Abstract
Low physical activity and sedentary behaviour characterise the lives of patients with chronic obstructive pulmonary disease (COPD). Using activity monitors, assessment of both aspects are possible, but many outcomes are not well validated. The aim of this study was to assess the accuracy and equivalency of three activity monitors regarding steps, body position and their ability to differentiate between periods of physical activity and inactivity.
Fifteen patients with COPD (8 females; median (interquartile range, IQR) age, 64 (59–69) years; forced expiratory volume in 1 second, 37 (28–48)% predicted; 6-minute walk distance, 444 (410–519) m) were enrolled. The DynaPort ADL-monitor, the DynaPort MiniMod monitor and the SenseWear Armband Pro 3 monitor were assessed. Subjects performed a structured protocol alternating physical activity and inactivity while simultaneously wearing all three monitors and being video recorded.
The mean difference (limits of agreement) in step count from monitors compared to manual step count was −69 (−443 to 305) for the ADL-monitor, −19 (−141 to 103) for the MiniMod and −479 (−855 to −103) for the SenseWear Armband. Compared to the video, the sitting time was 97 (94–100)% when measured by the ADL-monitor and 121 (110–139)% by the MiniMod. Standing time was 114 (107–122)% when measured by the ADL-monitor and 68 (47–106)% by the MiniMod.
Activity monitors are not equivalent in their abilities to detect steps or body positions. The choice of monitor should be based on the particular outcome of interest.
Introduction
Chronic obstructive pulmonary disease (COPD) is recognised as a condition that affects the body in several ways in addition to affecting respiratory function (Citation1). Subjects with COPD are less physically active and spend larger proportions of the day in sedentary behaviours compared to healthy controls (Citation2). Higher levels of physical activity lower the risk for hospitalisation and mortality (Citation3) and are accompanied by higher health-related quality of life (Citation4). Recommendations for physical activity aimed at maintaining or improving health emphasise that both increased physical activity, as well as decreased sedentary behaviour, are targets for intervention (Citation5).
The advances in wearable monitoring technology have made it possible to objectively assess physical activity and sedentary behaviour in great detail. These devices, often referred to as activity monitors, are worn on the body to register movements or accelerations in one, two or three planes of motion. The signal from the monitor is filtered, processed and converted through different, and often proprietary, algorithms into easily understood outcomes, such as number of steps, body positions and energy expenditure, or into more abstract forms, such as activity counts and vector magnitude units (Citation6). The multitude of outcomes reported from different monitors make comparisons between studies difficult (Citation7).
Physical activity is often defined as “[A]ny bodily movement produced by skeletal muscles that results in energy expenditure” (Citation8). Consequently, the majority of validation studies on activity monitors in COPD have focused on the aspect of energy expenditure (Citation9–11). However, disease specific characteristics of COPD, such as increased energy expenditure (Citation12) and altered mechanical efficiency (Citation13), are not likely to be captured by energy estimations from activity monitors relying only on motion sensor technology. Some authors have also reported on the accuracy of step count or body positions in COPD (Citation14–17). Recent reviews on validation studies have proposed that more attention should be given to direct monitor outputs such as steps (Citation18) and that information on body positions and activity recognition have great potential for improving the assessment of activity-related health outcomes (Citation19). However, even if the same outputs are reported in various studies, the different methods used to analyse and process the signals means that equivalence between systems cannot be assumed if validation and/or equivalence studies for the intended population are not available (Citation20).
There are relatively few studies that compare different activity monitors regarding accuracy of step count and recognition of body positions in subjects with COPD. Previous studies have reported total number of steps (Citation14), or have only used one advanced monitor compared to a pedometer (Citation10,Citation15). The different placements on the body and different walking pattern relating to walking at different speeds, with a rollator, with a backpack or stair walking, present specific challenges for activity monitors. Furthermore, accurate measures of outputs linked to specific behavioural changes, such as walking more or spending less time sitting or lying, would likely be valuable in addition to global measures of activity such as energy expenditure.
