Abstract
Background: Arterial rigidity and endothelial dysfunction are systemic manifestations of chronic obstructive pulmonary disease (COPD). The decrease in renal vascular resistance in order to adapt the increase in glomerular filtration rate after oral protein loading is known as normal renal functional reserve. We tested the hypothesis that COPD patients, even in those with mild-to-moderate airflow obstruction, are affected by systemic inflammation associated with abnormal renal functional reserve. Materials and Methods: The study enrolled 24 current smokers with a cigarette smoking history ^25 pack-years and 8 nonsmokers with normal spirometry as control. Doppler sonography detected the renal resistive index (RRI) before and after oral protein loading to determine the renal functional reserve. Pulmonary function and serum tumor necrosis factor 〈 (TNF-〈) levels were analyzed to compare with the renal functional reserve. Results: The smokers were stratified into 3 groups (Group 1: smokers with normal spirometry, Group 2: mild COPD, Group 3: moderate COPD); nonsmokers as Group 4. The baseline RRI levels were similar in Group 1 and Group 4. After protein loading, the RRI elevated in all smoking groups; moreover, Group 3 had the highest RRI and with longer duration than other groups. The smokers with higher serum TNF-〈 levels had a longer RRI elevation. Multiple linear regression revealed forced expiratory volume in one second (FEV1), serum TNF-〈 levels and aging were independently predictive factors of impaired renal functional reserve. Conclusions: A greater impairment in renal functional reserve of COPD patients was correlated with more severe airway obstruction and inflammation.
Introduction
To date, several risk factors have been identified for the development and progression of chronic kidney disease (CKD), such as ageing process, hypertension, diabetes, high body mass index and smoking (Citation1). However, the relationship between chronic obstructive pulmonary disease (COPD) and CKD has been less well examined. Incalzi and his colleagues recently investigated the association between COPD and CKD. They found that the prevalence of CKD in elderly patients with COPD was around 43%, and that COPD was an independent contributory factor for CKD (Citation2). van Gestel et al. also demonstrated that the prevalence of COPD was inversely related to renal function in patients who received vascular surgery, and moderate COPD was strongly independently associated with CKD. In addition, moderate and severe COPD have been found to be related to increased long-term mortality in patients with CKD (Citation3). However, the mechanisms responsible for this association remain largely unknown.
COPD is considered to be a systemic inflammatory disease (Citation4, 5) in which endothelial dysfunction (Citation6), atherosclerosis (Citation7), and cardiovascular events (Citation5) are co-morbidities. Moreover, increased inflammation levels are often seen in patients with kidney dysfunction (Citation8) and inflammatory factors or markers are associated with the risk of renal function decline and CKD development in a large population-based cohort during a 14-year follow-up (Citation9). Hence, we inferred that systemic inflammation might be one of the underlying mechanisms linking between COPD and CKD.
The majority of COPD patients have a reduced muscular mass (Citation10), thus serum creatinine levels may be falsely low as the result of decreased creatinine release. We therefore investigated whether a more sensitive index of renal function would show evidence of renal impairment in these patients. The increase in glomerular filtration rate (GFR) and renal blood flow in response to a dietary protein load or amino acid infusion, known as the renal functional reserve, is often impaired early in the course of renal disease, frequently before a fall in GFR (Citation11). Reduced renal functional reserve may thus be an early index of renovascular impairment (Citation12).
A series of articles published during the past decade have indicated the potential of using Doppler ultrasound to improve the assessment of renal dysfunction (Citation13–16). Changes in intra-renal arterial waveforms have been found in several types of intrinsic renal disorder and renal vascular disease (Citation17, 18). Doppler ultrasound of the renal vasculature therefore offers an excellent real-time measurement of renal arterial blood flow resistance, which is expressed in the renal resistive index (RRI). The RRI is a parameter of vascular downstream impedance which is dependent on resistance and compliance through the vessels (Citation19). Moreover, oral protein loading induces a significantly reversible increase in the GFR and renal blood flow (Citation20), and an increased RRI is a hallmark of arterial stiffness generating a higher resistance to increased blood flow (Citation21). Hence, RRI elevation can be a sensitive marker for an early detection of impaired renal functional reserve (Citation16, Citation22, Citation23).
