, we have shown that during cross-sectional analyses AQOL performs similarly to the SF36. In addition we identified that the clinical severity of COPD, as assessed by BODE, significantly influences reports of quality of life made using AQOL. The components of BODE that most strongly contributed to predicting HRQOL were dsypnea and exercise tolerance.
Introduction
Chronic obstructive pulmonary disease (COPD) is projected to become the third-leading cause of death worldwide by 2020 (Citation1). However, even this projection fails to fully represent the prolonged societal burden of COPD that occurs due to the disease's slowly-progressive time course. Affected patients usually experience many years of disability, daily discomfort with breathlessness and fatigue, and impaired quality of life.
Health-Related Quality of Life (HRQOL) is a multi-dimensional concept that includes an evaluation of physical, emotional and social well-being (Citation2). Because COPD is irreversible and progressive, HRQOL is increasingly coming to be considered one of the primary end-points of COPD management. The debilitating physical and emotional symptoms of COPD are known to have a negative impact on HRQOL (Citation3). Furthermore, HRQOL has been found to be an independent predictor of mortality, hospitalizations and outpatient health-care utilization amongst COPD patients (Citation4).
Currently the relationship between the physiologic severity of COPD and HRQOL is being established. Although lung function is often used as an index of COPD severity (Citation5), it is not necessarily linked to symptoms, disability or HRQOL; indeed, HRQOL appears more closely linked to the burden of respiratory symptoms than to lung function (Citation6). Recent research in COPD has found that a new prognostic tool that includes body mass index, bronchial obstruction, dyspnea and exercise (BODE index) is significantly associated with HRQOL (Citation7).
HRQOL measurement can be undertaken from either a generic or disease-specific standpoint. Using a generic tool enables patient well being in any disease state to be determined. Using generic tools it is feasible to compare the health status of populations with different diseases, and to explore utility (in terms of the years of life lived with disability). The most widely used generic HRQOL questionnaire in COPD research is the Medical Outcomes Short-Form SF36 (SF36, Citation8). The SF36 has been used in COPD both as a cross-sectional measure of health status and as an outcome measure for evaluating benefit and cost-utility of interventions (Citation9).
Disease-specific HRQOL measures for respiratory conditions include the St. George's Respiratory Questionnaire (SGRQ, Citation10) and the Chronic Respiratory Questionnaire (CRQ, Citation11). These instruments have demonstrated superior sensitivity and responsiveness in comparison with generic HRQOL tools when evaluating change after interventions such as pulmonary rehabilitation (Citation12, 13). Given their differing merits, there are benefits to using both generic and disease-specific tools in COPD research and management (Citation14).
The Assessment of Quality of Life (AQOL), like the SF36, has been validated for measuring generic HRQOL. It is a self-report questionnaire developed using Australian samples (Citation15), and has been used to examine HRQOL in a variety of clinical settings. These include following stroke (Citation16); joint replacement (Citation17); in those with depression (Citation18); and in community-dwelling elderly (Citation19, 20). The AQOL has also been used to examine HRQOL in non-small cell lung cancer patients and patients with pulmonary arterial hypertension (Citation21, 22), but it has not to date been applied to patients with COPD. In this study, our primary goal was to investigate how the generic measure, AQOL, functions in the setting of COPD compared to another generic measure (SF36) and a widely used disease specific measure (SGRQ). In this study we investigated both the convergent validity of AQOL and the extent to which measures of COPD disease severity predict AQOL scores.
We hypothesized that AQOL would be positively correlated with SF36 scores (where higher scores indicate better HRQOL) and negatively correlated with SGRQ scores (where higher scores indicate worse HRQOL). We hypothesized that AQOL would show a stronger correlation with the other generic measure of HRQOL, SF36, than with the disease-specific measure, SGRQ. We further hypothesized that those with more severe disease would have lower AQOL scores (worse HRQOL), and expected this effect to be present either when disease severity was represented using a composite global COPD severity score or when each of the four components of that global score were examined individually.
Method
The current study analyzed data from a consecutive series (Nov 2007 –Oct 2008) of COPD patients who were attending assessment clinics prior to entry to a pulmonary rehabilitation program at the Repatriation General Hospital, Adelaide, South Australia. Eighty-five of the patients met GOLD criteria for COPD. The remaining 33 patients had an FEV1/FVC>0.70, these patients had other chronic respiratory conditions including interstitial lung diseases and bronchiectasis.