The aim of this study was to investigate the accuracy and equivalency of three activity monitors against video recordings regarding the number of steps, time spent in different body positions and their ability to differentiate between short periods of physical activity and inactivity, in subjects with COPD.
Methods
A cross-sectional study was undertaken, and participants with COPD were recruited from a convenience sample from a patient registry at the Respiratory and Allergology Department at Uppsala University Hospital, Sweden. Seventeen subjects with COPD were approached, and fifteen accepted. The inclusion criteria were as follows: post-bronchodilator FEV1/FVC < 0.70, stable condition (no infection within Citation3 weeks) (). Exclusion criteria were FEV1 ≥ 80% predicted or long-term oxygen therapy (LTOT). The disease severities according to the GOLD-grade were II (n = 2), III (n = 8) and IV (n = 5). The study was approved by the regional ethical review board of Uppsala (2009/093), and written informed consent was obtained prior to the assessments.
Table 1. Subject characteristics, n = 15 (8 females), median (min-max)
Measurements
Lung function measurements from patient records were used if performed within six months of the study visit. Otherwise, post-bronchodilator spirometry was performed, and the predicted values according to European Community for Steal and Coal (Citation21) were used. Physical function was assessed by a 6-minute walk test (6MWT) (Citation22). Oxygen saturation was examined at the end of the 6MWT by a hand-held device (TuffSat, Hohenburg, Germany). Height and body weight were measured, and body mass index (BMI) was calculated (body weight (kg) / height (m) 2).
The activity monitors used were as follows: DynaPort ADL-monitor (ADL-monitor), DynaPort MiniMod (MiniMod) (McRoberts, The Hague, Netherlands) and SenseWear Armband Pro3 (SenseWear Armband) (SenseWear, BodyMedia, Pittsburgh, USA) ().
Table 2. Descriptions and details of the activity monitors
The data collected from the McRoberts systems were uploaded to the manufacturer's website for an in-house analysis of the raw data and downloaded in Microsoft Excel format. The data from the SenseWear Armband were analysed using Inner View PC software, version 6.1.
The subjects were video recorded using a digital video camera (Canon Legeria, model FS 36) for the duration of the protocol.
Setting and procedures
A 53-minute structured protocol of varying activities intended to mimic daily life in patients with COPD was constructed and performed at the clinic (). The protocol included all body positions (sitting, standing, lying) and locomotion (walking on level ground, stair walking, bicycling) classified by the monitors and consisted of alternating periods, ranging from 30 seconds to 6 minutes, of both physical activity (walking, stationary arm work, bicycling) and inactivity (sitting and lying). The protocol included some activities that were relevant for subjects on LTOT such as walking with a rollator and carrying a backpack with an oxygen cylinder. All three monitors were started simultaneously to allow for minute-by-minute comparisons throughout the protocol. The patients were guided through the protocol by the test leader (MA), who monitored the time and explained all subsequent steps of the protocol.
Table 3. Structured protocol performed at the hospital
Analysis
The data from the activity monitors were compared to that from the video recordings in terms of the number of steps and time spent lying down, sitting, standing and in locomotion. The video was analysed by two persons and the mean of their observations was used in the final analysis of steps and body positions. The analysis was completed by comparing the minute-by-minute data from all three monitors for each subject by manually counting steps and determining the time spent in different body positions from the video. The step count from the monitors and the manual count and the time spent in different body positions are presented as the medians (IQR) (Tables and ) and are shown as box plots () and Bland-Altman plots for agreement (A-C).
Figure 1. The total step count from each device during the protocol. The boxes represent the 1st and 3rd quartiles and the solid line is the median. Video (n) = 15, ADL-monitor (n) = 13, MiniMod (n) = 15, SenseWear Armband (n) = 14.