For pro-inflammatory cytokines, especially TNF-α, is identified as an important systemic biomarker involving in the disease process of COPD (Citation24, 25). The arterial stiffness and renal insufficiency has been found to associate with the serum levels of TNF-α in type 2 diabetic patients (Citation26). Additionally, anti-TNF-α therapy could improve vascular stiffness in patients with inflammatory arthropathies (Citation27). It is postulated that TNF-α may play a role in the arterial stiffness related renal insufficiency in patients with COPD.
An impaired renal functional reserve after protein loading is seen in patients with both severe COPD and hypercapnia (Citation28), however whether this occurs in patients with mild-to-moderate COPD using GOLD guideline staging is unclear. We hypothesized that, even in normocapnia, renal functional reserve could be affected in mild-to-moderate COPD because of the arterial stiffness induced by systemic inflammation (e.g., TNF-α) and micro-vascular dysfunction. Using oral protein loading with a standard formula for COPD patients, we assessed the renal blood flow resistance for current heavy smokers with different degrees of airway obstruction.
Materials and Methods
Subjects
The patients were enrolled prospectively from the Chest Outpatient Clinics of Taipei Veterans General Hospital from March 2005 to May 2005. Spring was chosen as the study period in order to reduce the variations of weather, temperature and circadian rhythms. Patients who were current smokers and had a cigarette smoking history of more than 25 pack-years were screened for eligibility. The study population was confined to those with airflow obstruction less than or equal to moderate stage of COPD (FEV1 ≥ 50% predicted). To prevent confusion by other vasoactive factors, the exclusion criteria were the presence of diabetes mellitus, hypertension, renal disease, hyperlipidemia, previous cerebrovascular accident, chronic liver or cardiac diseases.
We used the Charlson co-morbidity index to identify patients’ co-morbidities in their past history. Focusing on the subjects with more stable pulmonary function, patients with acute exacerbation in the previous 6 months or those who had asthma were also excluded from the study. To compare the RRI in healthy-control subjects, 8 age- and gender-matched nonsmokers without co-morbidities in exclusion criteria were enrolled from subjects received health check-up and with a normal spirometry at our outpatient clinics. All individuals gave written informed consent to participate in this study, which was approved by the Ethics Committee of Taipei Veterans General Hospital and followed the Helsinki recommendations (IRB approval number 940104A).
Study protocol
We started the renal sonographic examinations at 7:30 AM, after the patients had fasted overnight and all morning and had not taken any medications. We made clearly labeled marks on the body to confirm the anatomical position of the kidneys and the corresponding blood vessels. After recording the baseline level of RRI, protein loading with a consistent formula diet was given at 8 AM. All subsequent assessments were then scheduled as follows: sonography in the morning between 8:00 and 10:15 AM; arterial blood gas and pulmonary function tests between 10:30 and 11:30 AM; and blood and urine sampling between 11:30 and 12:00 PM. The serum was obtained immediately by centrifugation at 3000 rpm for 10 min and was stored at -80 ºC until further testing for TNF-α.
Ultrasonography and renal resistive index
Doppler sonography was performed using a Toshiba Nemio SSA-550A®. Patients received the examinations from a right translumbar route in a sitting position with a 3.7 MHz probe. Power Doppler was used to identify the interlobular arteries at the maximum longitudinal axis of the right kidney. It was then switched off to obtain better quality velocity signals in the pulse wave Doppler mode; the cursor was fixed at the position of the middle interlobular artery to detect pulsed flow waves. The patients in whom we had difficulty indentifying the interlobular arteries were excluded from the study. The RRI was defined as follows (Citation29):
Protein loading
After the overnight fasting and the basal blood flow resistance had been recorded, patients were protein-loaded by drinking a liquid formula for COPD patients (Pulmocare® liquid, Abbott Nutrition, USA) containing 16.7% protein and 55.1% fat, with an osmolality of 490 mOsm/Kg H2O over a period of 15 min. Patients with a body weight under 80 kg were given 2 cans of the formula (79.2 g of protein), and those over 80 kg were given 3 cans (118.7 g of protein). The RRI was then determined at 45, 90 and 135 min thereafter.
Pulmonary function tests
Spirometry was performed with a Sensormedics Vmax® computerized spirometer according to the American Thoracic Society guidelines. Subjects with less than 50% predicted values of forced expiratory volume in one second (FEV1), or those with more than 12% FEV1 and also in an absolute volume of 200 ml in response to 400 μg salbutamol were excluded from the study. Post-bronchodilator spirometry was performed to stratify the study subjects.
