Abstract
Morbid obesity may influence several aspects of airway function. However, the effect of morbid obesity on expiratory tracheal collapse in COPD patients is unknown. We thus prospectively studied 100 COPD patients who underwent full pulmonary function tests (PFTs), 6-minute walk test (6MWT), Saint George's Respiratory Questionnaire (SGRQ), and low-dose CT at total lung capacity and during dynamic exhalation with spirometric monitoring. We examined correlations between percentage dynamic expiratory tracheal collapse and body mass index (BMI). The association between tracheal collapse and BMI was compared to a control group of 53 volunteers without COPD. Patients included 48 women and 52 men with mean age 65 ± 7 years; BMI 30 ± 6; FEV1 64 ± 22% predicted and percentage expiratory collapse 59 ± 19%. Expiratory collapse was significantly associated with BMI (69 ± 12% tracheal collapse among 20 morbidly obese patients with BMI ≥35 compared to 57 ±19% in others, p = 0.002, t-test). In contrast, there was no significant difference in collapse between healthy volunteers with BMI ≥ 35 and < 35. COPD patients with BMI ≥ 35 also demonstrated shorter 6MWT distances (340 ± 139 m vs. 430 ± 139 m, p = 0.003) and higher (worse) total SGRQ scores (48 ± 19 vs. 36 ± 20, p = 0.013) compared to those with BMI < 35. In light of these results, clinicians should consider evaluating for excessive expiratory tracheal collapse when confronted with a morbidly obese COPD patient with greater quality of life impairment and worse exercise performance than expected based on functional measures.
Introduction
A previous prospective cohort study demonstrated that body weight is an important component of clinical COPD phenotypes (Citation1). Whereas low body weight is more prevalent among patients with emphysema, obesity is more prevalent among patients with chronic bronchitis (Citation1).
Physiologically, morbidly obese patients are likely to have elevated intrathoracic pressures that may cause excessive central airway narrowing during tidal breathing, particularly at end expiration (Citation2). Such narrowing could potentially be exacerbated among morbidly obese patients with coexisting COPD for several reasons. First, COPD may be associated with weakness of airway walls due to atrophy of elastic fibers in the posterior membranous portion of the trachea from chronic inflammation (Citation3,4). Second, flow limitation will develop in more peripheral airways at higher lung volumes and lower flow rates resulting in greater downstream pressure loss and more negative airway transmural pressure in the central airways. Finally, COPD may be associated with reduced lung elastic recoil, resulting in less tethering support of the central airways.
We thus hypothesized that morbidly obese COPD patients may have an enhanced susceptibility for excessive expiratory tracheal collapse. The presence of excessive expiratory tracheal collapse among COPD patients in general has recently gained attention due to reports of selected COPD patients with excessive expiratory tracheal collapse who demonstrated improved symptoms, quality of life and functional status following central airway stabilization (Citation5,6).
With these considerations in mind, the purpose of this study is to assess whether morbidly obese patients with COPD demonstrate greater expiratory tracheal collapse compared to non-morbidly obese and normal weight COPD patients.
Methods
This study was approved by our institutional review board and performed in compliance with Health Insurance Portability and Accountability Act guidelines. A description of the methodological design of this prospective, 5-year study of tracheal dynamics in COPD, including descriptions of the imaging technique and breathing instructions, has been published previously in greater detail (Citation7). The same COPD study cohort was in a previous publication which addressed a distinctly different research question (Citation7).
Study population
Between October 2008 and November 2010, we prospectively recruited subjects aged 35-75 years with COPD to participate in a study assessing the prevalence of excessive expiratory tracheal collapse using low-dose CT scanning. Exclusion criteria included: 1) pregnancy; 2) presence of other risk factors for tracheomalacia, including prior prolonged intubation, mediastinal radiation, tracheal surgery or tracheal stent placement; and 3) unstable coronary artery disease, including myocardial infarction within the past 6 months or unstable angina.
Once enrolled and following informed consent, participants underwent pulmonary function tests (PFTs) according to American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines (Citation8). Participants who met Global Initiative for Obstructive Lung Disease (GOLD) criteria for COPD (compatible history and symptoms along with post-bronchodilator FEV1/FVC < 0.7) (Citation9) were included in our initial study population.