The study was approved by the Southern Adelaide Health Service/Flinders University Human Research Ethics Committee. All patients attending the program were asked to consent to their data being used for a range of research purposes when completing their baseline questionnaires, and data collected were entered into an Excel spreadsheet. Anonymised data were extracted from this spreadsheet for the statistical analyses.
Quality-of-Life measures
AQOL is a utility measure of HRQOL (Citation15), consisting of 15 items which assess five dimensions: illness, independent living, social relationships, physical senses, and psychological well-being. Each item is rated on a 4-point scale, and utility weightings are applied to each item to make the resulting score suitable for calculation of quality adjusted life years and therefore useful for cost-utility analyses. The minimum AQOL score (–0.04) represents the worst possible HRQOL (health state worse than death). The maximum score (1.00) represents full HRQOL.
The Medical Outcomes Study 36-item Short Form Health Survey (SF36) questionnaire is a generic measure of HRQOL (Citation8), which, like the AQOL, has capacity for utility score generation. Mental and physical subscale scores were generated for the current analysis. The range of these scores is 0–100, with higher scores indicating better HRQOL.
The St. George Respiratory Questionnaire (SGRQ) is a COPD-specific measure of health-related quality of life (Citation10). This measure consists of 11 items rated on four or five point rating scales and 40 items rated on dichotomous scores. Items are summed to give three subscale scores (each with a range of 0 to 100) - Symptoms (distress due to respiratory symptoms), Activity (disturbance of physical activity), and Impacts (overall impact on daily life and well being). SGRQ total score is calculated from the average of the three subscales. The range of the total score is 0–100. Higher scores indicate worse HRQOL.
Measures of COPD clinical status
Patient height (meters) and weight (kilograms) were measured using stadiometer and scales, with the patient clothed but without shoes. Body mass index (BMI) was calculated as weight divided by squared height (kg/m2).
Subjective breathlessness was assessed using the modified MRC Dyspnea Scale (mMRC, Citation23). This assesses limitation of activities due to dyspnea on a 5-point scale with descriptive anchors ranging from 0 (Not troubled by breathlessness except on strenuous exercise) to 4 (Too breathless to leave the house, or breathless when dressing or undressing).
Forced expiratory volume in one second (FEV1) in liters per second and as a percentage of values predicted using the NHANES-III equations (% pred) was obtained from spirometry performed as part of baseline assessments pre-rehabilitation. The methodology used and result analyses met the ERS guidelines for standardization of spirometry (Citation24). The best FEV1 obtained following bronchodilator administration was used in the analyses for this study.
Exercise capacity at baseline was assessed using standardized six-minute walk distance (6MWD) test, carried out according to ATS instructions and scores were recorded as meters walked in 6 minutes (Citation25).
BODE scores (Citation26) were calculated by categorizing participants’ BMI as 0 (≥21.1) or 1(≤21); their FEV1%pred values were scored as: ≥ 65 = 0, 64-50 = 1, 49-36 = 2 and ≤35 = 3; raw scores (0 to 4) for mMRC were used. Participants’ 6MWDs (meters) were categorized as ≥350 m = 0; 349–250 m = 1; 249-150 m = 2; and ≤149 m = 3. Following these categorizations, component scores were summed to give a BODE index score (range 0–10, 10 indicating maximal functional impairment).
Statistical analyses
All statistical analyses were undertaken using SPSS version 17.1. After assessing the normality of the score distributions, Pearson's correlations were used to examine the strength of association between AQOL, SGRQ, and SF36 scores. Linear regressions were used to examined the ability of each BODE component to predict AQOL, and then to examine the ability of BODE index scores to predict AQOL. The above series of regressions was then repeated with the SGRQ and SF36 entered as the dependent variable of each model.
Prior to entry in to the regressions, both the predictor and dependent variables were standardized. An a priori decision was made to control for age and sex. These covariates, however, did not strongly influence the Beta weights identified for each measure of HRQOL (). ⊗R2 examines the proportion of variance explained during each univariate analysis. All analyses described here were conducted with the complete sample, and then with only those patients who met the GOLD criteria for COPD. The results were stable under both conditions; the results presented here are from analyses involving the complete sample.