The subjects who had FEV1/ forced vital capacity (FVC) ≥ 0.70; FEV1 ≥ 80% predicted; FVC ≥ 80% predicted were classified as Group 1 (smokers without COPD). Patients who fulfilled the 2003 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for mild and moderate COPD were classified as Group 2 (FEV1/FVC < 0.70; FEV1 ≥ 80% predicted) and Group 3 (FEV1/FVC < 0.70; 50% ≤ FEV1 < 80% predicted), respectively. This classification was similar to the updated 2010 GOLD guidelines. In addition, age-matched nonsmokers with normal spirometry were classified as Group 4. Arterial blood gas sampled from the radial arteries was analyzed before the measurement of pulmonary function.
Other laboratory tests
Serum levels of tumor necrosis factor α (TNF-α), urine and blood biochemistry examinations and osmolality, and renal clearance of creatinine were checked at the visit. Because the older COPD patients have less lean body mass, we took body weight and high into consideration for GFR estimation using the Jelliffe method by the formula (Citation30):
where BSA, indicating body surface area, was calculated based on the Du Bois Formula (Citation31):
TNF-α, a proinflammatory marker, was measured using an enzyme-linked immunosorbent assay (ELISA) kit according to the instructions of the manufacturer (R&D Systems, Minneapolis, MN, USA) and were performed in triplicate (the detection limit 0.5 pg/ml).
Statistical analysis
The planned sample size of 32 (8 in 4 groups) was estimated, on the basis of our previously described pilot study, to provide 80% power to detect a difference in the change in RRI of 0.1. Baseline characteristics were compared using ANOVA tests and least significant difference (LSD) post-hoc comparisons for continuous variables, and Fisher's exact tests for categorical variables between the three groups. We compared the differences in the RRI before and after a protein loading in the three groups of patients. A paired t-test was adopted to evaluate the change in baseline and post-protein-loading RRI of the patients. We also compared the differences between each of two groups at every time point using ANOVA tests and LSD post-hoc comparisons. Linear regression analyses were performed to correlate the levels of RRI with the percentage of predicted FEV1 and the values of serum TNF-α respectively.
To access the independent contributors to renal function reserve, age and the degrees of inflammation (TNF-α) and airway obstruction (FEV1), as well as other variables associated with renal function (GFR, urine/serum osmolality), arterial blood gas (PaO2 and PaCO2), and body mass index were forward stepwise selected in a multivariate linear regression model. A p-value less than 0.05 was considered to be statistically significant. All analyses were carried out using SPSS 17.0 for Windows software package (SPSS Inc., Chicago, IL, USA).
Results
Demographics and clinical data
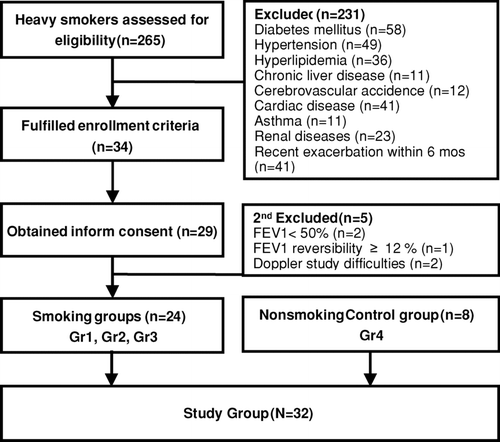
Originally, 265 heavy smokers enrolled in our clinic were assessed for this study, there were 34 (12.83%) of smokers met our study criteria. Twenty-nine of them consented to participate in the study; 5 of them were excluded due to unsatisfied status (n = 3) and difficulties in identifying the interlobar arteries (n = 2) (). These patients were primarily divided into 3 groups based on their severity of airflow obstruction. Group 1 comprised of 8 patients with normal spirometry, while Group 2 and Group 3 were composed of patients with mild (n = 8) and moderate COPD (n = 8), respectively. In addition, the age-matched nonsmoking control group (defined as Group 4, n = 8) also received the measurement of RRI based on the same protocol.
displays the baseline characteristics on the study day, the distributions of their blood pressure were negatively skewed and all levels were less than 140/90 mmHg. The enrolled patients were elderly with no statistical differences in age and body mass index among the 4 groups. The PaO2 disclosed similar borderline hypoxemia in the 3 smoker groups, yet the PaCO2 and pH levels were all within normal limits.
Table 1. Clinical characteristics, arterial blood gas analysis, and pulmonary function of the study population*.
