Abstract
Introduction: Smoking is a major risk factor for both cardiovascular disease (CVD) and chronic obstructive pulmonary disease (COPD). More individuals with COPD die from CVD than respiratory causes and the risk of developing CVD appears to be independent of smoking burden. Although CVD is a common comorbid condition within COPD, the nature of its relationships to COPD affection status and severity, and functional status is not well understood. Methods: The first 2,500 members of the COPDGene cohort were evaluated. Subjects were current and former smokers with a minimum 10 pack-year history of cigarette smoking. COPD was defined by spirometry as an FEV1/FVC < lower limit of normal (LLN) with further identification of severity by FEV1 percent of predicted (GOLD stages 2, 3, and 4) for the main analysis. The presence of physician-diagnosed self-reported CVD was determined from a medical history questionnaire administered by a trained staff member. Results: A total of 384 (15%) had pre-existing CVD. Self-reported CVD was independently related to COPD (Odds Ratio = 1.61, 95% CI = 1.18–2.20, p = 0.01) after adjustment for covariates with CHF having the greatest association with COPD. Within subjects with COPD, pre-existing self-reported CVD placed subjects at greater risk of hospitalization due to exacerbation, higher BODE index, and greater St. George's questionnaire score. The presence of self-reported CVD was associated with a shorter six-minute walk distance in those with COPD (p < 0.05). Conclusions: Self-reported CVD was independently related to COPD with presence of both self-reported CVD and COPD associated with a markedly reduced functional status and reduced quality of life. Identification of CVD in those with COPD is an important consideration in determining functional status.
Keywords: :
Introduction
In the United States, chronic obstructive pulmonary disease (COPD) is the third-leading cause of death (Citation1) affecting a minimum of 10 million adults (Citation2). Approximately 83 million individuals in the United States have cardiovascular disease (CVD) and it is the leading cause of death averaging one death every 39 seconds (Citation3). Smoking and age have been established as shared risk factors in both COPD and CVD. Smoking has been estimated to cause at least 75% of COPD related deaths (Citation24) and of the 443,000 premature deaths that occur each year due to smoking related illness, 32.7% of these deaths are related to CVD (Citation25). Shared risk factors lack to fully explain the relationship between both conditions as studies have shown a relationship between COPD and CVD adjusting for age, gender and smoking (Citation22).
Recently COPD has been recognized as a systemic disease possibly affecting the cardiovascular and cardiac autonomic system (Citation27,28). Subjects with COPD have been found to have higher levels of systemic inflammatory markers (Citation15–17). COPD has been associated with increased risk of myocardial infarction and stroke, which contribute substantially to the overall mortality of these patients. In a study done by Sin and Man a relationship was found between low FEV1pp and cardiovascular mortality (Citation23).
Alternatively, CVD is an important co-morbid condition within COPD with more patients with COPD suffering or dying from cardiovascular causes than from respiratory failure. The Lung Health Study found that 25% of deaths were due to cardiovascular causes and Mapel et al. found that cardiovascular complications such as arrhythmias, coronary artery disease, or congestive heart failure affected 38.9% of those with COPD versus 22.1% without COPD (Citation26, Citation13).
Despite the documented prevalence of coexistent CVD and COPD, the nature of this relationship remains understudied. It was hypothesized that individuals with physician diagnosed self-reported CVD are more likely to have COPD, greater COPD severity, and an increase in exacerbation related hospitalizations in a population of smokers. In addition, it was hypothesized that lower functional status was related to both CVD and COPD.
Methods
Study population
A case control design was conducted utilizing the first 2500 subjects from the COPDGene cohort, a multi-center study of the genetic susceptibility to COPD. COPD cases and controls were assigned by spirometry. Local IRB approval was obtained by each clinical site (Appendix 1) to enroll subjects and all subjects gave written informed consent prior to participation in the study. Detailed inclusion and exclusion criteria have been documented elsewhere (Citation4). In short, subjects consisted of Non-Hispanic White and non-Hispanic African Americans between the ages of 45 and 80 who were current and former smokers with at least 10 pack-years of smoking history.
