Abstract
Extrapulmonary manifestations are recognized to be of increasing clinical importance in Chronic Obstructive Pulmonary disease. To investigate cardiovascular and skeletal muscle manifestations of COPD, we developed a unique UK consortium funded by the Technology Strategy Board and Medical Research Council comprising industry in partnership with 5 academic centres. ERICA (Evaluating the Role of Inflammation in Chronic Airways disease) is a prospective, longitudinal, observational study investigating the prevalence and significance of cardiovascular and skeletal muscle manifestations of COPD in 800 subjects. Six monthly follow up will assess the predictive value of plasma fibrinogen, cardiovascular abnormalities and skeletal muscle weakness for death or hospitalization.
As ERICA is a multicentre study, to ensure data quality we sought to minimise systematic observer error due to variations in investigator skill, or adherence to operating procedures, by staff training followed by assessment of inter- and intra-observer reliability of the four key measurements used in the study: pulse wave velocity (PWV), carotid intima media thickness (CIMT), quadriceps maximal voluntary contraction force (QMVC) and 6-minute walk distance (6MWT). This report describes the objectives and methods of the ERICA trial, as well as the inter- and intra-observer reliability of these measurements.
Introduction
Although COPD is primarily a lung disease, it is now widely recognised that COPD is a heterogeneous condition with a range of extra-pulmonary manifestations including cachexia (Citation1), peripheral muscle dysfunction (Citation2, 3), cardiovascular disease (Citation4, 5) and osteoporosis (Citation5, 6) that have an effect on the severity of the condition.
Two of these extrapulmonary manifestations, namely cardiovascular and skeletal muscle dysfunction, represent a key unmet need in patients with COPD that require the development of new therapies. Cardiovascular disease is the second-leading cause of death in patients with COPD (Citation7), and even subjects with mild spirometric abnormalities have an increased risk of admission or death from cardiovascular causes (Citation8). Similarly skeletal muscle weakness (Citation2, 3) and biopsy abnormalities (Citation9) exist even in patients with mild airflow obstruction and are associated with an increased risk of death (Citation10).
A combination of systemic and local factors such as physical inactivity, oxidative stress, cachexia, exposure to cigarette smoke and inflammation are thought to contribute towards the skeletal muscle dysfunction seen in COPD (Citation11). Since pulmonary rehabilitation is a highly effective therapy in COPD that increases quadriceps strength (Citation12), targeting this abnormality is likely to translate into patient benefit. Objectively measured physical activity relates to muscle mass (Citation3), especially in mild disease, and in a survival analysis by Waschki and co-workers (Citation13), the combination of physical activity measurement and assessment of vascular status predicted mortality better than either alone, suggesting that the cardiovascular and skeletal muscle phenotypes are not identical.
Persistent systemic inflammation has been linked with poorer outcomes in COPD and has been identified as a novel COPD phenotype (Citation14). Recent data suggest that fibrinogen is a promising, stable biomarker of systemic inflammation, and that elevated fibrinogen levels relate to frequent exacerbations and mortality in COPD (Citation15–17). Previous studies have suggested that almost a third of COPD patients suffer from 2 or more exacerbations per year, with a fifth of COPD patients requiring hospitalizations over the course of 1 year (Citation18). For these reasons, the COPD Biomarkers Qualification Consortium (CBQC) has submitted fibrinogen for consideration for qualification as a drug development tool by the Food and Drug Administration (FDA). CBQC was established in 2010 with the aim of collating anonymised data from clinical and observational trials ().
Figure 1. Clinical and Observational studies contributing data towards the COPD Biomarker Qualification Consortium (CBQC) and their sources of funding.
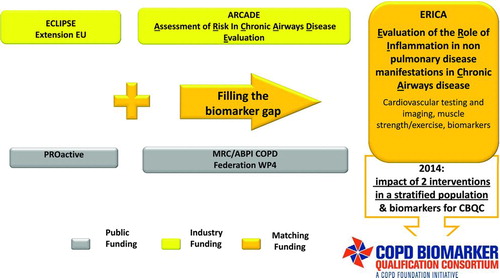
Trials such as ECLIPSE (Citation19), GSK-supported investigator-sponsored eclipse extension study NTR3221, ARCADE (Citation20), PROactive (Clinicaltrials.gov number NCT01388218) and MRC/ABPI WP4 (Clinicaltrials.gov number NCT01620645) will contribute data towards ERICA, thus allowing a sufficiently large dataset to conclusively establish the value of biomarkers or drug development tools (DDTs) as stratification tools (Citation21). Nevertheless there remains a paucity of data to assess whether fibrinogen will also be a satisfactory biomarker for extrapulmonary manifestations of COPD.