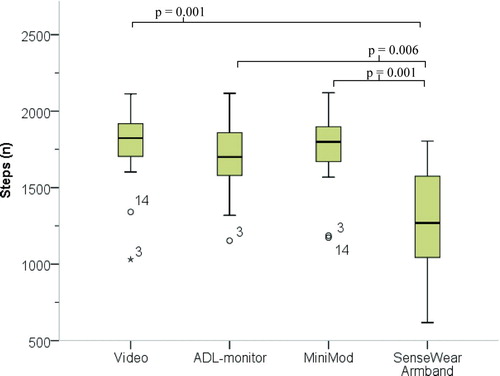
Figure 2 A-C. Bland-Altman plots for differences between activity monitors and manual step count (y-axis) plotted against the mean number of steps from monitors and manual count (x-axis). The thick line represents the bias (mean difference) and the dotted lines represent the upper and lower limit of agreement (LOA). For each monitor the bias (LOA) was as follows: ADL-monitor −69 (−443 to 305), MiniMod −19 (−141 to 103), SenseWear Armband −479 (−855 to −103). A = ADL-monitor (n = 13), B = MiniMod (n = 15) and C = SenseWear Armband monitor (n = 14).
Table 4. The number of steps during the walking activities of the protocol
Table 5. Time spent in different body positions and in locomotion, expressed as a percent of the time calculated from the video recording
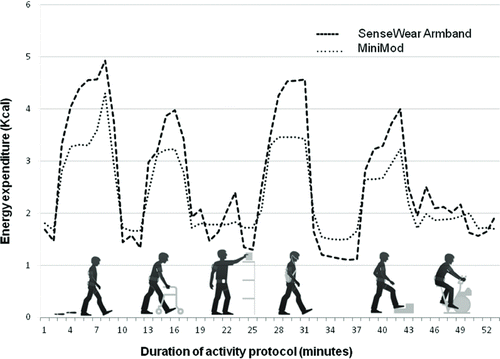
Friedman's ANOVA was used to determine the statistical significance of differences between the monitors and the manual step count, and the Wilcoxon signed-rank test was applied for post-hoc pair-wise comparisons between the different monitors and the videos. The correlation between the total number of steps recorded by the monitors and the video recordings was calculated using the intraclass correlation coefficient (ICC). Specifically, the selected ICC was that of single measures, two-way random effects with absolute agreement, ICC2.1. The significance level was set to p < 0.05 with a Bonferroni correction to account for multiple tests. The pattern of energy expenditure (kcal) is presented as a line graph () after the minute-by-minute watt data from the MiniMod were converted into kcal (watts × 0.01433) (Citation23).
Results
All 15 subjects completed the study and provided data from the standardised protocol measurement. In one subject, the energy expenditure measured by the SenseWear Armband was not altered during the duration of the protocol, and in two subjects the ADL-monitor reported a markedly low step count. Thus, these data were excluded from the analysis.
The median (IQR) number of total steps counted from the video was 1824 (252), and the corresponding number of steps reported by the activity monitors were as follows: ADL-monitor = 1700 (398), MiniMod = 1799 (290), and SenseWear Armband = 1269 (570) (). The mean difference between the number of steps recorded by the monitors and the manual count differed between monitors with the most accurate monitor being the MiniMod (A-C). When comparing the magnitude of the differences between monitors, only the difference of the SenseWear Armband reached statistical significance compared to the differences from the other monitors (p = 0.006 against ADL-monitor, p = 0.001 against the MiniMod). The accuracy of the step count was high for the ADL-monitor and the MiniMod for all types of walking activities compared to manual step count, whereas the accuracy of the SenseWear Armband was significantly lower than the manual step count during slow and fast walking (p = 0.001), rollator use (p = 0.001), walking with a backpack (p = 0.001) and intermittent and stair walking (p = 0.001) (). The correlations between the steps counted from the activity monitors and the video varied, with ICC2.1 values of 0.76 (p = 0.01) for the ADL-monitor, 0.97 (p = 0.01) for the MiniMod and 0.40 (p = 0.001) for the SenseWear Armband.