Control population
We created a control population of volunteers with normal pulmonary function and no history of smoking or respiratory illness who were comparable to the COPD study population with respect to age, gender, and BMI. As described below, we created this control population from an existing pool of volunteers who met these criteria and by strategically recruiting additional healthy volunteers >48 years of age with BMI ≥ 35.
As part of our larger study design, we previously recruited 81 adult healthy volunteers with normal pulmonary function and no history of smoking to undergo low-dose CT evaluation of expiratory tracheal collapse. The recruitment and study methods for these participants have been previously reported.(Citation10, 11) In order to obtain an overall comparable control group to our COPD population in terms of age distribution and BMI, an additional 11 volunteers ≥ 48 years of age with BMI ≥ 35 were recruited. From the potential total pool of 92 healthy volunteers (81 previous and 11 new recruits), we excluded 39 volunteers who were < 48 years old. Thus, our final control healthy group comprised 53 volunteers.
Medical record and medication review for COPD population
To determine whether a participant was receiving standard medical treatment for COPD, each participant's regimen was independently rated for concordance with current GOLD management guidelines (Citation9) by 2 board-certified pulmonologists with experience in managing patients with COPD. Discrepancies in ratings were adjudicated by a third, experienced pulmonologist. Each participant's available online medical records were also reviewed for history of previous diagnosis of obstructive sleep apnea.
Because of previous literature demonstrating an association between metabolic syndrome (MetS) and the airway phenotype of COPD (Citation12, 13), we sought to establish whether COPD participants met criteria for this syndrome. To achieve this goal, each participant's research data and medical records were reviewed by an experienced research nurse to determine if they met the definition of MetS: evidence of central obesity and the presence of at least 2 of the following criteria: 1) triglycerides >150 mg/dL (1.7 mmol/L) or treatment for elevated triglycerides; 2) HDL cholesterol <40 mg/dL(1.03 mmol/L) in men or <50 mg/dL (1.29 mmol/L) in women, or treatment for low HDL; 3) systolic blood pressure ≥130, diastolic blood pressure ≥85, or treatment for hypertension; and 4) fasting plasma glucose ≥100 mg/dL (5.6 mmol/L) or previously diagnosed type 2 diabetes (Citation14, 15). In accordance with published guidelines, it is not necessary to measure waist circumference in individuals with BMI ≥ 35, because central obesity can be assumed in these individuals (Citation15, 16). Using these modified criteria, the presence or absence of MetS was established in 94 participants.
Study procedures of the COPD patients
All COPD participants underwent: 1) St. George's Respiratory Questionnaire, a validated, 50-item instrument with 3 separate scales (symptoms, activity, and impact on daily life), with scores ranging from 0 to 100 (higher scores indicate worse health status) (Citation17); 2) a screening question for the presence or absence of chronic cough (>8 weeks in duration); 3) a 6MWT performed according to ATS guidelines along a 100-foot distance marked by cones and supervised by one of the researchers (CRO) (Citation18); and 4) CT imaging (below).
Low-dose, paired inspiratory-dynamic expiratory CT imaging
All participants (including COPD patients and healthy volunteers) were imaged according to a standardized, low-dose protocol using a 64-detector-row scanner (LightSpeed VCT, General Electric,Waukesha, WI) using the following parameters: 80 mA, 120 kVp, 0.625 mm detector collimation, 0.5 second gantry rotation time, and pitch of 1.375 (Citation7, Citation10). Helical scanning was performed in the cranio-caudal direction for both end-inspiratory (total lung capacity, TLC) and dynamic expiratory scans (obtained during a forced expiratory maneuver). For both sequences, images were reconstructed at 2.5 mm thickness with 1.25 mm reconstruction intervals and transferred to a Picture Archiving Communication System (PACS) (Centricity, Version 3.1, General Electric Medical Systems, Waukesha, WI) for analysis and interpretation. These parameters were selected based upon MDCT protocols validated by bronchoscopy for assessing tracheal collapsibility (Citation19–21). The total scanning time for each volumetric acquisition of the entire lungs was approximately 2.5 seconds.
CT breathing maneuvers
These maneuvers are described in greater detail in previous publications (Citation7, Citation10). Briefly, a researcher (CRO) monitored and coached subjects during CT studies. Participants were instructed in breathing maneuvers using a standard script and practiced each maneuver while on the CT table (Citation7, Citation22). Prior to separate TLC and dynamic expiratory maneuver CT sequences, the physiologist placed the mouthpiece and nose clips on the participant. A spirometric tracing was directly observed while the CT images were acquired in order to assure that maneuvers were adequate with respect to lung volume and forceful exhalation.