Definition of COPD
COPD was the main outcome of interest. Post bronchodilator spirometry was obtained using the ndd EasyOneTM spirometer (Zurich, Switzerland)(Citation5) with airflow obstruction defined as an FEV1/FVC ratio < lower limit of normal (LLN) in accordance to prediction equations by Hankinson and colleagues (Citation6) and usage previously published in COPDGene (Citation29). Levels of severity were further characterized in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification (Citation7). Control subjects were defined as subjects with a FEV1/FVC ≥ LLN and FEV1 > 80% of predicted (GOLD stage 0). Subjects with an FEV1/FVC < LLN but a FEV1 ≥ 80% predicted were identified as GOLD stage 1(n = 212) and subjects with an FEV1/FVC ≥ LLN but a FEV1 < 80% predicted were identified as GOLD-unclassified subjects (n = 227) and excluded from these analyses. COPD cases were defined as GOLD stage 2, 3, and 4 and smoking controls (GOLD stage 0) were defined as noted above.
Definition of CVD
Several questionnaires were utilized to acquire data for the study and were administered by a trained professional research coordinator (see full data collection forms on COPDGene web site at www.COPDGene.org). CVD was defined as subjects who answered “Yes” on the medical history questionnaire as having experienced at least one of the following conditions in the past: heart attack, congestive heart failure (CHF), coronary artery disease (CAD), peripheral vascular disease (PVD), angioplasty, coronary artery bypass graft(CABG), transient ischemic attack (TIA), and stroke.
Hospitalizations
Subjects were asked if they had a flare up in their chest in the past twelve months. For each exacerbation occurrence up to a maximum of six occurrences, subjects were asked to mark how the flare up was treated. Treatment options consisted of no special treatment, just increased your usual medication at home, took additional antibiotic or steroid medication which you keep at home, consulted your doctor who prescribed additional antibiotic and/or steroid treatment but did not admit you to the hospital or admitted to the hospital. We were specifically interested if the exacerbations lead to hospital admissions.
Smoking status
Smoking status was a created variable of current and former smokers using a modified questionnaire taken from the ATS adult respiratory questionnaire (Citation8). Subjects were classified as current smokers if they answered “Yes” to the following question: Do you now smoke cigarettes (as of a month ago)? Subjects who did not answer “Yes” to the aforementioned question were classified as former smokers.
Pack-years
Pack-years were calculated using standard questions from the ATS Adult Respiratory Questionnaire. The average number of cigarettes smoked per day was divided by 20 to convert to packs.
Hmg CoA reductase inhibitor (statin) use
A medication questionnaire was used to identify medications taken by each subject. A dichotomous variable was created if subjects identified themselves as taking any medication (brand name or generic), which fell into the statin category.
Additional covariates
Variables such as age, gender, and race were ascertained from a demographic questionnaire. Body mass index was a calculated variable dividing weight in kilograms by meters squared (kg/m2).
Six-minute walk test
The 6-minute walk test (6MWT) is recorded as the total distance in feet a subject can walk in 6 minutes. We used the 6MWT as a measure of functional status of the subjects using American Thoracic Society standardized guidelines (Citation9).
BODE index
The body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index is a multidimensional grading system utilized to assess respiratory mortality. The BODE index is derived from points in four categories; threshold value of FEV1, 6 minute walk distance, score on the Modified Medical Research Council Dyspnea Scale (MMRC), and BMI as previously described (Citation10). The BODE index is a total score ranging from 0 to 10 with a higher score indicating greater risk. We utilized the BODE index as a measure of functional status.
St. Georges Respiratory Questionnaire
The St. Georges Respiratory Questionnaire is a tool used to assess quality of life in those with chronic airflow limitation. It is a standardized questionnaire containing 76 items divided into 3 subcomponent scores: symptoms, activity, and impacts. The total score varies from 0 to 100 with higher scores indicating worse health status.
Statistical analysis
Data are presented as mean ± standard deviations, odds ratios, and 95% confidence intervals. The associations between individual demographic variables and COPD were tested with X2. The t-tests were used to test difference in means between groups. The trend of self-reported CVD across GOLD stages was tested using the Jonckheere Terpstra test. Univariate logistic regression was used to test individual CVD diagnoses and COPD (defined as GOLD 2–4), while multivariable logistic regression was used in identifying self-reported CVD as a primary risk factor for COPD adjusting for the following covariates: age, gender, race, smoking status, pack-years, BMI, hypertension, and statin use. Ordinal logistic regression was used to examine the relationship between self-reported CVD and COPD severity as defined by GOLD stage (Citation2–4) and BODE index in those with COPD after controlling for the covariates listed here.