The severity of airway obstruction is associated with impaired renal functional reserve
shows the RRI before and after protein loading in smokers with different severities of COPD and nonsmoking control subjects. The RRI baseline before the protein loading was almost indistinguishable between Groups 1 and 4 (smokers and nonsmokers with normal spirometry), which were lower than those in Groups 2 and 3 (mild and moderate COPD). However, the difference between the latters was not statistically significant. Oral protein loading induced a reversible decrease of the RRI values in Group 4, which demonstrated as normal renal function reserve. Forty-five minutes after the protein loading, the RRI values were elevated in all 3 smoker groups; however, their values returned to the baseline after 135 minutes in Group 1. For Group 3, the RRI values remained at a high level for more than 90 minutes (p < 0.01 compared with baseline RRI), and were significantly higher than Group 1 at 90 and 135 minutes after the protein loading (p < 0.05). The finding suggests that the changes of RRI occurs as early as within the first 45 minutes.
Figure 2. Renal resistive index before and after protein loading in smoker patients with different severities of COPD and nonsmoker control subjects. The postprandial RRIs were significantly increased compared with the baseline values in the heavy smoking patients. Moderate COPD patients (FEV1 50–80% predicted, Group 3) had persistent RRI elevation for more than 90 minutes, and the RRI elevation lasted for only 45 minutes in the other two smoker groups. Group 1: smokers with normal spirometry, 8 patients; Group 2: mild COPD, 8 patients; Group 3: moderate COPD, 8 patients; Group 4: nonsmoker control group, 8 subjects. *p < 0.05,**p < 0.01 compared with baseline RRI by paired t-tests. § p < 0.05 compared with Group 1 at the same time points by the Student's t-test.
Serum levels of TNF-〈 correlate with the severity of impaired renal functional reserve in smokers
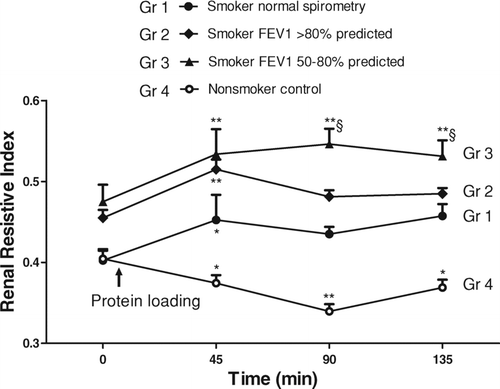
The serum TNF-α levels did not correlate with the severity of pulmonary function in our stable patients (Spearman Rank Correlation Coefficient = 0.08, p = 0.738). Their TNF-α levels were within the relatively lower range of ELISA measurement, a finding which is consistent with the notion that the patients were clinically stable and not exacerbating. In addition, the patients’ TNF-α levels did not correlate with their estimated values of GFR (Spearman Rank Correlation Coefficient = 0.189, p = 0.424). Therefore, we were interested to see whether the serum levels of the systemic proinflammatory cytokine TNF-α were correlated with the RRI values in smokers. According to the mean value of plasma TNF-α levels of 7 pg/ml, we reclassified the 24 smoking patients as high (> 7 pg/ml) and low (≤ 7 pg/ml) TNF-α groups. The RRI values at 45 minutes post-protein loading were significantly higher than the baseline; however, the elevation of RRI lasted longer in patients with high TNF-α levels (at 90 and 135 minutes, compared with baseline RRI, p < 0.05) ().
Figure 3. Renal resistive index before and after protein loading in patients with high/low serum TNF-α levels. The RRIs were significantly increased from the baseline values in patients with high TNF-α (> 7 pg/ml) and low TNF-α (≤ 7 pg/ml) levels 45 min postprandial, while the RRI elevation lasted longer in patients with high TNF-α levels (*p < 0.05,**p < 0.01 compared with baseline RRI by paired t-tests). The RRI was also significantly more elevated in patients with high TNF-α levels compared with those with low TNF-α levels at 135 min (§ p < 0.05 by the Student's t-test).
