ABSTRACT
Background: To evaluate the incidence of pulmonary embolism (PE) in patients with chronic obstructive pulmonary disease (COPD) in Taiwan. Methods: This was a retrospective population-based cohort study using data retrieved from Taiwan's National Health Insurance Research Database (2000 to 2008), which contains 99% of Taiwanese healthcare data. The evaluations included 355,878 COPD patients and 355,878 non-COPD patients for comparison. Results: The incidence of PE in the COPD cohort was 12.31 per 10,000 person-years (1.37/10,000 persons/y), which was approximately 4-times higher than in the comparison cohort (0.35/10,000 persons/y). In the COPD cohort, risk of PE was higher in the young age group (20-59 y, HR 4.64, 95% CI 3.06-7.03) than in other age groups. Risk of PE was higher in patients with COPD combined with hypertension, coronary artery disease, and cancer, or those with previous operation (HR 4.16, 4.75, 4.56, and 4.50 respectively) than in those with COPD and no comorbidity. Conclusions: The overall incidence of PE is lower in Taiwan than in western countries. However, the prevalence of PE in COPD patients is higher than in non-COPD patients and increases with age. It is crucial to incorporate PE into the differential diagnosis of COPD exacerbation for clinical physicians.
Introduction
The incidence of pulmonary embolism (PE) is increasing in hospitalized patients with the advances in new diagnostic methods and the vigilance of clinical physicians (Citation1). PE is the third most common acute cardiovascular disease after myocardial infarction and stroke (Citation2). The incidence of PE in hospitalized patients 18 years of age or older was reported 110 patients/100,000 adult population (0.77% of hospitalized adults), accounting for 30% of all venous thromboembolism (VTE) events (Citation3–7).
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of mortality worldwide with more than 3 million deaths annually (Citation8). Previous studies have reported dyspnea, malnutrition, osteoporosis, skeletal muscle weakness, and increased cardiovascular events in COPD patients (Citation9–11). As the disease progresses, patients become more dyspneic, leading to the development of a feedback cycle, in which dyspnea leads to immobility and immobility induces disuse muscle atrophy, which then worsens dyspnea.
Consistent with Virchow's triad, the risk of PE increases with immobility. Death of COPD patients typically occurs during a period of acute exacerbation (Citation12). Studies have shown that the most common cause of acute exacerbation is infection (50% to 70%), followed by environment pollution (10%) (Citation13).However, the causes of up to 30% of exacerbations remain unclear (Citation13). The incidence of PE is reported 24.7% or higher in hospitalized COPD patients with acute exacerbation (Citation14). Because of poor pulmonary reserve, COPD patients might suffer more severe consequences and increased mortality if PE occurs. However, PE is less common in Asian people than in Caucasian people (Citation3, 4, Citation6, Citation15–17). In Western countries, anticoagulant therapy have been widely used to prevent VTE events for several decades, leading to substantial decline in PE-specific mortality (Citation18). However, preventive anticoagulant therapy is difficult to put into clinical practice in Asians because of low incidence of PE and high risk of bleeding (Citation19).
In this study we aimed to identify the incidence of PE in patients with COPD in Taiwan, and to determine the association between different comorbidities and COPD with PE to identify high risk COPD patients who might benefit from anticoagulant therapy.
Materials and Methods
Data Source
Taiwan's National Health Insurance (NHI) is a universal insurance programs established in 1996, which covers almost 99% of the Taiwanese population. In this study, patient data were obtained from Taiwan's National Health Insurance Research Database (NHIRD), which contains claims data form the NHI. All personal information was encrypted before release to the public to protect patient privacy. Diseases were diagnosed according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) using inpatient clam data and the catastrophic illnesses registry. We confirm that all data was de-identified and analyzed anonymously. In addition, this study was also approved by the Ethics Review Board at China Medical University (CMU-REC-101–012).