Generalized linear models were used to test the association of self-reported CVD with St. George's questionnaire score and 6MWT distance. Bonferroni adjustment was conducted to account for multiple comparisons. An alpha level of 0.05 was considered statistically significant. The computer software SAS for Windows (version 9.2, SAS Institute Inc, Cary, NC) was used for the statistical analysis.
Results
Characteristics of study participants are shown separately for those with COPD (GOLD stage 2–4), and without COPD (). On average, COPD subjects were older and more likely to be non-Hispanic White. In subjects with COPD, there were a greater number of former smokers compared to current smokers (65% versus 48%, p < 0.0001). Although there was a greater frequency of former smokers in subjects with COPD, these same subjects had a much higher pack-year history compared to control subjects.
Table 1. Characteristics and distribution of COPD within COPDGene
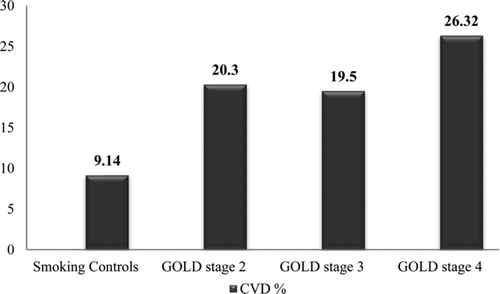
The proportion of individuals with self-reported CVD was greater in those with COPD than those without COPD (21.3% versus 9.1%, , p < 0.0001). Additionally, there was a statistically significant increased trend of higher proportion of those with self-reported CVD with increasing GOLD stages: 9.1%, 20.3%, 19.5%, and 26.3% (GOLD stage 0 and 2–4 respectively, p < 0.0001, ).
Figure 1. Prevalent self-reported cardiovascular disease by COPD severity. Jonckheere-Terpstra trend test significant at p < 0.0001. Smoking controls = subjects FEV1/FVC ≥ LLN and GOLD stage 0.
Characteristics of study participants are shown separately for those with and without self-reported CVD (). As expected CVD subjects were on average older and more likely to be male. Sixty-six percent of subjects with self-reported CVD were former smokers versus 34% who were current smokers (< 0.0001). Additionally, subjects with self-reported CVD had an average pack-year history of 56.5 compared to 43.4 in subjects without self-reported CVD (p < 0.0001). Both hypertension and high cholesterol were significantly associated with self-reported CVD (p < 0.0001 and p < 0.0001, respectively) with 67% of subjects with self-reported CVD having hypertension and 66% having elevated cholesterol.
Table 2. Characteristics and distribution of cardiovascular disease within COPDGene
Subjects with a history of CHF were 7.65 times more likely to have COPD compared to subjects without a history of CHF (, 95% CI = 3.44–17.01, <0.0001). Additionally, having a history of heart attack, peripheral vascular disease, and stroke placed subjects at a greater risk for COPD (p < 0.01, p < 0.05, and p < 0.01, respectively) with subjects who had suffered a prior heart attack were at a 1.9 times greater risk of developing COPD while history of peripheral vascular disease or stroke placed subjects at approximately 2.4 times the risk. Adjusting for covariates, self-reported CVD was independently associated with COPD (OR = 1.61, 95% CI = 1.18–2.20, p < 0.01).
Table 3. Odds of COPD by individual cardiovascular conditions
Statin use was strongly related to self-reported history of hypercholesterolemia (<0.0001) and self-reported CVD (p < 0.0001). When including statin use in the multivariable model assessing the relationship between self-reported CVD and COPD, we found that statin use was protective for COPD (OR = 0.62, 95% CI = 0.48–0.80, p < 0.001). Interactions between self-reported CVD with high cholesterol and self-reported CVD with statin use were not statistically significant (interaction term p = 0.2668, p = 0.8969, respectively) thus not included in the final model.
Subjects with COPD had 61% increased risk of CVD in adjusted models (OR = 1.61, 95% CI = 1.18–2.20, p < 0.01, , model 4). Additionally, within individuals with COPD, pre-existence of self-reported CVD put subjects at a two times greater risk of hospitalization due to an exacerbation (OR = 2.05, 95% CI = 1.27–3.31, p < 0.01) adjusting for demographics, smoking status, pack-years, BMI, lung function (FEV1% predicted), and statin use.
Table 4. Multivariable analysis looking at relationship between CVD and COPD
Six-minute walk test and BODE index were used as measures of functional status. We found that self-reported CVD was independently related to 6MWT distance adjusting for COPD, age, gender, race, smoking status, pack-years, and angina (p < 0.001).