Plasma fibrinogen independently predicts cardiovascular risk in the general, healthy population (Citation22), however, the value of fibrinogen in the prediction of the cardiovascular and skeletal muscle manifestations of COPD, and in the interplay between these phenotypes, requires further evaluation. For this reason we conceived the ERICA (Evaluating the Role of Inflammation in Chronic Airways disease) study. The study has three specific aims. First, to determine how effectively plasma fibrinogen predicts the cardiovascular and/or skeletal muscle manifestations of COPD. Second, to determine how fibrinogen and other specific measures of cardiovascular and muscle function predict longer-term outcomes including death, disability and hospital admission, and third, to determine the extent to which subsets of COPD patients with cardiovascular or muscle manifestations overlap. For this purpose, a cardiovascular manifestation is defined as an abnormally raised aortic pulse wave velocity and a skeletal muscle manifestation is defined as quadriceps muscle weakness.
The current report describes the objectives and methods of the ERICA trial, and the standardisation procedures undertaken with the objective to improve inter- and intra-observer reliability of measurements used in the study.
Methods
Subjects
A maximum of 800 COPD patients are to be recruited over a period of 2 years. The study is powered on the basis of a tertile analysis of the two key cardiovascular and muscular biomarkers, systemic arterial stiffness as measured by aortic pulse wave velocity (PWV) and skeletal muscle function, measured as quadriceps maximal voluntary contraction (QMVC). Assuming an average PWV of 10 (SD 1.0) m/s and a minimal clinically relevant difference of 0.4 m/s, 230 patients per tertile will provide 90% power at p < 0.01 to detect this difference between the top and bottom quartiles. For QMVC, assuming an average QMVC of 32 (SD 8) kg, 220 patients per tertile will provide 90% power at a significance of p < 0.01 in order to detect the minimum clinical difference of 3 kg between the top and bottom tertiles. Allowing for a 10% dropout rate and incomplete datasets, approximately 800 patients were calculated to be required. Recruitment is on target to finish in autumn 2013. describes the inclusion and exclusion criteria for subject participation in the study. All participants provided written, informed consent.
Table 1. Inclusion and exclusion criteria for study participants
Study design
ERICA is an on-going longitudinal, observational, prospective study being conducted at 5 centres in the UK, which is presently funded for 2 years by the UK Technology Strategy Board/MRC. As the study was not a trial, the study is registered with the UK Clinical Research Network Study Portfolio with UKCRN ID 11101 (http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID = 11101); the UKCRN is a publically searchable database.
Following baseline visits to perform study measurements, participants are followed up at 6 monthly intervals for 2 years with telephone or postal questionnaires to assess the frequency of COPD exacerbations. For this study, we have defined exacerbations as self-reported increase in COPD symptoms that required treatment with antibiotics and/or steroids and severe exacerbations as those that require hospital admission. The development of cardiac co-morbidity is assessed through new self-reported cardiac symptoms such as exertional chest pain or ankle swelling, physician diagnoses of angina, myocardial infarction, stroke or hypertension and the introduction of new concomitant cardiac medication since the last patient visit or questionnaire.
To assess the impact of COPD on the patient, we used Medical Research Council (MRC) dyspnoea scores, COPD Assessment Tool and the St George's Respiratory COPD Questionnaire, whilst physical activity is self-reported. There are no prohibited medications in the study. All subjects continued their routine prescribed medications throughout the study and the patient's physician may offer treatments (e.g. medication change, rehabilitation) in line with the patients’ needs. These treatment changes are captured at the 6 monthly calls/questionnaires. The study is being conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines.
The research was given a favourable opinion by the Cambridge South East Research Ethics Committee and the local research and development departments at each participating site. The trial steering committee comprises physicians and scientists from five UK academic centres, two academic physicians independent of the recruiting centres and representatives from GlaxoSmithKline.