Study population
This study used a retrospective population–based cohort design. A cohort of newly diagnosed COPD patients (ICD-9-CM 490, 491.0, 491.2, 491.9, 492, 493.2, 496 and V81.3) from 2000 to 2008 was established. The comparison group was formed by random selection from insured people without COPD and PE prior to baseline, with frequency matched by sex, age (every 5-year span), and index year. Patients who had experienced PE prior to the baseline year, or were aged less than 20 years at the baseline, were excluded. The principal outcome was PE (ICD-9-CM 415.1, 639.6 and 673.8). Follow-up was terminated upon observation of first tine of PE event, withdrawal from the NHI, or at the end of the evaluation period on December 31, 2009.
PE comorbidities were collected at the baseline as potential confounding factors. These comorbidities included hypertension (ICD-9-CM 401–405), coronary artery disease (CAD; ICD-9-CM 410–413, 414.0, 414.8 and 414.9), diabetes mellitus (DM; ICD-9-CM 250), stroke (ICD-9-CM 430–438), and hyperlipidemia (ICD-9-CM 272), which were identified using inpatient clam data and cancer (ICD-9-CM 140–208), identified using the catastrophic illnesses registry. Patients with previous operation were identified using the ICD code for the procedure prior to the baseline.
Statistical analysis
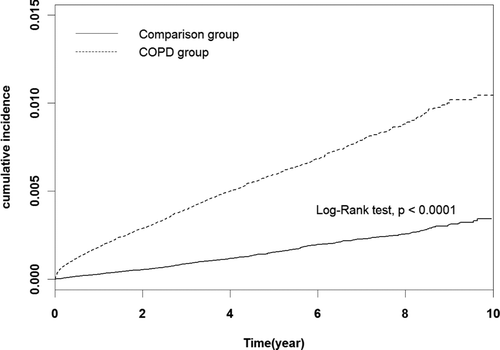
Chi-square test was used to analyze categorical variables and Student's t-test was used to assess continuous variables comparing the COPD and comparison cohorts at baseline demographic status. The PE incidence rate was calculated for each cohort, then the Kaplan-Meier method was used to prepare measured curves of cumulative PE incidence, and the log-rank test was used assess the differences between these curves. Cox proportional hazards regression was applied to estimate the hazard ratio (HR) and 95% confidence interval (CI) of PE in the COPD cohort compared to the comparison cohort.
All statistical analysis was performed using the SAS 9.1 statistical package (SAS Institute Inc., NC, USA). R software (R Foundation for Statistical Computing, Vienna, Austria) was used to prepare cumulative incidence curves. A P-value < 0.05 in 2-tailed tests was considered significant.
Results
In this study, we evaluated 355,878 COPD patients and 355,878 comparison patients (). Age (71.3 y) and sex ratio displayed nonsignificant differences between the groups. The COPD cohort had a higher proportion of patients with comorbidities and a higher rate of previous operation than the comparison cohort.
Table 1. Baseline demographic status and comorbidity compared between Comparison and COPD group
Our results indicated that the incidence of PE in the COPD cohort was 12.31 per 10,000 person-years (1.37/10,000 patients/y), which was approximately 4-times higher than that in the comparison cohort (3.16 per 10,000 persons-y or 0.35/10,000 patients/y; ). After adjustment for potential confounders, the COPD cohort displayed a 3.45-fold higher risk of developing PE than the comparison cohort (95% CI 3.10–3.83). Irrespective of age group, the COPD patients displayed higher risk of PE than the comparison cohort. We observed greatest differences in risk of PE between COPD and non-COPD patients in the young age group (20–59 y, HR 4.64, 95% CI 3.06–7.03). Risk of PE increased with increasing age in COPD patients. Kaplan-Meier estimates of the cumulative incidence of PE indicated that the incidence of PE in the COPD cohort become increasing higher than that in comparison cohort with time (; P < 0.0001 in log rank test). Sex-specific HR of PE was almost identical in each cohort (HR 2.83 vs. 3.87).