Table 1. Patient characteristics
Table 2. Correlations between AQOL and other quality-of-life measures
Table 3. BODE and BODE Component Scores as Predictors of HRQOL
Results
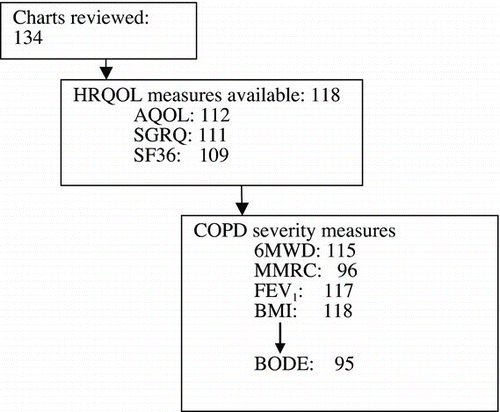
Of 134 consecutive patient charts reviewed, 118 had data available relating to at least one of the HRQOL measures of interest (). One hundred and one patients had completed all three questionnaires, 12 patients had completed two of the questionnaires, and 5 patients had completed only one of the questionnaires. The patient characteristics are described in .
The relationships between AQOL total score and the subscales of SF36 were moderately strong (), with the weakest relationship seen between AQOL and the general health subscale. Composite scores in SF36 for both mental and physical health (SF36 MCS and PCS respectively) showed moderate to strong relationships with the AQOL total score (). R2 values of 0.68 for the relationships between both SF36 composite scores and the AQOL total score indicate a shared variance of nearly 50% ().
Generic HRQOL scores (AQOL, SF36 MCS and SF36 PCS) correlated strongly with the Impacts subscale of SGRQ (). The SF36 PCS also demonstrated a strong correlation with SGRQ Activities subscale. There was only a small correlation between SGRQ Symptom score and AQOL.
Models examining the BODE index components individually showed that both mMRC score and 6MWD predicted total AQOL score (p<0.001, ). Each standard deviation of change in either mMRC score or 6MWD (either a 1.2 unit change in mMRC or a 121 m change in 6MWD) was associated with approximately one-third of a standard deviation change in AQOL score (). Neither BMI nor FEV1 significantly predicted AQOL score.
BODE component scores showed similar abilities to predict scores on the other HRQOL measures examined (SGRQ and SF36, ). In addition to significant predictive effects for both mMRC score and 6MWD, FEV1 also significantly predicted SGRQ (p<0.02). BMI showed a statistically significant ability to predict SF36-PCS (p<0.05), however the ⊗R2 associated with this effect (0.03) suggests it is unlikely to be of clinical importance.
BODE index score predicted AQOL score (p < 0.01, ), with every 2.3 unit increase in BODE index score (i.e., higher functional severity of COPD) associated with approximately a 1 unit decrease in AQOL score (i.e. decrease in HRQOL). This relationship (Beta = –0.306) is somewhat less than that identified between BODE and SF36 PCS (Beta = –0.375), although slightly stronger than the relationship identified between BODE and SF36 MCS (Beta = –0.251). BODE status had a stronger ability to predict SGRQ scores (Beta = 0.509) than scores on either of the generic measures of HRQOL.
In terms of the total variance explained, BODE score explained 10% of the variance in AQOL score (). As expected from the Beta weights reported above, this is greater than R2 when BODE was used to predict SF36 MCS (⊗R2 = 0.05) and slightly weaker than that seen for SF36 PCS (⊗R2 = 0.14). BODE score explained a greater proportion (⊗R2 = 0.27) of the variance in total SGRQ score. The same R2 pattern was found when mMRC and 6MWD scores were used to predict HRQOL (). However an unanticipated finding was that the BODE index did not explain a greater portion of the variance in any HRQOL score than either mMRC or 6MWD had achieved when used as univariate predictors.