Multiple linear regression of final RRI after protein loading in the COPD patients
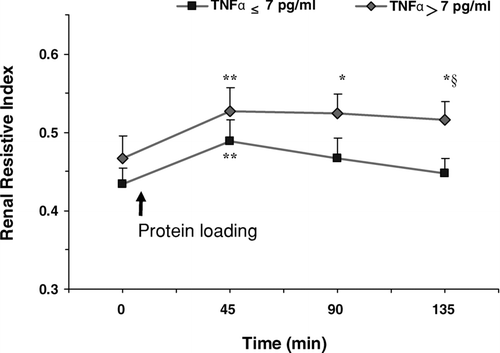
Since the degree of RRI elevation indicates renal functional reserve, we used the final RRI at 135 minutes after protein loading (the fourth time point of the RRI) as another indicator for renal functional reserve. Linear regression analyses revealed that final RRI was negatively correlated with the percentage of predicted FEV1 values (r = –0.563; p = 0.010) and positively correlated with serum TNF-α levels (r = 0.035; p = 0.01) (, and ).
Figure 4. Renal resistive index 135 min after protein loading against airway obstruction and TNF-α serum levels. (A) The RRI was negatively correlated with the percentage of predicted values of FEV1 (Correlation Coefficient = –0.563, P = 0.010). (B) The RRI was positively correlated with serum TNF-α levels (Correlation Coefficient = 0.473, P = 0.035). The solid lines indicate the regression line and the dashed lines indicate the 95% confidence intervals.
In addition, we constructed a predictive model using the important predictors of final RRI. The parameters of FEV1 and TNF-α were brought into the model due to their linear correlation with RRI, as well as the ratio of urine and serum osmolality for refracting fluid status, baseline GFR for estimating fluid excretion, age, body mass index, and PaO2, and PaCO2 for determining COPD outcome (Citation32). The forward stepwise multiple linear regression model showed that FEV1 predicted percentage, TNF-α levels and age were independently predictive factors of renal functional reserve (Adjusted R2 = 0.586; Regression Coefficients = –0.117, 0.030, and 0.004; p = 0.023, 0.003, and 0.001, respectively) ().
Table 2. Multiple linear regression of the final renal resistive indices.
Discussion
This study reveals several interesting and essential points: First, COPD patients with lower FEV1 exhibit a significantly slower recovery of renal vascular resistive changes after a protein challenge, and patients with higher serum TNF-α levels had a worse renal functional reserve than those with lower serum TNF-α levels. The impaired renal functional reserve in COPD patients might give some important clinical implications. First, it evokes a therapeutic concern about high protein diets or drug nephrotoxicity in patients with moderate COPD. Second, reduced renal functional reserve suggests an inability to increase renal blood flow (RBF), which may activate the renin/angiotensin system in stimulating aldosterone release and thereby cause a subsequent sodium and water retention to develop edema (Citation33). Third, this report offers a reasonable explanation for the recently disclosed association of CKD and COPD in epidemiology cohort study (Citation2).
Fourth, patients with mild-to-moderate COPD in this study suffered from concealed renal insufficiency (normal serum creatinine but reduced GFR). Given that early stages of impaired kidney function are associated with an increased risk of death, cardiovascular events and hospitalization (Citation34), it is important to identify and to consequently treat these patients in order to improve prognosis. Studying renal vascular resistance before and after protein loading provides a value to prospectively notice COPD patients with potential risk in developing progressive renal damages.
To the best of our knowledge, the relationship between systemic inflammation and abnormal renal vascular function in stable COPD patients has not previously been explored. The multiple linear regression model provided an important finding that the final RRI was affected by FEV1, TNF-α and age (adjusted coefficient of determination = 0.586). There was a positive correlation between the serum levels of the inflammatory mediator, TNF-α, and impaired renal functional reserve in the stable COPD patients. COPD is generally recognized as a cause of systemic inflammation (Citation4), and pro-inflammatory cytokines, especially TNF-α, play an important role in the disease process (Citation24–26).
This systemic inflammatory state in patients with COPD is associated with the increased risk of cardiac injury (Citation5), and cardiovascular events (Citation35). In addition, both endothelium-dependent and endothelium-independent vasodilatation in peripheral arteries are significantly impaired in patients with stable COPD (Citation6). Sabit et al. recently reported arterial stiffness in patients with COPD compared with control subjects after adjusting for age, sex, blood pressure, and other cardiovascular risk factors (Citation7). The authors postulated that the increased systemic inflammation seen in COPD may have been responsible for the observed increase in arterial stiffness.