Table 2. Incidence of PE and multivariate Cox proportional hazards regression analysis measured hazard ratio for study cohort
shows the combined effects of COPD and comorbidity on risk of PE development. We included patients without COPD or comorbidity in the reference group. Patients with COPD and comorbidity, such as hypertension (HR 4.16), CAD (HR 4.75), and cancer (HR 4.56), or those with COPD and previous operation (HR 4.50) displayed increased risk of PE. The incidence of PE in patients with previous operation was nearly 6 times higher than that in patients without COPD or previous operation (13.19/10,000 person-y vs. 2.47/10,000 person-y). Using patients without COPD or previous operation for comparison, patients with COPD and previous displayed higher risk of PE (HR 4.50, 95% CI 3.90–5.19) than patients with COPD and no previous operation (HR 3.94, 95% CI 3.35–4.62) and non-COPD patients with previous operation (HR 1.43, 95% CI 1.21–1.70).
Table 3. Joint effect between comorbidity in association with PE in study population
Discussion
In this study, we identified that the incidence of PE is much higher in patients with COPD than in patients without COPD. COPD is a systemic disease that severely weakens patients. The feedback cycle of dyspnea, immobility, muscle atrophy, and dyspnea associated with COPD increases the risk of subsequent VTE and PE. In addition, cardiopulmonary reserves deplete with increasing age. This trend can explain the increasing rate of PE with increasing age in both cohorts, especially in the COPD patients. We based comparisons of HR on age stratification. The HR therefore indicated that COPD patients had higher risk of PE compared to non-COPD patients in the same age group. This result differed from those of Stein et al, which indicated equal risk of PE in all age groups (Citation20). In our study, the cumulative incidence of PE increased after long-term follow-up in both groups. However, HR did not increase with patient age. HR of PE is higher in the young age group (20–59 y) than in older age groups. The possible explanation might be that old patients apt to have more comorbid diseases in each COPD and non-COPD cohorts, which might be risk factors of thromboembolism events. Therefore, the HR of old age groups was lower than young age group.
The second major finding of our study was the higher prevalence of PE in women compared to men in both the COPD and non-COPD groups. The finding was similar to the results of previous studies (Citation4, Citation21). Investigators have proposed several mechanisms to explain the influence of sex on VTE such as use of oral contraceptives, use of hormone therapy, and pregnancy (Citation22–24). In our study, we observed that COPD increased the risk of PE. This finding might be caused by the described dyspnea, immobility, muscle atrophy, dyspnea feedback cycle and effects of vascular dysfunction in COPD patients (Citation11, Citation25, Citation26).
In this study, we further investigated the cumulative risk of PE in patients with COPD and other comorbidity. Few studies have evaluated the effects of comorbid conditions on patients with COPD. It is likely that COPD patients have increased risk of complications of other comorbid diseases, such as hypertension, diabetes, stroke, and cancer. These diseases are more prevalent in older adults and all are risk factors for VTE (Citation27–29). In this study, the COPD group was associated with increased risk of PE than in non-COPD patients with comorbidity such as hypertension, diabetes, coronary disease, cancer, stroke, and hyperlipidemia, or those with previous operation (). The HR of PE in these patients was up to 4 times higher than in the reference group. However, not all comorbidities increased the risk of PE in COPD patients. For example, diabetes, stroke, and hyperlipidemia did not increase the risk of PE in patients with COPD and no other comorbidity. It is possible that during the long follow-up period, physicians might have provided anti-platelet or statin therapies to these patients, leading to observations of relatively low risk of PE in comorbidity-complicated COPD patients than in COPD patients without comorbidity. Cancer might induce thrombotic potential, which might explain the high risk of PE in patients with COPD and cancer. It was reported that patients with DM have significantly higher prevalence of PE (Citation30). In our study, diabetes did not increase the risk of PE in COPD patients. However, the underlying mechanism required further investigations.