Outcome measurements
Study assessments are performed at baseline over two visits. Standardised procedures are used in all centres as defined in the study procedure manual. Measurements that are of primary interest are plasma fibrinogen, aortic PWV, carotid intima media thickness (CIMT), 6-minute walk distance (6MWT) and QMVC. Methods for these 5 procedures are described here, but all study parameters are listed in . For all study procedures a Standard Operating Procedure (an SOP) was generated to which all partners adhered. Patients will be registered for long-term health outcomes through Hospital Episode Statistics (HES), a central UK database recording all admissions to National Health Service (NHS) hospitals, and the NHS Information Centre from the Office for National Statistics (previously the Medical Research Information Service), which can report on the status of study participants and provide follow up data for longitudinal studies within the UK.
Table 2. Description of standardized assessments carried out on all study subjects as described in the study manual and existing studies that are carrying out these assessments for the COPD Biomarker Qualification Consortium
Fibrinogen
For determination of plasma fibrinogen, whole blood is collected into a vacutainer tube (sodium citrate as the anticoagulant) by venepuncture from a peripheral vein after a 4-hour fast. Plasma is prepared by centrifugation at 2000 × g for 10 min. Plasma fibrinogen is measured in fresh plasma samples using an automated, modified Clauss method [HemosIL Fibrinogen-C XL, Instrumentation Laboratories(Citation23)]. The assay method is a direct measurement of functional fibrinogen and is the method most commonly used in clinical laboratories. Daily testing on a fibrinogen calibrator was carried out at the Royal Brompton and Harefield NHS hospital laboratory and Addenbrookes hospital laboratory to assess inter-assay variability.
Pulse wave velocity
Following 10 minutes of supine rest, brachial blood pressure was measured three times, and an average of the final two readings was used for analysis. Aortic PWV is measured via the SphygmoCor device (AtCor, West Ryde, Australia), between the carotid and femoral arteries, using a piezoelectric tonometer placed over the artery and ECG gating, as previously described in detail (Citation24). The path length is calculated by subtracting the distance between the carotid pulse and supra-sternal notch, from the femoral artery supra-sternal notch distance. Measurements are made following 4 hours of fasting, and 6 hours without bronchodilator use.
Carotid intima media thickness
Carotid intima media thickness (Citation25) was measured via B-mode ultrasound, using a 7–12 MHz linear probe. Measurements were taken after 10 minutes of supine rest. Both the right and left common carotid arteries are scanned at a distance of 1 cm from the carotid bulb. Images are not ECG gated, and three 10 second loops are recorded for each carotid artery. Images are then transferred in DICOM format to be analysed via Vascular Tools 5 software (Medical Imaging Application LLC, Coralville, USA).
Six-minute walk test
Six-minute walk distance is measured in accordance with the guidelines of the American Thoracic Society (Citation26) except that a practice walk was not performed due to time constraints. Although subjects could set their own walking pace, it was emphasized that they cover as many laps as possible over a standard 30 m, level track during the 6 minutes. Subjects were permitted to use their usual medications prior to the test, and were given standardised encouragement only at the end of each minute during the walking test. Where oxygen was required during the walking test, an additional researcher carried the oxygen cylinder for the patient, but behind the patient in order not to influence the patient's pace.
Quadriceps maximal volitional contraction
QMVC force was measured using the technique of Edwards et al. (Citation27) and expressed as a percentage of predicted values using the equations developed by Seymour and co-workers (Citation2). Patients were verbally encouraged to make a maximal contraction by pushing out (i.e. extension) against an inextensible strap placed above the ankle. The manoeuvre is repeated six times with a minimum 20-second interval between efforts. We used the highest value of contraction which could be sustained for 1 second for analysis.
Harmonising inter-site data collection
Prior to study recruitment, all centres participated in centralised training, individual site training and standardisation visits. Amongst study measurements, QMVC, 6MWT, PWV and CIMT were identified as most prone to systematic error due to variability in equipment and expertise across sites. Intra- observer reliability measurements were therefore carried out using 10 volunteer subjects at each site. Additionally, unlike the 6MWT, QMVC measurement was novel to most sites, and requires the observer to verbally encourage as well as correct patient technique, therefore inter-observer reliability measurements were carried out on 10 volunteers at each site. Intra-class correlation coefficients were used to measure inter- and intra-user reliability. Statistical analysis was carried out using IPB SPSS v 19.