CT interpretation
An experienced, fellowship-trained thoracic radiologist (PMB) prospectively interpreted each scan. The radiologist was aware that the study participants met GOLD criteria for COPD, but was blinded to other study data. All exams were interpreted using a PACS workstation with standard lung (level, -650 HU; width, 1500 HU) and soft tissue (level, 50 HU; width, 350 HU) window display settings (Citation7, Citation10, Citation23). For each imaging sequence, measurements were obtained at a standard level of 1 cm above the aortic arch (mid-trachea). Using a previously validated technique, the cross-sectional area (CSA) of the airway lumen was measured by tracing the inner wall of the airway with an electronic tracing tool (Citation7, Citation19, Citation21). The percentage expiratory luminal collapse was calculated as:% Luminal Collapse = 100 x [1 –(luminal area at dynamic expiration/luminal area at end-inspiration)]. In addition to performing measurements at the standard level, the entire imaged trachea was visually assessed on both sequences in order to ensure that there was not a localized segment in the trachea that demonstrated substantially greater collapse compared to the site of standard measurement (Citation7). Additionally, the paratracheal region was visually assessed for any potential causes of extrinsic compression.
Statistical considerations
Our initial sample size of 100 COPD participants was selected to provide >90% power to demonstrate a relationship between the percentage expiratory tracheal collapse measured at CT and each of various clinical and physiological parameters, as long as R2 is ≥ 0.10.
Sample characteristics are presented as means and standard deviations for continuous variables; counts and proportions for nominal and ordinal variables. Normal distribution of continuous variables was examined by the Kolmogorov-Smirnov one-sample test. Between-group comparisons were assessed by t-test for continuous variables, and the Fisher exact probability test for categorical variables. Univariate ANOVA was used to assess differences in the primary outcome measure by subject type and BMI.
Results
Study population
The final study population of COPD patients included 48 women and 52 men with a mean age of 65 ± 7. The control population included 28 women and 25 men with a mean age of 60 ± 8. Age, height, weight, and body mass index (BMI), are provided in .
Table 1. Descriptive characteristics of the COPD and healthy populations
Twenty of 100 (20.0%) COPD patients and 13 of 53 (24.5%) controls had BMI ≥ 35 (p = 0.52 by chi-square analysis). The COPD subgroup with BMI ≥ 35 was similar to the COPD subgroup with BMI < 35 in terms of age and sex distribution. The mean age of COPD participants with BMI ≥ 35 was 63 ± 7 compared with 65 ± 7 among COPD participants with BMI < 35 (p = 0.35). Eleven of 20 (55.0%) COPD participants with BMI ≥ 35 were women as compared with 37 of 80 (46.3%) COPD participants with BMI < 35 (p = 0.46). Of the 20 COPD participants with BMI ≥ 35, coexisting metabolic syndrome was present in 16 (80%).
Functional characteristics of the COPD study population (including FVC, FEV1, FEV1/FVC, DLCO, GOLD-stage distribution and 6MWT results) are documented in , along with SGRQ scores. A majority of participants (74%) had moderate to severe COPD (Gold Stages II-IV).
Table 2. Functional characteristics of the entire COPD study population
Tracheal cross-sectional area measurements
Among COPD participants, the mean CSA of the mid trachea decreased from 283 ± 66 mm2 at end-inspiration to 113 ± 56 mm2 during forced expiration. The mean percentage expiratory reduction in mid tracheal lumen CSA for COPD participants was 59 ± 19% and was similar for men and women (58 ± 20% and 60 ± 17% respectively, p = 0.61).
The expiratory reduction of the mid trachea was not different for controls (mean percentage expiratory reduction = 57 ± 20%, p = 0.55). There were no cases in which visual assessment demonstrated evidence of either a localized tracheal segment with substantially greater collapse compared to the site of standard measurement or evidence of extrinsic tracheal compression by adjacent vascular structures or a mass.