Several studies have described that patients with COPD display increased risk of deep vein thrombosis (DVT) with the incidence of DVT ranging from 9.2% to 62% in hospitalized COPD patients (Citation20, Citation31, Citation32). Pek et al reported that DVT is uncommon in Asian patients with COPD(Citation33). Therefore, routine screening for DVT is not recommended. In this study, we identified that the incidence of DVT is very low in COPD patients with PE, which is compatible with the result of Pek et al. This finding indicated that DVT and PE in patients with COPD are independent events. We agree that routine screening for DVT in COPD patients might be unnecessary in Taiwan; however, we recommend screening for pulmonary embolism in COPD patients with acute exacerbation even if patients display no signs of DVT.
Limitations
This study's strengths were its large sample and population-based design, which increased the generalizability of results. However, it was also subject to a few limitations. First, the NHIRD did not provide information on selected variables that would have been relevant to our investigation, such as the detailed demographic information on smoking habits, alcohol consumption, body mass index, socioeconomic status, and family history of systemic disease. These could be major risk factors for COPD and PE. Thus, our analyses were limited to the available data, and we were unable to conduct more sophisticated tests including adjustment for such variables. Second, the medication history is also not provided. Usages of anticoagulant or antiplatelet agents can reduce the incidence of PE/DVT in patients with cardiovascular diseases or accepting orthopedic surgery, which has been well documented. Patients already accepted the anticoagulant or antiplatelet drugs maybe have lower risk of PE, which might influence on the result. Third, the NHIRD did not provide data of pulmonary function tests to define the severity of patients with COPD. The diagnosis of COPD is based on the ICD-9 code and the impression of clinical physicians. The diagnosis of COPD might be lack of accuracy. Both factors might influence the results. At last, evidence deriving from any cohort study is generally considered of a poorer methodological quality than data obtained from randomized trials, because a cohort study design is associated with higher likelihood of biases related to confounder adjustment. Therefore, despite our meticulous study design and efforts to control confounding variables, a key limitation was that bias could remain for unmeasured or unknown confounders. However, the data we obtained on COPD and PE diagnoses were highly reliable.
In conclusion, results from our population-based retrospective cohort study indicate that the incidence of subsequent PE is approximately 4-times higher in COPD patients than in non-COPD comparison patients. Clinical physicians should be alert that COPD is a risk factor of PE. However, further large-scale controlled prospective studies are needed to confirm our findings.
Declaration of Interest Statement
All authors report no conflicts of interest.
Acknowledgments
The study was supported in part by the study projects of DMR-102-014, DMR-102-023; Taiwan Department of Health Clinical Trial and Research Center and for Excellence (DOH102-TD-B-111-004), Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03, Taiwan); and International Research-Intensive Centers of Excellence in Taiwan (I-RiCE) (NSC101-2911-I-002-303).
References
- DeMonaco NA, Dang Q, Kapoor WN, Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am J Med 2008; 121(7):611–617.
- Giuntini C, Di Ricco G, Marini C, Melillo E, Palla A. Pulmonary embolism: epidemiology. Chest 1995; 107(1 Suppl):3S–9S.
- Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest 1995; 108(4):978–981.
- Stein PD, Huang H, Afzal A, Noor HA. Incidence of acute pulmonary embolism in a general hospital: relation to age, sex, and race. Chest 1999; 116(4):909–913.
- Stein PD, Patel KC, Kalra NK, Estimated incidence of acute pulmonary embolism in a community/teaching general hospital. Chest 2002; 121(3): 802–805.
- Yang Y, Liang L, Zhai Z, Pulmonary embolism incidence and fatality trends in chinese hospitals from 1997 to 2008: a multicenter registration study. PLoS One 2011; 6(11):e26861.
- Stein PD, Matta F. Acute pulmonary embolism. Curr Probl Cardiol 2010; 35(7):314–376.
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997; 349 (9061):1269–1276.
- Madsen H, Brixen K, Hallas J. Screening, prevention and treatment of osteoporosis in patients with chronic obstructive pulmonary disease - a population-based database study. Clin Respir J. 2010; 4(1):22–29.
- Sabit R, Bolton CE, Edwards PH, Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007; 175(12):1259–1265.