The ADL-monitor and MiniMod detected lying down, sitting, standing and in locomotion, whereas the SenseWear Armband did not detect any positions. The median (IQR) time spent in different body positions, expressed as a percent of the time recorded from the video, is presented in . Compared to video recordings, the ADL-monitor overestimated time spent standing (114%, p = 0.004) and underestimated time in locomotion (92%, p = 0.001) whereas the MiniMod overestimated sitting (121%, p = 0.001) and underestimated time in locomotion (77%, p = 0.001). Differences between the ADL-monitor and the MiniMod existed for time lying (p = 0.002), sitting (p = 0.001) and in locomotion (p = 0.001). The MiniMod overestimated sitting time because it did not detect a period of lighter arm work performed while standing and partially classified stationary bicycling as sitting or shuffling.
The pattern of physical activity and inactivity measured minute-by-minute during the protocol was captured well by both the MiniMod and the SenseWear Armband (). Lower energy expenditure was reported by the MiniMod compared to the SenseWear Armband, with the exception of lying down, which was reported at a lower rate by the SenseWear Armband.
Discussion
In this study, we demonstrated that both the MiniMod and ADL-monitor were accurate for measuring steps, while the ADL-monitor was the most accurate for detecting time spent sitting and in locomotion. Furthermore, the steps measured using the SenseWear Armband was significantly lower during all tasks of walking compared to the video recordings. The MiniMod and the SenseWear Armband captured the pattern of physically active and inactive periods equally.
The accuracy of the step count was the highest for the MiniMod and the lowest for the SenseWear Armband. To our knowledge, the MiniMod has been assessed for step count accuracy in one previous study of COPD subjects in which the SenseWear Armband was also included (Citation14). They found that the MiniMod underestimated steps compared to the manual step count (-43), but that the SenseWear Armband had an even larger underestimation of steps (-465).
We included a period of walking with a backpack and with a rollator because these are common in subjects on LTOT. The SenseWear Armband underestimated the step count for the total protocol as well as during all the different walking activities in the protocol. These results are similar to that reported by Cavalheri et al., who found that the SenseWear Armband underestimated step count in all types of activities investigated (walking on the level, with a backpack, stair walking, rising/sitting in chairs) compared to video recordings (Citation10). In the present study, the most striking difference between the manual step count and activity monitor occurred with rollator use, with an underestimation of 85% for the SenseWear Armband.
This is not surprising given that the positioning of the SenseWear Armband on the right upper arm will dampen movements while walking with an arm supported by a rollator. Hill et al. found that a significant underestimation of step rate (steps/minute) occurred at slow walking speeds (<50 m/min) and that the use of a rollator further worsened the accuracy at slow walking speeds (Citation16). The MiniMod was not affected by a backpack or rollator, most likely due to its location at the lower back.
In the present study, some differences between the MiniMod and the ADL-monitor were seen in ability to detect body positions and time spent in locomotion. This result is partially in disagreement with the study by Langer et al., who reported excellent agreement between the ADL-monitor and the MiniMod for the percent of time spent standing, lying down, sitting, and walking (Citation14). The differences between the study by Langer et al. and our study are likely due to differences in the protocols used. We chose to include a period of stationary bicycling, whereas Langer et al. only used walking activities.
The MiniMod consists of a single unit at the lumbar region and lacks the ability to identify stationary bicycling, in contrast to the ADL-monitor, which incorporates a leg sensor and can identify bicycling. In our study, the MiniMod classified the cycling time as locomotion or shuffling or as a body position (sitting or standing). If cycling time was excluded from our analysis, the accuracy for locomotion time by the MiniMod monitor was 96%, which is in agreement with the study by Langer et al.
The other source of inaccuracy in the MiniMod stems from the period of standing up lifting/moving objects on a shelf. This period of standing was not detected in 12 of the subjects. Instead, the time was included as sitting time, contributing to both the overestimation of sitting and the underestimation of standing. The high accuracy for the ADL-monitor in the present study confirms the results of previous studies (Citation14,Citation17).
The SenseWear Armband should be able to detect lying down as a distinct body position, but for reasons unknown to us, no classification of lying down was attributed to any of the subjects.