Discussion
Our results support the convergent validity of AQOL as a generic measure of HRQOL in COPD patients as well as patients with other respiratory disorders. The strength of correlations between AQOL, SF36, and SGRQ supports the notion that AQOL functions as a generic measure of HRQOL. While the AQOL was developed and validated in Australian populations, no features of the questionnaire make it specific to Australians to preclude its use in other populations. Our results also establish that AQOL scores are systematically related to the clinical severity of COPD, as measured by the composite global severity (BODE) score. These cross-sectional findings support the utility of AQOL as a measure of generic HRQOL in COPD research.
The average HRQOL reported by our sample was substantially reduced when compared to the age-matched AQOL norm values reported by Hawthorne and Osborne (2004). This finding adds to the growing body of data documenting the negative life impacts associated with COPD (Citation27). The generic nature of the AQOL allows for comparison across populations. The level of HRQOL reported by our COPD patients was similar to that reported by patients with Major Depression (Citation18), better than stroke patients (Citation16), and worse than patients with influenza (Citation28).
In settings including osteoarthritis, acute stroke, and community-dwelling older adults, the AQOL, like the SF36, has been shown to function as a generic measure of HRQOL (Citation19, Citation29, Citation30). In our sample of COPD patients, the strength of the correlation we observed between AQOL and SF36 scores (.68) further supports the convergent validity of AQOL as a generic measure of HRQOL. The observation that, of the three SGRQ subscales, AQOL showed the weakest correlation (–.26) with the most disease-specific SGRQ subscale (symptoms subscale) also supports the notion that AQOL functions primarily as a generic measure of HRQOL.
BODE, a prognostic tool which includes body mass index, bronchial obstruction, dyspnea and exercise, has been found to be significantly associated with HRQOL (Citation7, Citation31). In their recent study examining the relationship between BODE and SGRQ scores, Medinas-Amoros et al categorized their BODE data, and found that higher BODE quartiles were generally associated with worse HRQOL. We retained the continuous nature of BODE scores and examined the ability of BODE scores to predict HRQOL scores. This approach allowed us to compare the magnitude of effect identified when disease-specific, versus generic measures of HRQOL were used. As expected, the effect size was largest for the disease-specific measure, SGRQ. However, BODE also significantly predicted HRQOL assessments made using generic tools. The relationship between BODE and AQOL was of a similar magnitude to the relationship between BODE and SF36 scores.
Dissecting the BODE index and examining each of its components separately provided further detail. Dyspnea and functional impairment (six-minute walk distance) showed the strongest abilities to predict AQOL scores. Relationships between dyspnea and quality of life have been identified across multiple clinical settings, including COPD and congestive heart failure (Citation32–34). Using AQOL to assess HRQOL, we found that dyspnea explained 11% of the variance in the HRQOL scores. This effect size is similar to that reported by Hu and Meek when SF36 subscales were used as the outcome of interest (Citation35).
Using both generic and disease-specific measures, significant correlations have been demonstrated between exercise tolerance/intolerance and HRQOL (Citation33, 34). Our results show that 6MWD predicts AQOL and SF36-MCS scores to a similar extent. The magnitude of these effects is however less than that seen when 6MWD is used to predict SF36-PCS or SGRQ scores. Compared to AQOL items, a greater proportion SF36-PCS and the SGRQ items are oriented towards capturing HRQOL as represented by abilities to be physically active.
Compared to patients whose weight was in the normal range, Katsura et al (Citation34) found that underweight patients tended to report greater impairment in SGRQ scores. In our data BMI did not have a clinically significance ability to predict scores on any of the HRQOL measures examined, although this analysis was limited by the paucity of participants (19 or 16% of participants) with BMI equal to or less than 21, who may be considered approaching cachexia.
Finally, the relationship between HRQOL and FEV1 is much contested in existing literature. Some studies support a weak or absent relationship (for example, Citation36), while other results have identified a moderate association (for example, Citation37, 38). In our data FEV1 did not predict AQOL scores. One explanation put forward to account for this lack of relationship, and for the lack of strength in relationships noted between clinical indicators of COPD severity and HRQOL generally, is that HRQOL reports are a consequence of interactions between multiple physical, psychological and social factors (Citation10), and therefore do not map simply onto observations of single physiologic predictors.