- Clarenbach CF, Thurnheer R, Kohler M. Vascular dysfunction in chronic obstructive pulmonary disease: current evidence and perspectives. Expert Rev Respir Med. 2012; 6(1):37–43.
- Seneff MG, Wagner DP, Wagner, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995; 274(23):1852–1857.
- Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax. 2006; 61(3):250–258.
- Rizkallah J, Man SF, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3):786–793.
- Choi KJ, Cha SI, Shin KM, Prevalence and Predictors of Pulmonary Embolism in Korean Patients with Exacerbation of Chronic Obstructive Pulmonary Disease. Respiration 2012.
- Stein PD, Beemath A, Olson RE. Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients. Am J Cardiol. 2005; 95 (12):1525–1526.
- Stein PD, Kayali F, Olson RE, Milford CE. Pulmonary thromboembolism in Asians/Pacific Islanders in the United States: analysis of data from the National Hospital Discharge Survey and the United States Bureau of the Census. Am J Med. 2004; 116(7):435–442.
- Guyatt GH, Akl EA, Crowther M, Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):7S–47S.
- Mehta RH, Parsons L, Peterson ED, National Registry of Myocardial Infarction Investigators. Comparison of bleeding and in-hospital mortality in Asian- Americans versus Caucasian-Americans with ST-elevation myocardial infarction receiving reperfusion therapy. Am J Cardiol. 2012; 109(7):925–931.
- Stein PD, Beemath A, Meyers FA, Olson RE. Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease. J Cardiovasc Med (Hagerstown). 2007; 8(4):253–257.
- Geibel A, Olschewski M, Zehender M, Possible gender-related differences in the risk-to-benefit ratio of thrombolysis for acute submassive pulmonary embolism. Am J Cardiol. 2007; 99(1):103–107.
- Ferguson JN. Pulmonary embolization and oral contraceptive. JAMA. 1967; 200 (6):560.
- Evans GL, Dalen JE, Dexter L. Pulmonary embolism during pregnancy. JAMA. 1968; 206(2):320–326.
- Grady D, Sawaya G.. Postmenopausal hormone therapy increases risk of deep vein thrombosis and pulmonary embolism. Am J Med. 1998; 105(1):41–43.
- Barr RG. The epidemiology of vascular dysfunction relating to chronic obstructive pulmonary disease and emphysema. Proc Am Thorac Soc. 2011; 8(6):522–527.
- Yang Q, Underwood MJ, Hsin MK, Liu XC, He GW. Dysfunction of pulmonary vascular endothelium in chronic obstructive pulmonary disease: basic considerations for future drug development. Curr Drug Metab. 2008; 9(7):661–667.
- Li G, Lu WX, Wang C. [Pulmonary embolism in cancer: clinical analysis of 60 cases]. Zhonghua Zhong Liu Za Zhi. 2009; 31(7):550–553.
- Stein PD, Beemath A, Meters FA, Kayali F, Skaf E, Olson RE. Pulmonary embolism as a cause of death in patients who died with cancer. Am J Med. 2006; 119(2): 163–165.
- Sipola P, Hedman M, Jakala P, Prevalence of pulmonary embolism in patients with suspected cardioembolic ischemic stroke. J Thorac Imaging. 2011; 26(1):32–35.
- Movahed MR, Hashemzadeh M, Jamal MM. The prevalence of pulmonary embolism and pulmonary hypertension in patients with type II diabetes mellitus. Chest. 2005; 128(5):3568–3571.
- Shetty R, Seddighzadeh A, Piazza G, Goldhaber SZ. Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination. J Thromb Thrombolysis. 2008; 26(1):35–40.
- Schonhofer B, Kohler D. Prevalence of deep-vein thrombosis of the leg in patients with acute exacerbation of chronic obstructive pulmonary disease. Respiration. 1998; 65(3):173–177.
- Pek WY, Johan A, Stan S, Lee P, Chee CB, Wang YT. Deep vein thrombosis in patients admitted for exacerbation of chronic obstructive pulmonary disease. Singapore Med J. 2001; 42(7):308–311.