Abstract
It is increasingly recognised that new measures of disease ‘activity’ for COPD are required, however the relationship between markers of disease ‘activity’, ‘severity’ and ‘impact’ though closely linked, is poorly understood. Additionally, while change in markers of disease ‘severity’ (e.g. change in FEV1) may be considered a marker of disease ‘activity’, these quantify a single aspect of disease ‘activity’ in COPD rather than measuring the overall disease process and this has stimulated the search for new biomarkers of COPD that reflect the ‘activity’ of the disease process.
The ideal biomarker of disease ‘activity’ would be stable with respect to time since measurement at any time point would then relate to subsequent disease progression. This would allow the influence of a therapeutic intervention to be assessed early, facilitating both phase 2 and 3 clinical trials. Although a number of potential biomarkers of COPD disease ‘activity’ have been studied, to date none have been shown to conclusively relate to disease progression and the stability of underlying disease ‘activity’ therefore requires further consideration. Interestingly, while the variability of disease ‘activity’ of COPD is rarely mentioned in the current literature, and there is uncertainty whether ‘activity’ is constant or highly variable, there are clues from available data as discussed in the current article. Finally we consider how markers of disease ‘activity’, ‘severity’ and ‘impact’ may relate, which is of utmost importance in the ongoing search for new biomarkers in COPD and a greater understanding of the pathogenesis of the disease process.
Keywords :
Disease ‘activity’, ‘severity’ and ‘impact’: interrelationships in COPD
Is a measure of disease ‘activity’ the Holy Grail for COPD, or a variable impossible to quantify? It has recently been suggested that markers of COPD should be categorised into 3 distinct groups (disease ‘activity’, ‘severity’ and ‘impact’), which provide differing information about the patient (Citation1). Disease ‘activity’ describes the underlying pathophysiological process at a given moment in time resulting in end-organ damage that can be measured by a marker of disease ‘severity’ and leading to an ‘impact’ producing an adverse influence on the quality of life of a patient, and there is likely to be a temporal relationship between these factors, which is considered further in the current article.
Disease ‘activity’ is recognised to be distinct from ‘severity’ since subjects with limited impairment of a marker of disease ‘severity’ (e.g. FEV1) may have very active disease and may or may not progress at a different rate to those with ‘severe disease’ (Citation1, 2). An ideal marker of disease ‘activity’ would be a measure (biomarker) of the underlying pathophysiological process which leads to end-organ damage, with the associated changes in a marker of disease ‘severity’ and development of symptoms. In general, a biomarker of COPD disease ‘activity’ would therefore relate to the inflammatory processes, which are widely accepted to be important in the pathogenesis of COPD, however, the final mediators remain uncertain and thus disease ‘activity’ remains nebulous in the absence of an understanding of the key destructive mechanisms with an associated dearth of specific markers.
Measures of disease ‘severity’ include spirometry, gas transfer, exercise capacity and HRCT thorax scans, while it has been suggested that measures of disease ‘impact’ include the mMRC symptom score, daily ‘activity’ score and COPD assessment test (Citation1). However, the 6-minute walk test (and equivalent tests) could arguably be described as either a measure of disease ‘severity’ (since those with more severe disease may be expected to walk a shorter distance in 6 minutes) or a measure of ‘impact,’ because it is a measure of the influence of the ‘severity’ of the disease (muscular dysfunction, breathlessness, fatigability) on an everyday ‘activity’.
Additionally, the ‘impact’ of a disease relates to the complex interaction between the individual's disease ‘severity’, their perception of symptoms and the influence of the disease on their everyday life (which may also vary depending on lifestyle choices). This may differ considerably between people who otherwise have similar disease ‘severity’ or even similar symptoms, and could also be considered in terms of disability (symptoms) and handicap (‘impact’). Therefore the ‘impact’ of a disease may only be truly measured by a marker of quality of life, while a fourth group of tests may be required to measure the symptoms of the disease, since moderate symptoms may have little ‘impact’ on the quality of life experienced by some subjects yet warrant inclusion in any composite measure of COPD.