Percentage expiratory tracheal collapse and BMI
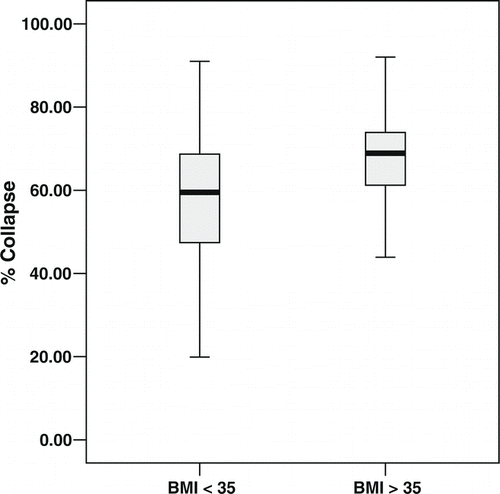
A scatter plot of tracheal collapse () in the study population reveals substantial variability and little correlation with BMI (R2 = .003). However, analysis of variance of tracheal collapse shows a significant interaction of subject type by BMI (p = 0.004). We therefore analyzed the relationship between tracheal collapse and BMI separately by subject type. There is significantly less variance in collapsibility among the subset of COPD participants with morbid obesity (n = 20) than among non-morbidly obese COPD participants (F = 6.98, p = 0.01); however, this is not the case for controls (F = 2.76, p = 0.1). Examination of stem-and-leaf plots reveals that COPD participants with BMI ≥ 35 have greater tracheal collapse with a smaller interquartile range (). Mean expiratory mid-tracheal collapse was 69% ± 12% (coefficient of variation [CV] = 18%) among COPD participants with BMI ≥ 35, compared to 57% ± 19% (CV = 33%) among COPD participants with BMI < 35 (p = 0.002). In contrast, among controls, mean expiratory mid-tracheal collapse was 49% ± 20% (CV = 41%) among subjects with BMI ≥ 35, compared to 60% ± 20% (CV = 33%) among subjects with BMI < 35 (p = 0.12).
Figure 1. Scatter plot of percentage expiratory tracheal collapse as a function of BMI shows considerable variability.
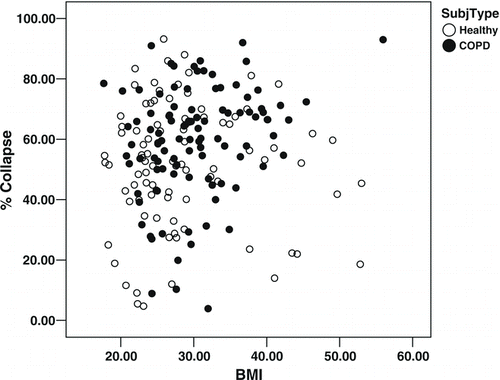
Figure 2. Stem-and-leaf plots of percentage expiratory tracheal collapse in COPD patients by BMI category.
Among the subgroup of COPD patients with BMI ≥ 35, we compared mean SGRQ symptom subscale values between participants with mean expiratory mid-tracheal tracheal collapse ≥ 70% (n = 8) and <70% (n = 12). Mean SGRQ symptom subscale scores were 59 ± 17 for participants with tracheal collapse ≥ 70% compared to 41 ± 19 for those with tracheal collapse < 70% (p = 0.04).
COPD patients in the higher BMI category also demonstrated shorter 6MWT distances and higher (worse) total SGRQ scores compared to those in the lower BMI category as shown in (p = 0.003 and 0.013, respectively). However, the SGRQ symptom subscale score did not differ significantly by BMI category (p = 0.588). There was no significant difference in mean FEV1 values or the percentages of patients receiving standard medical therapy for COPD by BMI category. Additionally, there was no association between the presence of chronic cough and BMI level in our cohort. Among 80 participants with BMI < 35, 32 (40%) reported a chronic cough; among 20 participants with BMI ≥ 35, 8 (40%) reported a chronic cough (p = 1.0).
Table 3. Functional, symptom and treatment indices by BMI category in COPD study population
Among 75 COPD participants with available data in the medical record to assess the presence or absence of OSA, 16 (21.3%) had been previously diagnosed with and prescribed treatment for this condition. As expected, the prevalence of OSA was greater among morbidly obese subjects than among other subjects (43.0% and 16.0%, respectively); however, this difference did not reach statistical significance (p = 0.06 by Fisher's exact probability test). There was no statistically significant difference in percentage expiratory tracheal collapse between COPD participants with and without OSA (60.6% and 62.4%, respectively, p = 0.73).