Subjects with COPD had a shorter walk distance compared to those without either condition (1160 ± 418 feet p < 0.05 versus 1482 ± 408 feet, respectively). Subjects with both self-reported CVD and COPD had the shortest 6MWT distance (1068 ± 414, p < 0.05). Within COPD subjects, self-reported CVD was related to a higher BODE index (OR = 1.42, 95% CI 1.06–1.91, p < 0.05) adjusting for demographics (age, gender, race), smoking status, pack-years, and statin use.
We found that self-reported CVD was related to worse quality of life as indicated by the St. George's questionnaire score. In COPD subjects, those with self-reported CVD had a greater total St. George's score compared to subjects without self-reported CVD (45.4 versus 40.1, p < 0.001, respectively) adjusting for demographics, smoking status, pack-years, BMI, and statin use. Subjects with self-reported CVD had on average an almost 6-point increase in their total score compared to those without self-reported CVD.
Discussion
This study examined the relationship between self-reported CVD and COPD in the COPDGene cohort. Self-reported CVD was independently associated with COPD adjusting for known risk factors. There was a statistically significant increased trend in the proportion of individuals with self-reported CVD by higher GOLD stage. Finally, the presence of concurrent self-reported CVD and COPD dramatically decreased functional status and quality of life as measured by 6MWT distance, BODE index, and St. George's questionnaire score compared to having only one condition or in the absence of both self-reported CVD and COPD.
Similarly to our findings, others have previously shown that CVD occurred more commonly in those with COPD compared to those without COPD (Citation13); however, this was the first study to report the relationships between individual self-reported CVD conditions and COPD in addition to displaying an increasing trend of prior self-reported CVD with greater COPD severity.
Prior studies have shown inflammation as a common link between CVD and COPD (Citation14–17). After analyzing various individual cardiovascular risk factors and COPD it was found that the highest risk occurring in subjects having CHF followed by stroke, peripheral vascular disease, and heart attack. This could be explained by inflammatory mechanisms leading to atherogenesis, cardiac remodeling, and subsequent ventricular dysfunction. Due to similar inflammatory processes we would have expected all independent self-reported CVD variables to be associated with COPD risk. Subjects with less severe COPD may have less inflammatory burden leading to specific CVD outcomes. Including GOLD 1's in model 4, the odds of COPD was attenuated (OR = 1.36, 95% CI 1.04–1.78, p < 0.05) yet still showed a significant relationship between self-reported CVD and COPD. By including GOLD 2–4 in our COPD definition, we showed a stronger relationship between self-reported CVD and COPD.
In addition, our study showed the influence of having both self-reported CVD and COPD on functional status specifically identifying that subjects with both self-reported CVD and COPD had worse functional status compared to having either condition alone. Other studies have looked at the influence of each individual condition on functional status and resultant mortality (Citation18–20) however; this is the first study illustrating the synergistic impact of both self-reported CVD and COPD on 6MWT. BODE index was used as another measure of functional status. We found that the BODE index was significantly increased in subjects having both self-reported CVD and COPD compared to COPD alone. The use of the 6MWT and BODE index are simple and effective strategies in gauging the condition of patients living with chronic illnesses.
The St. George's questionnaire is used to assess quality of life in subjects with chronic conditions (Citation11–12). We found that within COPD subjects, those with pre-existent self-reported CVD had on average an increase of 6 points in their total score compared to subjects without self-reported CVD. A change in total score of 4 points considered a clinically important difference (Citation21).
Limitations and strengths
The main limitation with our study is its case control design, which precludes the ability to assess causality. Despite this, we were still able to examine the relationship between CVD and COPD specifically looking at the association between self-reported CVD and COPD severity as measured by GOLD stage and determine if this association was independent of known risk factors.
It is possible that there was survival bias with our study as subjects with COPD are at increased risk for arrhythmias, CAD, and CHF (Citation13); however, if survival bias exists the apparent bias would result in a protective effect of self-reported CVD on COPD in contrast to the current results.
Another potential weakness was in the manner in which CHF was captured. The CVD data was self-reported which limited the ability to verify that subjects had prior CHF versus cor pulmonale. After excluding CHF, self-reported CVD was not significantly related to COPD (OR = 1.29, 95% CI = 0.93–1.78, p = 0.1283) indicating the possibility that CHF may exert a greater impact on the relationship between self-reported CVD and COPD.