Results
Inter-assay%CV values based on a fibrinogen calibrator tested daily are 6.7% for Royal Brompton Hospital and 9.4% for Cambridge University Hospital.
Intra- and inter-observer variability are shown in . The initial intra-class correlation coefficient (ICC) at one centre for QMVC was entered erroneously; a repeat set revealed an ICC of 0.60. As this was less than the agreed target of 0.85, recommendations were implemented and a final intra-observer repeatability ICC of 0.96 was observed.
Table 3. Intra-observer reliability for Pulse Wave Velocity (PWV), 6-minute walk test (6MWT) and quadriceps maximal volitional contraction (QMVC) and inter-observer reliability measurements for QMVC in 10 volunteers as measured by final intra-class correlation coefficients
Once a site demonstrated competency in all relevant research techniques, they were allowed to recruit study participants. reports the baseline characteristics of the first 10 subjects recruited at each site, in order to provide an example of the likely eventual type of patients who will be recruited to the cohort.
Table 4. Baseline characteristics of the first 50 study recruits at participating sites
Discussion
The main conclusions drawn from setting up ERICA are first that technically demanding measurements, like PWV, carotid media thickness and maximal voluntary contraction force can be made in patients with COPD. Secondly, with relatively little training measurements can be made with good repeatability and low inter-observer variability. Finally, participants recruited to the trial so far appear representative of a typical convenience cohort for COPD trials and there does not appear to be a great deal of variance between individual sites.
Harmonising inter-site data collection is essential in multi-centre studies for the production of valid, reliable results. Centralised training followed by individual site visits has enabled standardisation of techniques, and collection of inter- and intra-observer reliability allowed identification of problems prior to the commencement of study recruitment. We would therefore endorse the current practice that researcher competency in performing novel techniques should be formally evaluated in multi-site trials prior to subject recruitment to ensure good quality data.
Currently if planning a large trial of either an anabolic or cardiovascular therapeutic that addresses extrapulmonary disease manifestations in COPD, an investigator would be hampered by insufficient detail regarding subsets of patients who are most likely to benefit. Although cardiovascular and skeletal muscle dysfunction are serious and common co-morbidities in COPD, they are not observed in all patients diagnosed with the disease. At this time it is also unclear whether there is an association between manifestations of skeletal muscle or cardiovascular dysfunction in COPD and the knowledge of their functional consequences is limited. It would be particularly attractive to have a blood biomarker which permitted selection of patients with these disease manifestations for clinical trials assessing efficacy as large all comer trials are likely to fail (Citation28).
More data are available for cardiovascular disease than skeletal muscle weakness, but in both cases the evidence is that these extra-pulmonary manifestations of COPD are present only in a minority of patients. In a review of approximately 46,000 case records of patients managed in a Kaiser Permanante program several cardiovascular co-morbidities were identified in a minority of patients (at most 25%) though the group as a whole had a 2–3-fold increased risk of subsequent cardiovascular-related admission (Citation8). In relation to the measures used in this study only very small data sets exist; in the study of Maclay et al. (Citation29) roughly half the participants were above the threshold of 10 m/s considered to represent increased future cardiovascular risk. Skeletal muscle dysfunction, while common, is present in only a minority of patients with COPD whether judged by weakness (Citation2) or muscle size (Citation3). Interestingly the prevalence of skeletal muscle weakness, probably around 30% of patients, is not greatly influenced by disease severity judged by FEV1 (Citation3). We have previously discussed the difficulties of developing an anabolic agent for patients with COPD elsewhere (Citation28).
Fibrinogen is attractive as a biomarker, as it is a commonly available test, acceptable, relatively inexpensive and easy measure in clinical practice. Inflammatory markers such as TNF-alpha, IL-6, CRP and p-selectin have been shown to relate to disease severity in some studies, however individual variability for these markers is high (Citation30). A panel of 34 inflammatory markers was recently assessed in the ECLIPSE study, with plasma fibrinogen emerging as the most repeatable biomarker in stable patients with COPD. Although other inflammatory markers such as C-reactive protein and interleukin-6 were also raised in COPD patients as compared to healthy controls, these biomarkers displayed wide variability in stable subjects with COPD over 3 months. Fibrinogen has additionally been shown to relate to disease severity and is predictive of death both in COPD and other conditions (Citation15,Citation31).