Discussion
In this study, we found that morbidly obese COPD patients demonstrated greater expiratory tracheal collapse than non-morbidly obese and normal weight COPD patients. In contrast, no difference in expiratory tracheal collapse was found between morbidly obese and non-morbidly obese and normal weight controls.
Whereas a variety of respiratory alterations have been associated with obesity (Citation24), to our knowledge this is the first reported association between morbid obesity and excessive expiratory tracheal collapse among COPD patients (). Notably, a link between obesity and COPD has recently been recognized (Citation25). Although the mechanisms underlying this association are unclear, it has been postulated that there may be a potential interaction between abnormal adipose tissue function, systemic inflammation, and COPD (Citation25, 26). A previous prospective cohort study found that obesity is more prevalent among patients with chronic bronchitis, whereas low body weight is more prevalent among patients with emphysema (Citation1). More recently, attention has shifted from body weight alone to the presence of the complex metabolic syndrome. For example, investigators have reported a substantial prevalence of MetS among patients with COPD, especially those with chronic bronchitis (Citation12,13). As 80% of morbidly obese COPD patients in our study had coexisting MetS, it is intriguing to consider whether excessive dynamic expiratory tracheal collapse is part of an inflammatory airway phenotype of COPD in association with MetS. Future studies are first necessary to determine whether expiratory tracheal collapse is primarily a consequence of obesity and COPD or if the presence of MetS adds further risk.
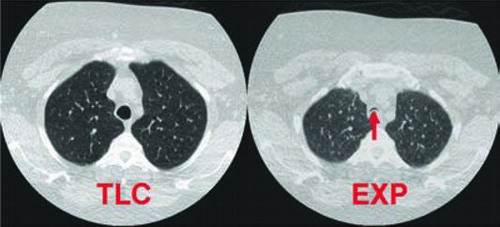
Figure 3. Dynamic expiratory tracheal collapse in a 51-year-old, morbidly obese woman with COPD. Paired axial CT images 1 cm above aortic arch obtained at total lung capacity (TLC) and during forced expiration (EXP) demonstrate near complete dynamic expiratory tracheal collapse (arrow), with 93% expiratory reduction in tracheal luminal cross-sectional area. Image is displayed using large field of view in order to also illustrate obese body habitus.
From a practical standpoint, the association between morbid obesity and excessive tracheal collapse suggests that clinicians should consider evaluating for excessive expiratory tracheal collapse when confronted with a morbidly obese COPD patient having greater quality-of-life impairment and worse exercise performance than expected based on functional measures. However, because obesity is independently associated with reduced exercise performance among COPD patients, further studies are necessary to determine whether there are specific clinical or laboratory tests able to predict which morbidly obese COPD patients are most likely to have excessive expiratory tracheal collapse. Future studies would also be helpful to more fully characterize the imaging and clinical phenotype of morbidly obese COPD patients.
We acknowledge several limitations of our study. First, our study population is relatively small and our results should thus be validated in a larger population. Second, we acknowledge that excessive expiratory tracheal narrowing may be greater in the supine posture than the upright posture as additional mass loading of the chest wall further restricts lung volume. However, spirometry monitoring demonstrated no difference in volume excursion during forced expiratory image acquisition between morbidly obese and non-morbidly obese COPD subjects. Furthermore, there was no association of expiratory tracheal collapsibility with morbid obesity among control subjects. It thus seems unlikely that supine posture contributes substantially to our findings in the COPD population.
Third, the study is limited by a lack of direct respiratory pressure measurements. Although esophageal manometry could have provided more direct information regarding the uniformity of expiratory efforts, several noninvasive techniques were used to ensure maximal expiratory effort. These included standardized respiratory instructions, practice maneuvers, direct observation, and spirometric monitoring. Additionally, we have reported high reliability of measurements among a subset of individuals with repeated testing over a 1-year interval (Citation27). Thus, it seems unlikely that our results were substantially biased due to effort dependence of forced expiratory tracheal collapse.
Fourth, our measurements were obtained at a standard level in order to ensure consistency with previous studies. As we have previously shown that there is < 2% difference in expiratory collapse between the upper and lower trachea in COPD patients (Citation7) and controls (Citation10), this level is representative of the entire trachea.