The SenseWear Armband was designed as a portable metabolic monitor and has been proven to agree with the gold-standard for measuring free living energy expenditure, the doubly labelled water technique (Citation24). In the present study, the SenseWear Armband recognized the period in the protocol during which lying down occurred as the period of lowest energy expenditure and was able to capture the increased activity associated with arm movements. Furthermore, the SenseWear Armband did recognise increased energy expenditure during the part of the protocol where subjects walked with a rollator, despite not detecting steps accurately. Hill et al. found that rollator use increased the variability of energy expenditure estimates from the SenseWear Armband, therefore making them less useful (Citation16). Hill et al. subsequently investigated whether energy expenditure actually was altered by walking with a rollator and, through indirect calorimetry, was able to show that it was not (Citation25).
The ability of the SenseWear Armband to explain energy expenditure has been reported to rely on the added information from its physiological sensors as opposed to only using the accelerometer data (Citation9). Our results suggest that the pattern of energy expenditure detection was fairly similar for the MiniMod and the SenseWear Armband. The change in movement intensity, represented by the change in walking speed from slow to fast 6 minutes into the protocol, was also reflected by increased energy expenditure by both systems. Recently, Van Remoortel et al. reported that the increased energy expenditure resulting from increased walking speed was better captured by the MiniMod than the SenseWear Armband (Citation11). On the other hand, they found that the SenseWear Armband was able to account for a larger proportion of mean VO2 than the MiniMod (58 vs. 21%). This could likely be a result of the added physiological information registered from the SenseWear Armband.
There are some potential limitations of this study. The sample was small and was not recruited randomly. Additionally, no control group was used, potentially introducing selection bias. Because the objective was to assess monitor accuracy, not to establish whether subjects with COPD differed from controls, we deemed this appropriate. Furthermore, it is well accepted that subjects with COPD are less physically active, whereas little is known regarding activity monitor equivalency in this sedentary group. It should be noted that both SenseWear Armband and MiniMod have undergone further development regarding both hardware and software after this study was completed.
The strengths of this study were the simultaneous comparison of the MiniMod and SenseWear Armband, which have been reported to be among the most accurate activity monitors for assessing energy expenditure in COPD (Citation11). The importance of accurate assessment of walking should be evident because it is the type of physical activity that is most frequently reported by adults, regardless of age or gender (Citation26). This is, to our knowledge, the first study to report on the accuracy of multiple advanced activity monitors regarding step count in different walking activities mimicking daily life in a detailed manner.
Outcomes such as steps and body positions or time spent walking are all easily comprehensible to patients and would therefore be valuable in goal setting and for forming activity plans in a rehabilitation setting. In order to assess a behavioural change, i.e., change from a sedentary to a more physically active behaviour, the ability to assess body positions and locomotion, are more informative than assessing energy expenditure.
Using our methodology, we were able to extend the accuracy of step count from the MiniMod to subjects using a rollator and to provide information on the limitations of the SenseWear Armband in the same population. We also confirmed the accuracy of the MiniMod for detecting body positions and time in locomotion and simultaneously showed the potential limitations in detecting transitions from sitting to standing. The results presented here should be valuable to those designing an intervention study targeting either sedentary behaviours (sitting, lying, or standing) or attempting to increase walking, measured as steps, in subjects with moderate-to-severe COPD.
Conclusion
Activity monitors are not equivalent in their abilities to detect steps or body positions, and the differences between systems highlight the fact that the choice of activity monitor should be based on the facets of physical activity or inactivity that one wishes to measure. Both the MiniMod and SenseWear Armband are capable of distinguishing between periods of physical activity and sedentary behaviours. However, if detailed step count or body position information is the target, the MiniMod monitor is more suitable.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The study was supported by grants from the Swedish Heart and Lung Foundation, Stockholm, Sweden and the Bror Hjerpstedt foundation, Uppsala, Sweden. McRoberts supplied the ADL-monitor and the MiniMod for this study free of charge. We acknowledge Professor Thierry Troosters for assistance in developing the structured protocol and Drs Charlotte Urell and Morgan Emtner for their valuable assistance in data collection and data management. Graphical assistance was supplied from www.alexunderstands.com.