However it is largely unknown to what extent fibrinogen has a predictive value to diagnose cardiovascular dysfunction and especially skeletal muscle dysfunction in COPD. Data from very large studies have demonstrated that COPD patients with a self-reported history of cardiovascular disease have higher fibrinogen levels (Citation16), but the relationship between fibrinogen and other measures with predictive value for future cardiovascular disease, such as pulse wave velocity and carotid intima media thickness used in ERICA, is unknown. Eickhoff and colleague used a third detailed and predictive measure, flow mediated dilatation, and found no relationship with fibrinogen, but their cohort was limited to 60 patients (Citation32). Several studies that have investigated fibrinogen and cardiovascular disease in COPD have often excluded those with severe cardiovascular disease (Citation15), which runs the risk of biasing results, and also leaves a data gap for patients most at risk from cardiovascular death. Importantly therefore pre-existing cardiovascular disease is not an exclusion criterion for our study.
Although both quadriceps strength, measured as QMVC and fibrinogen were related to lower physical activity in COPD patients measured by accelerometry in a prior smaller report (Citation33), its relationship to skeletal muscle strength has not been examined before in a large cohort of patients. Our study additionally measures several aspects of muscle function, specifically QMVC, Sniff Nasal Inspiratory Pressure (SNIP) (Citation34), Short Physical Performance Battery (SPPB) (Citation35) and 6-minute walk distance. The short physical performance battery may prove to be of particular interest from a regulatory perspective since it is widely used in academic gerontology. The 4-metre gait speed, which is a component of the SPPB predicts death in elderly people and is reproducible in COPD (Citation36).
Conclusions
In conclusion, ERICA is the first large prospective study to examine the interplay between fibrinogen, skeletal muscle and cardiovascular manifestations of COPD, as well as their relation to exacerbations and mortality. At the conclusion of the study we will be able to determine whether cardiovascular and muscle dysfunction phenotypic “sets” commonly overlap and to what extent fibrinogen is a useful marker of these sets. Identification of the relationship between co-morbidities and potential predictive biomarkers of COPD will help the development of future therapies, and may be useful diagnostically.
Acknowledgments
Authors Mohan, Wilkinson, Singer, and Polkey contributed equally to this work.
With special thanks to Sridevi Nagarajan and, Mellone Marchong and Jessica Middlemiss from the Cambridge Clinical Trials Unit, Addenbrookes Hospital, Cambridge, CB2 2QQ, the research teams at the participating centres and Suresh Chahwala from the Technology Strategy Board, North Star House, North Star Avenue, Swindon, SN2 1UE.
This work was supported by the NIHR Respiratory Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College who part fund the salaries of MIP and DM. IBW and CMM are funded by the British Heart Foundation and the Cambridge Biomedical Research Centre, and receive support for the East Anglian CLRN. All 5 study centres are supported by the NIHR Clinical Research Network.
Declaration of Interest Statement
The authors have no conflicts of interest. Michael Polkey, Divya Mohan, Nichola Gale, Carmel McEniery and Ian Wilkinson have had grants paid to their institute by the Technology Strategy Board. Charlotte Bolton was paid for an educational, non-promotional lecture in 2011 and her institute received consultancy fees from an advisory board in 2012. John Cockcroft has received consultancy fees from Merck, Forest Pharmaceuticals and GlaxoSmithKline (GSK), lecturing fees from Menarini and Sanofi, and his institute has received grants from the Technology Strategy Board and Medical Research Council. William MacNee has received royalties from Health Press Ltd, Hodder & Stoughton Ltd, People's Medical Publishing House, Imperial College Press Ltd, consultancy fees from Pfizer, GSK, Novartis, Almirall, Janssen and received payments from GSK, Novartis and Pfizer and their institute has received money from the Technology Strategy Board. David Lomas has received board membership, lecture, travel expenses and consultancy fees from GSK, consultancy fees from Talecris/Grifols and travel expenses from Boehringer Ingelheim (BI), and his institute has received grants from GlaxoSmithKline. Peter Calverley has received travel expenses from the Medical Research Council, board membership fees from Nycomed, BI, consultancy fees from Novartis, expert testimony fees from Forest, payment for lectures from GSK, Astrazeneca, Takeda and travel expenses from BI, and his institute has received lecture fees from Novartis and Pfizer. Bruce Miller is an employee of GSK, and is married to a GSK employee and shareholder. Ruth Tal-Singer is employed by GSK and owns shares in GSK. Michael Polkey has received consulting fees from Novartis and Philips, travel support from GSK Almirall and BI and his institute has received consulting fees from BI, Lilly, Regeneron and GSK, lecture fees from Chiesi as well as grants from Technology Strategy Board, AstraZeneca and GSK. The authors alone are responsible for the writing and the content of the paper.