Current Measures of Disease ‘Activity’
Measures of disease ‘severity’ and disease ‘impact’ are useful in cross-sectional studies. However measures of disease ‘activity’ are crucial, particularly in interventional studies. Although the rate of change of markers of disease ‘severity’ (e.g. change in FEV1) have long been considered to be markers of disease ‘activity’ (Citation1), one must add the caveat that they measure a single aspect of disease ‘activity’ in COPD (namely, that leading to increased airflow obstruction) rather than representing a measure of the activity of the overall disease process. The rate of change of FEV1 for instance could not be considered a gold standard marker of the ‘activity’ of the disease that specifically causes emphysema development and progression, nor could it be considered a direct marker of underlying pathophysiological disease ‘activity’.
There is also uncertainty regarding the most appropriate methods to measure the rates of change of markers of disease ‘severity’ such as FEV1 since the impact of the change in an absolute value of FEV1 is dependent on the baseline value (Citation3). For example, a 60 ml change in FEV1 may have less ‘impact’ in someone with mild airflow obstruction than in an individual with severe airflow obstruction. The use of change in% predicted or standardised residual values partly (but not completely (Citation4)) compensates for this differential although the ‘impact’ of a similar change is still likely to remain greater in more severe disease. Thus the description of different rates of disease ‘activity’ at different disease ‘severity’ stages should be interpreted with caution.
Furthermore, the rate of change of FEV1 is essentially a surrogate, rather than direct, marker of a single aspect of the underlying pathophysiological disease ‘activity’ in patients with COPD since a change in FEV1 will only occur in situations where the underlying disease process specifically alters airflow obstruction. Additionally, FEV1 relates to overall mortality and is therefore at least partly a surrogate marker of overall health, rather than being a specific marker of disease ‘severity’ (or ‘activity’ in the case of rate of change of FEV1) (Citation5). Furthermore it should be remembered that even with constant disease ‘activity’ the rate of change of FEV1 with time may not be linear, especially early and late in the disease process. All these factors further support the need for new biomarkers of disease ‘activity’ for use in highly characterised patients who have or are at risk of developing COPD and emphysema.
Biomarkers of disease ‘activity’
A number of biomarkers of disease ‘activity’ have been considered for use in patients with COPD, and although early study results appeared promising since (for example) fibrinogen relates to disease severity, exacerbations and mortality in patients with COPD (Citation6), to date no biomarker has shown a convincing association with disease progression. Recently, the ECLIPSE study demonstrated an association between disease progression measured by FEV1 decline over a 3-year period and Clara Cell Secretory Protein 16 (CC-16), but not fibrinogen, IL-6, IL-8, TNF-α, CRP or SPD (Citation7). However, the association with CC-16 was weak, and, there was no relationship between it and emphysema severity measured by CT densitometry (Citation8). There is therefore no biomarker, which currently fulfils the criteria for an ideal biomarker for use in patients with COPD (Citation9), namely:
Being central to the pathophysiology of the disease process
Being stable (although varying with events known to relate to disease progression)
Predicting disease progression
Being sensitive to therapeutic factors
Relating to disease severity
The relationship between ‘activity’ and severity in COPD should be considered carefully, since this is not only important for the development of novel biomarkers but may also be central to achieving a greater understanding of the pathogenesis of COPD and the factors related to disease progression. Although ‘activity’ and ‘severity’ are two different things, there may be a temporal relationship between them. For example, if ‘activity’ is stable throughout the course of the disease then ‘activity’ and ‘severity’ would be related in a proportional time dependent manner (), and as such severe disease at a young age indicates preceding high ‘activity’ (or at least frequent periods of high ‘activity’) and mild disease at an older age infers low ‘activity’.
Figure 1. A theoretical model of disease activity and severity with time. If disease activity is stable with a similar age of onset, there will be a proportional relationship between disease severity and activity and age which will reflect preceding activity. The presence of more severe disease at a younger age therefore implies either a younger age of disease onset or more likely (based on the current understanding of the pathogenesis of COPD) this indicates a more active disease process.