Strengths of the study include that COPDGene is a large multicenter study with individuals having a substantial smoking history and include a biracial population of both non-Hispanic African-American and non-Hispanic Whites. Functional status (6MWT, BODE index), and quality of life (St. George's) were also examined in the study participants, which illustrates the synergistic impact of both CVD and COPD on functional decline.
Conclusions
Patients with COPD are more likely to have self-reported CVD even after accounting for smoking history demographics, CVD risk factors, and statin medication use. Overall, self-reported CVD is associated with greater respiratory disease burden among patients with COPD. Subjects having concurrent self-reported CVD and COPD had a markedly reduced functional status. Understanding the burden of CVD in individuals with COPD is important to assessing the course of the disease and subsequent effect on an individual's functional abilities and quality of life.
Declaration of Interest Statement
The project described is being supported by Award Numbers U01HL089897 and U01HL089856 from the National Heart, Lung, And Blood Institute. The content of this manuscript is solely the responsibility of the Jennifer L. Black-Shinn and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. This work is also supported by the Monfort Family Foundation and the COPD Foundation.
The authors are responsible for the content and the writing of this article.
Preliminary results from the current manuscript were accepted and presented as an abstract/thematic poster at the 2010 American Thoracic Society meeting in New Orleans.
References
- Sahyoun NR, Lentzner H, Hoyert D, Trends in causes of death among the elderly. Aging Trends 2001; 1–10.
- Mannino DM, Homa DM, Akinbami LJ, Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ 2002; 51:1–16.
- American Heart Association. Heart disease and stroke statistics—2011 update. Dallas, TX: American Heart Association, 2011.
- Regan EA, Hokanson JE, Murphy JR, Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7:32–43.
- Miller MR, Hankinson J, Brusasco V, Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–187.
- Rodriguez-Roisin R, Anzueto A, Bourbeau J, Calverley P, DeGuia T, Fukuchi Y, Hui D, Jenkins C, Kocabas A, Martinez F, DeOca M, VanWeel C, Vestbo J, Woodhead M, Agusti A, Barnes P, Decramer M, Fabbri L, Jones P, Sin D, Wedzicha J. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2009. http://www.goldcopd.org/Guidelines/guidelines-resources.html.
- Ferris BG. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis 1978; 118:1–120.
- ATS statement. guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Celli BR, Cote CG, Marin JM, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012.
- Jones PW. Quality of life measurement for patients with diseases of the airways. Thorax 1991; 46:676–682.
- Jones PW, Quirk FH, Baveystock CM, A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327.
- Mapel DW, Robinson SB, Dastani HB, The direct medical costs of undiagnosed chronic obstructive pulmonary disease. Value Health 2008; 11:628–636.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107:1514–1519.
- Danesh J, Wheeler JG, Hirschfield GM, C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–1397.
- Kosmala W, Derzhko R, Przewlocka-Kosmala M, Plasma levels of TNF-alpha, IL-6, and IL-10 and their relationship with left ventricular diastolic function in patients with stable angina pectoris and preserved left ventricular systolic performance. Coron Artery Dis 2008; 19:375–382.
- Sakkinen P, Abbott RD, Curb JD, C-reactive protein and myocardial infarction. J Clin Epidemiol 2002; 55:445–451.
- Bittner V, Weiner DH, Yusuf S, Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993; 270:1702–1707.
- Curtis JP, Rathore SS, Wang Y, The association of 6-minute walk performance and outcomes in stable outpatients with heart failure. J Card Fail 2004; 10:9–14.
- Pinto-Plata VM, Cote C, Cabral H, The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004; 23:28–33.
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991; 85 Suppl B:25–31.
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012; 379:1341–1351.
- Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2005; 2:8–11.
- Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 1997–2001. MMWR Morb Mortal Wkly Rep 2005; 54:625–628.
- Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep 2008; 57:1226–1228.
- Anthonisen NR, Connett JE, Enright PL, Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002; 166:333–339.
- Stein PK, Nelson P, Rottman JN, Howard D, Ward SM, Kleiger RE, Heart rate variability reflects severity of COPD in PiZ α1-antitrypsin deficiency. Chest 1998; 113:327–333.
- Stewart AG, Waterhouse JC, Howard P. Cardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary disease. Eur Respir J 1991; 4:1207–1214.
- Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, Crapo JD, Silverman EK, and the COPDGene Investigators. Clinical and radiographic predictors of GOLD–Unclassified smokers in the COPDGene study. Amer J Respir Criti Care Med 2011; 184:57–63.