References
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171(9):972–977.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61(9):772–778.
- Arne M, Lundin F, Boman G, Janson C, Janson S, Emtner M. Factors associated with good self-rated health and quality of life in subjects with self-reported COPD. Int J Chron Obstruct Pulmon Dis 2011; 6:511–519.
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007; 116(9):1094–1105.
- Chen KY, Bassett DR Jr. The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc 2005; 37(11 Suppl):S490–500.
- Taraldsen K, Chastin SFM, Riphagen II, Vereijken B, Helbostad JL. Physical activity monitoring by use of accelerometer-based body-worn sensors in older adults: A systematic literature review of current knowledge and applications. Maturitas. 2012; 71(1):13–19.
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep Wash DC 1985; 100(2):126–131.
- Patel SA, Benzo RP, Slivka WA, Sciurba FC. Activity monitoring and energy expenditure in COPD patients: a validation study. COPD 2007; 4(2):107–112.
- Cavalheri V, Donária L, Ferreira T, Finatti M, Camillo CA, Cipulo Ramos EM, Energy expenditure during daily activities as measured by two motion sensors in patients with COPD. Respir Med 2011; 105(6):922–929.
- Van Remoortel H, Raste Y, Louvaris Z, Giavedoni S, Burtin C, Langer D, Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PloS One 2012; 7(6):e39198.
- Baarends EM, Schols AM, Westerterp KR, Wouters EF. Total daily energy expenditure relative to resting energy expenditure in clinically stable patients with COPD. Thorax 1997; 52(9):780–785.
- Baarends EM, Schols AM, Akkermans MA, Wouters EF. Decreased mechanical efficiency in clinically stable patients with COPD. Thorax 1997; 52(11):981–986.
- Langer D, Gosselink R, Sena R, Burtin C, Decramer M, Troosters T. Validation of two activity monitors in patients with COPD. Thorax 2009; 64(7):641–642.
- Furlanetto KC, Bisca GW, Oldemberg N, Sant’Anna TJ, Morakami FK, Camillo CA, Step Counting and Energy Expenditure Estimation in Patients With Chronic Obstructive Pulmonary Disease and Healthy Elderly: Accuracy of 2 Motion Sensors. Arch Phys Med Rehabil 2010; 91(2):261–267.
- Hill K, Dolmage TE, Woon L, Goldstein R, Brooks D. Measurement properties of the SenseWear armband in adults with chronic obstructive pulmonary disease. Thorax 2010; 65(6):486–491.
- Pitta F, Troosters T, Spruit MA, Decramer M, Gosselink R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2005; 86(10):1979–1985.
- Van Remoortel H, Giavedoni S, Raste Y, Burtin C, Louvaris Z, Gimeno-Santos E, Validity of activity monitors in health and chronic disease: a systematic review. Int J Behav Nutr Phys Act 2012; 9(1):84.
- Plasqui G, Bonomi AG, Westerterp KR. Daily physical activity assessment with accelerometers: new insights and validation studies. Obes Rev 2013; 14(6):451–462.
- Welk GJ, McClain J, Ainsworth BE. Protocols for evaluating equivalency of accelerometry-based activity monitors. Med Sci Sports Exerc 2012; 44(1 Suppl 1):S39–49.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:5–40.
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166(1):111–117.
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011; 43(8):1575–1581.
- Mackey DC, Manini TM, Schoeller DA, Koster A, Glynn NW, Goodpaster BH, Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci 2011; 66(10):1108–1113.
- Hill K, Dolmage TE, Woon LJ, Brooks D, Goldstein RS. Rollator use does not consistently change the metabolic cost of walking in people with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2012; 93(6):1077–1080.
- Simpson ME, Serdula M, Galuska DA, Gillespie C, Donehoo R, Macera C, Walking trends among U.S. adults: the Behavioral Risk Factor Surveillance System, 1987–2000. Am J Prev Med 2003; 25(2):95–100.