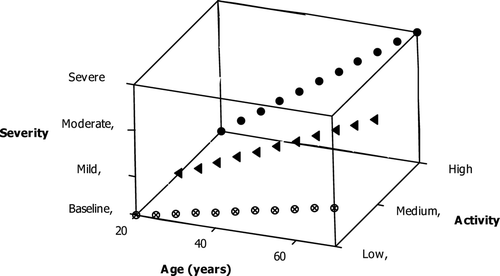
Thus, if the processes of progression are constant, any correlation between the severity and putative ‘activity’ at a given age more likely reflect cause than effect. In addition there will be a further relationship between disease ‘activity’ and ‘impact’ in which, for example, there may be a lesser ‘impact’ if there is low ‘activity’ resulting in a greater delay between disease onset and the development of severe disease. Alternatively if progression is intermittent and depends on specific events like exacerbations, ‘activity’ during these events (rather than the stable state) will be more compatible with subsequent ‘severity’ (and hence with ‘impact’). Conversely, if disease ‘activity’ is highly variable, then there should be no clear temporal relationship between these factors and again any relationship between a proposed marker of ‘activity’ and severity will reflect effect not cause.
Is Disease ‘activity’ Stable?
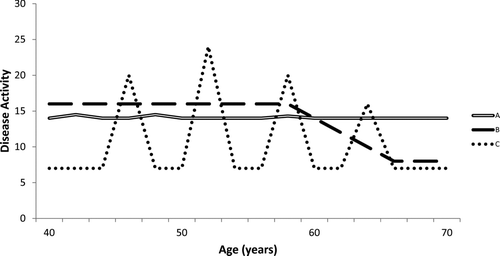
In the absence of an intervention which influences disease progression, an ideal biomarker of disease ‘activity’ would be constant over time (Citation9), since a measurement obtained at any time point would therefore relate to subsequent disease progression. Additionally the stability in a placebo arm would allow the benefit of a therapeutic intervention to be assessed early by its effect on the biomarker. However, the underlying disease ‘activity’ responsible for most of the progression in COPD must also be stable in line with the marker of ‘activity’. Since there are currently no effective markers of disease ‘activity’ this question has yet to be answered (), however there are clues in currently available data.
Figure 2. The variability of disease activity in COPD is currently unknown, although current data suggest it is relatively stable (line A) with a small contribution from exacerbations (minor peaks). Alternatively, activity may be exacerbation predominant (line C) with exacerbations contributing significantly to disease progression (although this degree of contribution is not compatible with more recent data) or disease activity may decline with time/increasing severity (line B).
Firstly, if disease ‘activity’ were highly variable there would be no relationship between a measure of disease ‘activity’ at a single time point and either disease ‘severity’ (in a cross-sectional study) or subsequent disease progression (in a longitudinal study). However, if there are periods of relative stability, when biomarkers are measured in large cross-sectional studies it is more likely that they will be obtained at a point which reflects the predominant (and presumably longstanding) level of disease ‘activity’ with a resulting relationship between disease ‘severity’ and ‘activity’ at least across the population, even if this relationship is not observed for each individual.
For instance, since 4 potential biomarkers of disease ‘activity’ related to the pathophysiology of emphysema (fibrinogen, Aα-Val360, urinary desmosine and sputum neutrophilic markers) are associated with disease ‘severity’(Citation6, Citation10–13) and, importantly, future progression (Citation12, 13), it is likely that there are prolonged periods of relatively stable ‘activity’ through the course of the disease. It should also be recognised that these periods of stable ‘activity’ may not be reflected by the stability (or stable decline) of markers of disease severity such as FEV1 since importantly the rate of change of FEV1 is not necessarily linear and may even demonstrate improvement in 15% of subjects (Citation7). This indicates an even more complex relationship between FEV1 decline and pathological changes within the airway and parenchyma rather than simply reflecting variability in disease ‘activity’.
Second, if disease ‘activity’ is variable it could be anticipated that significant increases in ‘activity’ would be associated with an increase in symptoms (as in exacerbations) which would thus be strongly linked with disease progression especially if these were regular events (frequent exacerbators) and associated with increased inflammation (infective events). However, while exacerbations in general appear to accelerate disease progression, this contribution is relatively small (Citation7) perhaps reflecting the heterogeneity of the events (purulent or inflamed versus non-purulent or not inflamed (Citation14)) and also suggesting that clinically stable state disease ‘activity’ is of greater overall importance even in FEV1 decline.
Third, there is a highly significant difference in the rate of (FEV1) decline in patients who currently smoke versus ex-smokers (Citation7), indicating that modification of a known driver of disease ‘activity’ in COPD will reset the underlying ‘activity’ in a relatively consistent (and presumably stable) manner.