A recent study by Iwamoto et al. found airflow limitation to be associated with increased mean thickness of carotid intima in smokers and never-smokers (Citation36). This suggests that airflow limitation, instead of smoking status, is independently related to subclinical atherosclerosis. Subsequently, arterial stiffness and atherosclerosis might affect the vasculature in the kidneys leading to kidney dysfunction (Citation37, 38). There are still many variables that may play a role in predicting renal function declined in COPD patients. Previous reports have shown that RRI is altered if arterial CO2 tension is above 51 mmHg or oxygen saturation declines to 88–90% (Citation39–41). Cigarette smoking elicits abundant oxidative-inflammatory molecules and can induce systemic endothelial dysfunction, arteriosclerosis, and chronic kidney disease (Citation42, 43).
There are several mechanisms that may explain the association of elevated serum concentrations of TNF-α with impaired renal functional reserve. TNF-α has been identified in human atherosclerotic plaques (Citation44) and been found to play a role in the development and progression of atherosclerosis (Citation45). TNF-α also contributes to the development of renal tubulointerstitial fibrosis (Citation46). These suggest that TNF-α is important in both the early inflammatory phase and the later scarring phase of renal disease. Thus TNF-α may contribute to a possible athe-rosclerogenesis in the kidneys leading to impaired renal functional reserve. Another inflammatory molecule, C-reactive protein, has been applied to predict abnormal RRI in several different populations with essential hypertension (Citation47, 48)
Diurnal and climate changes may also have an effect in changes of vascular tone; therefore in this study we controlled these confounding factors by having all the subjects to receive examinations at the same time over the morning in spring. We chose the middle interlobar artery and measured the early diastolic velocity for two reasons: first, the anatomical location of the vessel allows for repeated measurements; second, the blood flow velocity at the early diastolic phase is more constant. Consequently, the measurements of our results were slightly lower than in previous reports (Citation28).
A lot of systemic diseases can affect renal vascular resistance. Hypertension and diabetes mellitus directly damage the renal vasculature (Citation2, Citation49), and RRI elevation is associated with long-term progression in renal function insufficiency and tubular-interstitial injury (Citation16, Citation50). Liver cirrhosis can lead to portal hypertension complicated with renal function impairment (Citation51). Moreover, cardiovascular disease and hyperlipidemia both contribute to endothelial dysfunction (Citation52). Recently, Casanova et al. disclosed microalbuminuria in COPD patients with hypoxemia (Citation53). This notion suggests that our smoker groups, with borderline hypoxemia, might have a declined renal function.
Incalzi et al. just reported an epidemiological study suggesting that chronic renal failure is often being neglected as a co-morbidity of COPD (Citation2). Our study provides new insight on the relationship between the renal function impairment and the development of mild-to-moderate COPD. The progressive increase in blood urine nitrogen and urine osmolality with a decrease in GFR, combined with increased RRI after the protein loading from Group 1 to Group 3 may indicate that a decrease of renal functional reserve is associated with the severity of airway obstruction in early-stage COPD patients (). However, the final RRI levels did not correlate with GFR, creatinine, and BUN values (Spearman Rank Correlation Coefficient = –0.119, –0.46, –0.6; p = 0.616, 0.846, 0.616, respectively).
One major drawback of this report is that the sample size used in this study may not provide a non-disputable statistics. The RRI dynamic observation after protein loading was relative short-term in this study. Thus, a larger scale case-controlled study may be necessary to address this issue in the future. This study only analyzed one inflammatory biomarker (TNF-α), but other biomarkers may be deserved to further explore the linkage of systemic inflammation and renal function reserves in COPD.
It is also worth mentioning that our intention was to use smokers as a model for COPD to test the relationship between airflow limitation and impaired renal function reserve, how this result applied in non-smoking related COPD may need further study to clarify. In conclusion, this study demonstrates that a greater impairment in the renal functional reserve of COPD patients is correlated to a greater decrease in airflow and higher circulating levels of systemic inflammatory cytokines. Impaired renal functional reserve can be recognized early in stable COPD patients with moderate airway obstruction, normal creatinine values, and even normocapnia.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
This work was supported by National Science Council Grant 95-2314-B075-034, and 100-2314-B-075-047-MY3 (to KYY), 100-2314-B-010-001-MY2 and 100-2314-B-010-044-MY3 (to CYC); Taipei Veterans General Hospital Grants V93-101, V94-301, V97C1-143, V98C1-072, and V99C1-167 (to KYY); and National Yang Ming University Grant RD2011-07 (to CYC).
References
- Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004; 291(7):844–850.
- Incalzi RA, Corsonello A, Pedone C, Battaglia S, Paglino G, Bellia V. Chronic renal failure: a neglected comorbidity of COPD. Chest 2010; 137(4):831–837.