References
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157(6 Pt 1):1791–1797.
- Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Euro Respir J 2010; 36(1):81–88.
- Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, Puthucheary ZA, Quadriceps wasting and physical inactivity in patients with COPD. ERJ 2012; 40(5):1115–1122.
- McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176(12):1208–1214.
- Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175(12):1259–1265.
- Bolton CE, Ionescu AA, Shiels KM, Pettit RJ, Edwards PH, Stone MD, Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170(12):1286–93.
- Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006; 100(1):115–122.
- Sidney S, Sorel M, Quesenberry CP, Jr., DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005; 128(4):2068–2075.
- Natanek SA, Gosker HR, Slot IG, Marsh GS, Hopkinson NS, Man WD, Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve 2013; 48(4):488–497.
- Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62(2):115–120.
- Gea J, Agusti A, Roca J. Pathophysiology of muscle dysfunction in COPD. J Appl Physiol 2013; 114(9):1222–1234.
- Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med 2000; 109(3):207–212.
- Waschki B, Kirsten A, Holz O, Muller KC, Meyer T, Watz H, Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140(2):331–342.
- Agusti A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PloS One 2012; 7(5):e37483.
- Duvoix A, Dickens J, Haq I, Mannino D, Miller B, Tal-Singer R, Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax 2013; 68:670–676.
- Valvi D, Mannino DM, Mullerova H, Tal-Singer R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int J Chron Obstruct Pulmon Dis 2012; 7:173–182.
- Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186(10):982–988.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128–1138.
- Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). European Respir J 2008; 31(4):869–873.
- Gale NS, Munnery M, Albarrati A, Munnery I, Shale DJ, Cockcroft JR. Assessment of risk factors in chronic airways disease evaluation (ARCADE). Artery Res 2012; 6(4):158.
- Casaburi R, Celli B, Crapo J, Criner G, Croxton T, Gaw A, The COPD Biomarker Qualification Consortium (CBQC). J COPD. 2013; 10(3):367–377.
- Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012; 367(14):1310–1320.
- Clauss A. [Rapid physiological coagulation method in determination of fibrinogen]. Acta Haematol 1957; 17(4):237–246.
- Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 1998; 16(12 Pt 2):2079–2084.
- Csanyi A, Egervari A. Simple clinical method of average intima-media thickness measurement in the common carotid artery. VASA Zeitschrift fur Gefasskrankheiten 1996;25(3):242–248.
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117.
- Edwards RH, Young A, Hosking GP, Jones DA. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med 1977; 52(3):283–290.
- Steiner MC, Roubenoff R, Tal-Singer R, Polkey MI. Prospects for the development of effective pharmacotherapy targeted at the skeletal muscles in chronic obstructive pulmonary disease: a translational review. Thorax 2012; 67(12):1102–1109.
- Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180(6):513–520.
- MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med 2013; 45(3):291–300.
- Dickens JA, Miller BE, Edwards LD, Silverman EK, Lomas DA, Tal-Singer R. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res 2011; 12:146.
- Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 178(12):1211–1218.
- Waschki B, Spruit MA, Watz H, Albert PS, Shrikrishna D, Groenen M, Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med 2012; 106(4):522–530.
- Moore AJ, Soler RS, Cetti EJ, Amanda Sathyapala S, Hopkinson NS, Roughton M, Sniff nasal inspiratory pressure versus IC/TLC ratio as predictors of mortality in COPD. Respir Med. 2010; 104(9):1319–1325.
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49(2):M85–94.
- Kon SS, Patel MS, Canavan JL, Clark AL, Jones SE, Nolan CM, Reliability and validity of the four metre gait speed in COPD. ERJ 2013; 42(2):333–340.