Unfortunately to date, no biomarker has demonstrated a convincing association with disease progression measured by physiological or radiological parameters which would thus become the gold standard measure of both the biomarker and stability of underlying disease. Additionally to date many biomarkers exhibit significant variability even in the short-term (Citation15), and while this probably relates partly to the assay characteristics or selection of an inappropriate biomarker which is not central to the underlying pathophysiological disease process, it may also indicate a level of variability of the underlying disease ‘activity,’ which is difficult to overcome.
Nevertheless, for advances to be made in the field of COPD (particularly therapeutics), a greater understanding of the natural history of COPD and its disease ‘activity’ has to be achieved. This will only be answered by large longitudinal studies conducted over a number of years, with repeated measures of appropriate and relevant biomarkers which can be compared with progression of a range of markers of COPD disease ‘severity’, symptoms and ‘impact’. If repeated measures change and correlate with physiological deterioration it is likely the marker reflects a disease effect, whereas if the baseline correlates with subsequent physiological deterioration, particularly if the marker is stable, it is more likely to indicate disease ‘activity’.
Conclusion
In summary separating disease ‘activity’ from severity is complex. As inflammation is central to the pathophysiology of COPD, the key (rather than generic) mediators and/or their effects should reflect ‘activity’, although cross-sectional correlations with severity may indicate either cause or effect. Cause can be implied if the ‘activity’ relates to severity within a limited age (and hence time) range in a cross-sectional study, assuming a similar age of onset. Alternatively, cause can be implied from longitudinal studies of changing severity. A further option is to relate ‘activity’ biomarkers to a time/severity index although this becomes less relevant when factors such as smoking are not continuous up to the time of assessment. A similar relationship to ‘impact’ should also be explored as it may be that the speed of progression (increasing severity) is an important determinant of individual ‘impact’ because (for example) of the potential for compensatory behaviours to develop with time. Future studies should attempt to identify and define these interactions in detail (i.e. above and beyond simple correlations).
Declaration of Interest Statement
A patent exists on the Aα-Val360 assay (held by Merck, Rahway, NJ, USA), which was developed and validated jointly by Merck and Professor Stockley. Merck played no role in the current manuscript. The authors are responsible for the content and the writing of this paper.
References
- Agusti A, Macnee W. The COPD control panel: towards personalised medicine in COPD. Thorax 2013;68:7: 687–690.
- Vestbo J, Rennard S. Chronic obstructive pulmonary disease biomarker(s) for disease activity needed—urgently. Am J Respir Crit Care Med 2010; 182(7):863–864.
- Calverley PM, Burge PS, Spencer S, Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003; 58(8):659–664.
- Quanjer PH, Schouten JP, Miller MR, Avoiding bias in the annualized rate of change of FEV1. Am J Respir Crit Care Med 2007; 175(3):291–292; author reply 292.
- Stavem K, Aaser E, Sandvik L, Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur Respir J 2005; 25(4):618–625.
- Duvoix A, Dickens J, Haq I, Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax 2013;68:7: 670–676.
- Vestbo J, Edwards LD, Scanlon PD, Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011; 365(13):1184–1192.
- Lomas DA, Silverman EK, Edwards LD, Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax 2008; 63(12):1058–1063.
- Stockley RA. Biomarkers in COPD: time for a deep breath. Thorax 2007; 62(8):657–660.
- Lindberg CA, Engstrom G, de Verdier MG, Total desmosines in plasma and urine correlate with lung function. Eur Respir J 2012; 39(4):839–845.
- Carter RI, Mumford RA, Treonze KM, The fibrinogen cleavage product A{alpha}-Val360, a specific marker of neutrophil elastase activity in vivo. Thorax 2011; 66(8):686–691.
- Carter RI, Ungurs MJ, Mumford RA, Aalpha-Val360: A marker of neutrophil elastase and COPD disease activity. Eur Respir J 2013; 41(1):31–38.
- Parr DG, White AJ, Bayley DL, Inflammation in sputum relates to progression of disease in subjects with COPD: a prospective descriptive study. Respir Res 2006; 7:136.
- Gompertz S, O'Brien C, Bayley DL, Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Euro Respir J: Off J Euro Soc Clin Respir Physiol 2001; 17(6):1112–1119.
- Dickens JA, Miller BE, Edwards LD, COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res 2011; 12:146.
