Abstract
The prevalence of COPD and its risk factor pattern varies between different areas of the world. The aim of this study was to investigate the prevalence of COPD by disease severity in men and women and risk factors for COPD in northern Vietnam. From all 5782 responders to a questionnaire survey, a randomly selected sample of 1500 subjects was invited to a clinical follow-up study. The methods included a structured interview using a modified GA2LEN study questionnaire for registration of symptoms and possible determinants of disease. Spirometry was performed before and after bronchodilation. The age distribution of the sample was 23–72 years. Of 684 subjects attending, 565 completed acceptable spirometric measurements. The prevalence of COPD defined by the GOLD criteria was 7.1%; in men 10.9% and in women 3.9% (p = 0.002). Of those 3.4% had a mild disease, 2.8% a moderate and 0.9% a severe disease. In ages >50 years, 23.5% of men and 6.8% of women had COPD. Among smokers aged >60 years (all men), 47.8% had COPD. None of the women with COPD had been smokers. Increasing age, smoking and male sex were the dominating risk factors, although male sex lost its significance in a multivariate setting. The prevalence of COPD among adults in northern Vietnam was 7.1% and was considerably higher among men than women. The prevalence increased considerably with age. Increasing age and smoking, the latter among men only, were the most important determinants of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by airflow limitation that is not fully reversible (Citation1). It is expected to be the third leading cause of death worldwide by 2020 (Citation2). Male gender, advanced age, cigarette smoking, occupational exposure to gases, dust and fumes, and low socio-economic status are well-known independent risk factors for COPD (Citation1). Smoking cessation reduces the risk of developing COPD, or slows its progress and reduces the risk of hospital admissions (Citation3). The true burden of COPD is probably underestimated because the disease is usually not diagnosed until it is clinically apparent (Citation1). A combination of validated questionnaires and spirometry, allow for an early diagnosis of COPD (Citation4). However, the prevalence of COPD varies throughout the world due to different study methods, diagnostic criteria and analytic approaches (Citation1). By using GOLD criteria to diagnose COPD (Citation1) results also in East Asia show large differences in prevalence ranging from 3.7% to more than 20%, (Citation5,6) partly due to different age compositions of the studied samples. Prevalence of COPD in population studies of middle aged and elderly in so call developed countries has been estimated at result15–20% with smoking and increasing age being the dominating risk factors (Citation4,Citation7).
Following a network study in 12 Asia Pacific countries and regions, a model to estimate the prevalence of COPD based on smoking habits indicated the ranged from a minimum of 3.5% in Hongkong and Singapore to a maximum of 6.7% in Vietnam (Citation8). Vietnam was found to have the highest prevalence of smoking men in the world (Citation9). Using biomass fuel on open fire for cooking is common. Furthermore, the incidence of tuberculosis, a disease known to cause airway obstruction, is high in Vietnam (Citation10). Thus, there was a need for a population based epidemiologic study in Vietnam to assess the true prevalence of COPD using standardized definitions and objective measurements by spirometry. Therefore, the aim of this study was to estimate the prevalence of COPD by disease severity and to identify risk factors for the disease in northern Vietnam.
Methods
Study Sample
In 2007–2008 a questionnaire survey was conducted in Hoankiem and Bavi, an urban and a rural area of Hanoi in northern Vietnam (Citation11,12). The two areas were selected as they represent different indoor and outdoor exposures and living conditions in urban and rural areas of the country. The response rate was 82.5%. Among the 5782 responders to a questionnaire survey (Citation11), a sample of 1500, 23–72 years old subjects, 750 from each of the two areas, was randomly selected for clinical examinations. Signed informed consent with adequate information was obtained before the start of the study procedures. In total, 684 subjects (46%) attended the clinical study. There were no significant differences in respiratory symptoms or diagnosis between those who had participated in the questionnaire survey and those who attended the clinical examinations (Citation13).
Methods
The clinical study was conducted from March 2009 to April 2010. The data were collected by trained nurses and medical doctors from Bachmai hospital, Hanoi. The study was conducted following the Helsinki guidelines and was approved by the Medical Ethics Research Committee of Hanoi Medical University.
Questionnaire
The interview questionnaire used was the Global Allergy and Asthma European Network (GA2LEN) Study questionnaire (Citation15), which is mainly based on the ECRHS and the ARIA questionnaires (Citation16,17). Additional questions were taken from the Swedish OLIN-questionnaire (Citation18). The questionnaire included questions about respiratory and allergic symptoms, diagnoses, medication use, smoking habits and exposure, use of open fires for cooking, occupation, urban or rural childhood living and other possible determinants of disease.
Lung function tests
The lung function tests were performed using a portable spirometer (Multi-functional spirometer HI-801). Calibration of the spirometer was performed daily. A pulmonary function test following the ATS/ERS recommendations (Citation19) was performed including a bronchodilation test with 400 μg salbulamol (Ventoline® MDI). The reference values were taken from the equation calculated for the East Asian population. The difference between the two best FVC and the two best FEV1 values had to be <5% or <100 ml in case the values were < 2.0 l. All out-prints of the lung function tests were quality controlled by the supervisors of the study (ER and BL).
Definitions
COPD
East Asian reference data of lower limit of normal for the ratio of FEV1/FVC have not been validated for Vietnam, thus the ATS/ERS standards for defining COPD (Citation20) could not be fully met, while COPD was defined using the fixed ratio of FEV1/FVC <0.70 (Citation1). The highest FEV1 and FVC were used including post bronchodilator results. The severity of COPD was determined by the spirometric cut off values used proposed by GOLD (Citation1):
Due to few cases of severe and very severe COPD, these two groups were combined. The wording of the symptoms longstanding cough, sputum production, chronic productive cough, SOB when hurrying, any medicine for lung disorders and previous diagnosed COPD has been previously described (Citation11).