- van Gestel YR, Chonchol M, Hoeks SE, Welten GM, Stam H, Mertens FW, van Domburg RT, Poldermans D. Association between chronic obstructive pulmonary disease and chronic kidney disease in vascular surgery patients. Nephrology, Dialysis, Transplantation: Official publication of the European Dialysis and Transplant Association - European Renal Association 2009; 24(9):2763–2767.
- Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007; 370(9589):797–799.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107(11):1514–1519.
- Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Kohansal R, Burghuber OC. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2008; 178(12):1211–1218.
- Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockcroft JR, Shale DJ. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2007; 175(12):1259–1265.
- Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2003; 107(1):87–92.
- Bash LD, Erlinger TP, Coresh J, Marsh-Manzi J, Folsom AR, Astor BC. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. Amer J Kidney Dis: Off J Natl Kidney Foun 2009; 53(4):596–605.
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest 2007; 132(1):164–169.
- Bosch JP, Lew S, Glabman S, Lauer A. Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Amer J Med 1986; 81(5):809–815.
- Kawai T, Kamide K, Onishi M, Yamamoto-Hanasaki H, Baba Y, Hongyo K, Shimaoka I, Tatara Y, Takeya Y, Ohishi M, Usefulness of the resistive index in renal Doppler ultrasonography as an indicator of vascular damage in patients with risks of atherosclerosis. Nephrology, Dialysis, Transplantation: Official publication of the European Dialysis and Transplant Association - European Renal Association 2011; 26(10):3256–3262.
- Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, Gebel MJ, Galanski M, Koch KM, Haller H. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. New Engl J Med 2001; 344(6):410–417.
- Jansen O, Rob PM, Schmidtke V, Marienhoff N, Rinast E, Weiss HD. Follow-up study of renal transplants by duplex Doppler and gray-scale ultrasound. Euro J Radiol 1992; 15(1):26–31.
- Kirkpantur A, Yilmaz R, Baydar DE, Aki T, Cil B, Arici M, Altun B, Erdem Y, Erkan I, Bakkaloglu M Utility of the Doppler ultrasound parameter, resistive index, in renal transplant histopathology. Transplant Proc 2008; 40(1):104–106.
- Parolini C, Noce A, Staffolani E, Giarrizzo GF, Costanzi S, Splendiani G. Renal resistive index and long-term outcome in chronic nephropathies. Radiology 2009; 252(3):888–896.
- Helenon O, Melki P, Correas JM, Boyer JC, Moreau JF. Renovascular disease: Doppler ultrasound. Seminars in ultrasound, CT, and MR 1997; 18(2):136–146.
- Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol 2003; 180(4):885–892.
- Colli A, Cocciolo M, Riva C, Martinez E. Abnormal renovascular impedance in patients with hepatic cirrhosis: detection with duplex US. Radiology 1993; 187(2):561–563.
- Woods LL. Mechanisms of renal hemodynamic regulation in response to protein feeding. Kidney Inter 1993; 44(4):659–675.
- Ratto E, Leoncini G, Viazzi F, Vaccaro V, Falqui V, Parodi A, Conti N, Tomolillo C, Deferrari G, Pontremoli R. Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens 2006; 24(10):2033–2038.
- Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease: results of a 4-year follow-up. Clin Exp Nephrol 2011; 15(1):114–120.
- Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension 2002; 39(2 Pt 2):699–703.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59(7):574–580.
- Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2000; 161(4 Pt 1):1179–1184.
- Hirota T, Suzuki E, Ito I, Coronary artery calcification, arterial stiffness and renal insufficiency associate with serum levels of tumor necrosis factor-alpha in Japanese type 2 diabetic patients. Diabetes Res Clin Pract 2008; 82(1):58–65.
- Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension 2010; 55(2):333–338.
- Sharkey RA, Mulloy EM, Kilgallen IA, O'Neill SJ. Renal functional reserve in patients with severe chronic obstructive pulmonary disease. Thorax 1997; 52(5):411–415.
- Makino Y, Ogawa M, Ueda S, Hori J, Ohto M, Wakashin M, Akikusa B, Yamamoto S. Intrarenal arterial Doppler sonography in patients with various renal disease: correlation of resistive index with biopsy findings. Nihon Jinzo Gakkai Shi 1992; 34(2):207–212.