Representing disease severity using multivariable index scores begins the process of trying to represent elements of the complexity described above. However, in contrast to the setting of mortality predictions (the context in which the BODE index was originally derived), the process of categorization followed by summation used to generate BODE scores did not improve our ability to predict HRQOL; in our data mMRC and 6MWD scores individually had similar power to the summed BODE index to predict HRQOL. Although the BODE index is an advance in describing COPD global severity beyond FEV1, an important direction for future research will be to improve the ability of global measures to capture and model the interacting predictors that influence HRQOL in COPD.
A limitation of the current work is that the nature of the study population may have biased the findings. Our participants were considered to require pulmonary rehabilitation because of symptoms, yet were also considered to be functionally capable of achieving benefit from exercise training. A sample with different characteristics, particularly more severe disease, may have yielded different results. However, the stability of the current findings when COPD only or more generalized chronic respiratory patients (the complete sample) were included supports the robustness of the current findings. In addition, while this work supports the convergent validity of the AQOL as a generic measure of HRQL, the important question of whether this utility measure is sensitive enough to capture HRQOL change induced by COPD interventions remains unanswered. Establishing the answer to this question represents one of the most important directions for future research using AQOL in COPD.
The current study represents a first step in the process of establishing that AQOL, a generic measure of HRQOL, can be applied to investigations of HRQOL in COPD patients. This observation has relevance to situations where COPD populations are being compared to populations with other chronic diseases, or when the utility and cost-effectiveness of specific interventions is being evaluated. We have shown that the clinical index of disease severity, BODE, significantly predicts HRQOL assessments made using AQOL.
The components of this index that most strongly contributed to predicting HRQOL were dsypnea and exercise tolerance. SGRQ scores provided HRQOL scores that were more strongly predicted by BODE status than either of the generic HRQOL tools used in this analysis. For future studies wishing to examine HRQOL from a generic perspective, we have shown that AQOL performs similarly to the SF36 in a cross-sectional setting. An important direction for future research is to examine AQOL's responsiveness to change with treatment interventions.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Murray CL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet 1997;249(9064):1498–504.
- Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med 1993;118(8):622–9.
- Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Euro Respir J 2007;30:993–1013.
- Sprenkle MD, Niewoehner DE, Nelson DB, Nichol KL. The Veterans’ Short Form 36 questionnaire is predictive of mortality and health-care utilization in a population of veterans with a self-reported diagnosis of asthma or COPD. Chest 2004;126(1):81–9.
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease 2010 [cited August 2011]; Available from: http://www.goldcopd.org.
- Voll-Aanerud M, Eagan TML, Plana E, Omenaas ER, Bakke PS, Svanes C, Respiratory symptoms in adults are related to impaired quality of life, regardless of asthma and COPD: results from the European community respiratory health survey. Health Qual Life Outcomes 2010;8:107–15.
- Medinas-Amoros M, Mas-Tous C, Renom-Sotorra F, Rubi-Ponseti M, Centeno-Flores MJ, Gorriz-Dolz MT. Health-related quality of life is associated with COPD severity: a comparison between the GOLD staging and the BODE index. Chronic Respir Dis 2009;6:75–80.
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). Med Care 1992;30(6):473–83.
- Griffiths TL, Phillips CJ, Davies S, Burr ML, Campbell IA. Cost effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programme. Thorax 2001;56:779–84.
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991;85(Suppl B):25–31.
- Williams JEA, Singh SJ, Sewell L, Guyatt GH, Morgan MDL. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR). Thorax 2001;56:954–9.
- Ringbaek T, Brondum E, Martinez G, Lange P. EuroQoL in assessment of the effect of pulmonary rehabilitation COPD patients. Respir Med 2008;102(11):1563–7.
- Puhan MA, Behnke M, Laschke M, Lichtenschopf A, Brandli O, Guyatt GH, Self-Administration and Standardisation of the Chronic Respiratory Questionnaire: A Randomised Trial in Three German-Speaking Countries. Respir Med 2004;98(4):342–50.
- Engstrom CP, Persson LO, Larsson S, Sullivan M. Health related quality of life in COPD: why both disease-specific and generic measures should be used. Euro Respir J 2001;18(1):69–76.