Conclusions
In summary, because morbid obesity is positively correlated with the degree of expiratory tracheal collapse among COPD patients, one should consider assessing for excessive expiratory tracheal collapse when confronted with a morbidly obese COPD patient with worse exercise performance and greater quality-of-life impairment than anticipated for their GOLD stage. Future studies are necessary to determine whether the presence of coexisting MetS further increases the risk for excessive expiratory tracheal collapse among morbidly obese COPD patients.
Declaration of Interest Statement
This work was supported by the National Institutes of Health (Grant R01HL084331).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 2002; 122(4):1256–1263.
- Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol 2010; 108(1):212–218.
- Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults. An in-depth review. Chest 2005; 127(3):984–1005.
- Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006; 11(4):388–406.
- Ernst A, Majid A, Feller-Kopman D, Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest 2007; 132: 609–616.
- Majid A, Guerrero J, Gangadharan S, Tracheobronchoplasty for severe tracheobronchomalacia: a prospective outcome analysis. Chest 2008; 134:801–807.
- Boiselle PM, Michaud G, Roberts DH, Loring SH, Womble HM, Millett ME, O'Donnell CR. Dynamic expiratory tracheal collapse in COPD: Correlation with clinical and physiological parameters. Chest 2012; 142:1539–1544.
- Miller MR, Crapo R, Hankinson J, ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J 2005; 26(1):153–161.
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2011. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed December 6, 2012.
- Boiselle PM, O'Donnell CR, Bankier AA, Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT. Radiology 2009; 252(1):255–262.
- O'Donnell CR, Litmanovich D, Loring SH, Boiselle PM. Age and gender dependence of forced expiratory central airway collapse in healthy volunteers. Chest 2011 epub Dec 22, 2011; doi: 10.1378/chest.11-2361.
- Watz H, Waschki B, Kirsten A, The metabolic syndrome in patients with chronic bronchitis and COPD: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009; 136(4):1039–1046.
- Marquis K, Maltais F, Duquay V, The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005; 25(4):226–232.
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006; 23(5):469–480.
- NHLBI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report; NHLBI electronic textbook. www.nhlbi.nih.gov/guidelines/obesity/e_txtbk/txgd/4142.htm. Accessed: December 6, 2012.
- Guidelines for the care of adults with prediabetes and/or the metabolic syndrome in clinical settings. www.ihs.gov/MedicalPrograms/Diabetes/HomeDocs/Tools/ClinicalGuidelines/PreDiabetes_Guidelines_0209.pdf. Date last updated: September 2008. Accessed December 6, 2012.
- Jones PW, Quirk FH, Baveystock CM. The St. George's respiratory questionnaire. Respir Med 1991; 85(suppl B):25–31.
- American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1):111–117.
- Lee KS, Sun ME, Ernst A, Feller-Kopman D, Majid A, Boiselle PM. Comparison of dynamic expiratory CT with bronchoscopy in diagnosing airway malacia: A pilot evaluation. Chest 2007; 131(3):758–764.
- Gilkeson RC, Ciancibello LM, Hejal RB, Montenegro HD, Lange P. Tracheobronchomalacia: dynamic airway evaluation with multidetector CT. AJR Am J Roentgenol 2001; 176(1):205–210.
- Zhang J, Hasegawa I, Feller-Kopman D, Boiselle PM. 2003 AUR Memorial Award: dynamic expiratory volumetric CT imaging of the central airways: comparison of standard-dose and low-dose techniques. Acad Radiol 2003; 10(7):719–724.
- Bankier AA, O'Donnell CR, Boiselle PM. Quality initiatives. Respiratory instructions for CT examinations of the lungs: a hands-on guide. Radiographics 2008; 28(4):919–931.
- Baroni RH, Feller-Kopman D, Nishino M, Tracheobronchomalacia: comparison between end-expiratory and dynamic expiratory CT for evaluation of central airway collapse. Radiology 2005; 235(2):635–641.
- Franssen FM, O'Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5 obesity and COPD. Thorax 2008; 63(12):1110–1117.
- Poulain M, Doucet M, Major GC, The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ 2006; 174(9):1293–1299.
- Tkacova R. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspectives. Mediators Inflamm, epub April 20, 2010; doi:10.1155/2010/585989.
- Boiselle PM, O'Donnell CR, Loring SH, Bankier AA. Reproducibility of forced expiratory tracheal collapse: assessment with MDCT in healthy volunteers. Acad Radiol 2010; 17(9):1186–1189.