- Lamb EJ, Webb MC, Simpson DE, Coakley AJ, Newman DJ, O'Riordan SE. Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: is the modification of diet in renal disease formula an improvement? J Amer Geriatr Soc 2003; 51(7):1012–1017.
- Du Bois D, Du Bois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; 17:863–871.
- Coleta KD, Silveira LV, Lima DF, Rampinelli EA, Godoy I. Predictors of first-year survival in patients with advanced COPD treated using long-term oxygen therapy. Respir Med 2008; 102(4):512–518.
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part two. Amer J Respir Crit Care Med 1994; 150(4):1158–1168.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med 2004; 351(13):1296–1305.
- Ross R. Atherosclerosis—An inflammatory disease. New Engl J Med 1999; 340(2):115–126.
- Iwamoto H, Yokoyama A, Kitahara Y, Ishikawa N, Haruta Y, Yamane K, Hattori N, Hara H, Kohno N. Airflow limitation in smokers is associated with subclinical atherosclerosis. Amer J Respir Crit Care Med 2009; 179(1):35–40.
- Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis 2009; 204(1):298–303.
- Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE. Cardiovascular disease and subsequent kidney disease. Arch Intern Med 2007; 167(11):1130–1136.
- Mills NL, Miller JJ, Anand A, Robinson SD, Frazer GA, Anderson D, Breen L, Wilkinson IB, McEniery CM, Donaldson K, Increased arterial stiffness in patients with chronic obstructive pulmonary disease: A mechanism for increased cardiovascular risk. Thorax 2008; 63(4):306–311.
- Sharkey RA, Mulloy EM, O'Neill SJ. Acute effects of hypoxaemia, hyperoxaemia and hypercapnia on renal blood flow in normal and renal transplant subjects. Euro Respir J: Off J Euro Soc Clin Respir Physiol 1998; 12(3):653–657.
- Darmon M, Schortgen F, Leon R, Moutereau S, Mayaux J, Di Marco F, Devaquet J, Brun-Buisson C, Brochard L. Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intens Care Med 2009; 35(6):1031–1038.
- Mendes ES, Campos MA, Wanner A. Airway blood flow reactivity in healthy smokers and in ex-smokers with or without COPD. Chest 2006; 129(4):893–898.
- Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Amer J Epidemiol 2006; 164(3):263–271.
- Rus HG, Niculescu F, Vlaicu R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis 1991; 89(2-3):247–254.
- Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005; 180(1):11–17.
- Guo G, Morrissey J, McCracken R, Tolley T, Liapis H, Klahr S. Contributions of angiotensin II and tumor necrosis factor-alpha to the development of renal fibrosis. Amer J Physiol Renal Physiol 2001; 280(5):F777–785.
- Berni A, Ciani E, Bernetti M, Cecioni I, Berardino S, Poggesi L, Abbate R, Boddi M. Renal resistive index and low-grade inflammation in patients with essential hypertension. J Hum Hypertens 2011; 10:1–8.
- Ozelsancak R, Torun D, Koc Z, Sezer S, Ozdemir FN, Niron EA. Relationship between renal resistive index and inflammation in untreated hypertensive patients. Int Heart J 2009; 50(6):753–761.
- Raff U, Schwarz TK, Schmidt BM, Schneider MP, Schmieder RE. Renal resistive index–a valid tool to assess renal endothelial function in humans? Nephrology, Dialysis, Transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 2010; 25(6):1869–1874.
- Petersen LJ, Petersen JR, Talleruphuus U, Ladefoged SD, Mehlsen J, Jensen HA. The pulsatility index and the resistive index in renal arteries. Associations with long-term progression in chronic renal failure. Nephrology, Dialysis, Transplantation: Official publication of the European Dialysis and Transplant Association - European Renal Association 1997; 12(7):1376–1380.
- Colli A, Fraquelli M, Pometta R, Cocciolo M, Visentin S, Conte D. Renovascular impedance and esophageal varices in patients with Child-Pugh class A cirrhosis. Radiology 2001; 219(3):712–715.
- Okon EB, Chung AW, Zhang H, Laher I, van Breemen C. Hyperglycemia and hyperlipidemia are associated with endothelial dysfunction during the development of type 2 diabetes. Can J Physiol Pharmacol 2007; 85(5):562–567.
- Casanova C, de Torres JP, Navarro J, Aguirre-Jaime A, Toledo P, Cordoba E, Baz R, Celli BR. Microalbuminuria and hypoxemia in patients with chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2010; 182(8):1004–1010.