- Hawthorne G, Richarson J, Osborne RH. The Assessment of Quality of Life (AQoL) instrument: A psychometric measure of health related quality of life. Qual Life Res 1999;8:209–24.
- Sturm JW, Osborne RH, Dewey HM, Donnan GA, Macdonell RA, Thrift AG. Brief comprehensive quality of life assessment after stroke: The assessment of quality of life instrument in the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2002;33(12):2888–94.
- Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg 2008;16(1):58–65.
- Hawthorne G, Cheok F, Goldney R, Fisher L. The excess cost of depression in South Australia: A comparative study of two methods of calculating burden. Austral New Zeal J Psych 2003;37(3):362–73.
- Osborne RH, Hawthorne G, Lew EA, Gray LC. Quality of life assessments in the community-dwelling elderly: Validation of the Assessment of Quality of Life (AQoL) instrument and comparison with the SF-36. J Clin Epidemiol 2003;56(2):138–47.
- Holland R, Smith R, Harvey I, Swift L, Lenaghan E. Assessing quality of life in the elderly: A direct comparison of the EQ-50 and AQoL. Health Econ 2004;13(8):793–805.
- Manser RL, Wright G, Byrnes G, Hart D, Conron M, Carter R, Validity of the Assessment of Quality of Life (AQoL) utility instrument in patients with operable and inoperable lung cancer. Lung Cancer 2006;53:217–29.
- Chen H, Taichman DB, Doyle RL. Health-related quality of life and patient-reported outcomes in pulmonary arterial hypertension. Proc Amer Thorac Soc. 2008;5:623–630.
- Brooks SM. Surveillance for respiratory hazards. ATS News 1982;8:12–16.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Standardisation of spirometry. Euro Respir J 2005;26:319–38.
- Crapo RO, Casaburi R, Coates AL, Enright PL, Mac Intyre NR, McKay RT, ATS Statement: Guidelines for the Six-Minute Walk Test. Amer J Respir Crit Care Med 2002;166:111–7.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. New Engl J Med 2004;350:1005–12.
- Tkac J, Man SF, Sin DD. Systemic consequences of COPD. Therapeut Advan Respir Dis 2007;1:47–59.
- Osborne RH, Hawthorne G, Papanicolaou M, Wegmueller Y. Measurement of rapid changes in health outcomes in people with influenza symptoms. J Outcomes Res 2000;4:15–30.
- Whitfield K, Buchbinder R, Segal L, Osborne RH. Parsimonious and efficient assessment of health-related quality of life in osteoarthritis research: validation of the Assessment of Quality of Life (AQoL) instrument. Health Qual Life Outcomes 2006;4:19–29.
- Mortimer D, Segal L, Sturm J. Can we derive an ‘exchange rate’ between descriptive and preference-based outcome measures for stroke? Results from the transfer to utility (TTU) technique. Health Qual Life Outcomes 2009;7: 33–52.
- Lin Y, Xu W, Liang L, Pang B, Nie X, Zhang J, The cross-sectional and longitudinal association of the BODE index with quality of life in patients with chronic obstructive pulmonary disease. Chin Med J 2009;122(24):2939–44.
- Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients’ perceptions of the effects of heart failure on their quality of life. J Cardiac Failure 2006;2:87–92.
- Gonzalez E, Herrejon A, Inchaurraga I, Blanquer R. Determinants of health-related quality of life in patients with pulmonary emphysema. Respir Med 2005;99:638–44.
- Katsura H, Yamada K, Kida K. Both generic and disease specific health-related quality of life are deteriorated in patients with underweight COPD. Respir Med 2005;99:624–30.
- Hu J, Meek P. Health-related quality of life in individuals with chronic obstructive pulmonary disease. Heart Lung 2005;34:415–22.
- Wijnhoven HA, Kriegsman DM, Hesselink AE, Penninx BW, de Haan M. Determinants of different dimensions of disease severity in asthma and COPD: pulmonary function and health-related quality of life. Chest 2001;119:1034–1042.
- Ferrer M, Alonso J, Morera J, Marrades RM, Khalaf A, Agua MC, Chronic obstructive pulmonary disease stage and health-related quality of life. Ann Intern Med 1997;127:1072–9.
- Spencer S, Calverley PM, Sherwood BP, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2001;163:122–8.