Smoking habits
Current smokers are those who are currently smoking or who had stopped smoking within 12 months prior to the study.
Ex-smokers are those who had stopped smoking more than 12 months prior to the study.
Non-smokers are those who never have been smokers or ex-smokers.
1 pack year = smoking of 20 cigarettes per day for one year.
Exposures and possible determinants of disease have been previously defined (Citation11,12).
Analysis
The Predictive Analytics Soft Ware (PASW) version 18.0 was used for statistical analysis. Difference in prevalence was calculated using χ2 test or, when appropriate χ2 test for trends. A p value < 0.05 was considered statistically significant. The 95% confidence intervals (CI) for prevalence were calculated exactly from the binomial distributions. Risk factors were calculated by using both uni-variate and multiple logistic regression analysis. Demographic and exposure data were used as independent variables and included age, sex, area, smoking habits, use of open fires for cooking, and occupational exposure to gas, dust or fumes. Because of uncertainty of classification of disease by self-reporting, a report of asthma was not included among the risk factors of COPD in the multivariate model. In the multivariate analysis, smoking habits and age class were analysed both separately and combined. The results are expressed as odds ratios (OR) with 95% confidence intervals (CI).
Results
Basic characteristics
Of the 684 participants 565 (83%) presented fully acceptable lung function measurements. The distribution by age, sex, smoking habits, and lung function (FEV1 < 80% of predicted) are shown in . Among men 63% were current smokers versus 0.5% (p < 0.001) among women. Of women, 99% had never been smokers. Among all subjects aged >50 years, 12% were ex-smokers compared to 5% (p = 0.001) among the younger. A significantly greater proportion of men than women had FEV1 < 80% of predicted values ().
Table 1. Basic characteristics (%) of the study population. Difference (p-value) by age and sex.
Prevalence of COPD by disease severity and smoking habits
The prevalence of COPD among men was 10.9% versus 3.9% (p = 0.002) among women (), and the overall prevalence was 7.1% (in Hoankiem 7.2% and in Bavi 7.0%). By disease severity, 3.4% had a mild COPD, 2.8% had moderate COPD and 0.9% had severe COPD (GOLD stage 3+4). COPD was considerably more common among subjects aged >50 years, among men 23.5% and in women 6.8%, compared with those ≤50 years: men 2.6%; women 2.1% ().
Table 2. Prevalence (%) and severity of COPD in men and women using the GOLD criteria.
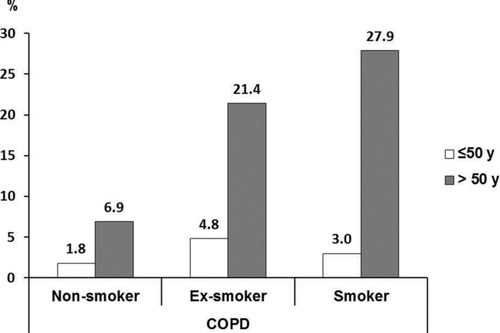
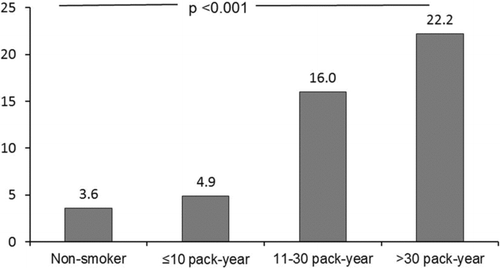
Of the 28 men fulfilling the criteria of COPD only one was a non-smoker, while all 12 women with COPD had never been smokers. Among subjects aged >50 years, COPD was strongly associated with smoking habits: COPD was found in 27.9% of smokers, 21.4 of ex-smokers and in 6.9% of non-smokers (). Further, among male smokers aged >60, 47.8% had COPD. Smoking exposure expressed as pack-years correlated significantly with the prevalence COPD, p < 0.001 (). The severity of COPD followed a similar pattern in relation to pack-years.
COPD in relation to symptoms and diseases
The prevalence of most respiratory symptoms increased with severity of disease (), and a large majority those with COPD reported either one or more of recurrent or chronic symptoms. The increase was most obvious from mild to moderate COPD, while the evaluation regarding severe COPD lacks power due to few subjects in this group. Of the 35 subjects with mild or moderate COPD, 3 had previously been diagnosed as having asthma, while 4 out of 5 with severe COPD had previously been diagnosed with asthma. Of those with COPD, 35% were using respiratory medicines, and the same proportion had previously been diagnosed as having COPD ().
Table 3. Proportion (%) of respiratory conditions among subjects without COPD and with COPD by disease severity.
Univariate and multivariate relationships
COPD was strongly related to increasing age and smoking in the univariate analysis. Further, mild and moderate COPD tended to be associated with living in the rural area, but severe COPD tended to be associated with urban living. In the univariate analysis, also asthma was associated with COPD. Passive smoking, occupational exposure to gas dust or fumes and open fire for cooking were not significantly associated with COPD.
In the multivariate setting, male sex was no longer significantly associated with COPD. Using the category age ≤50 y and non-smoker as reference, age >50 but ≤60 years and ever smoker yielded an OR of 13.5 (95% CI 2.2–84.0) and age >60 and ever smoker an OR of 36.5 (95% CI 6.2–215.4). Also age >60 and non-smoker was a risk factor for COPD with an OR of 6.2 (95% CI 1.7–22.7) (). When age category and smoking habits were analysed separately, age 50–60 years yielded an OR of 4.3 (95% CI 1.7–11.0) and age >60 years an OR of 11.5 (95% CI 4.8–27.7), while ever smoker did not reach statistical significance, OR 4.4 (95% CI 0.9–20.8).
Table 4. Risk factors for COPD (GOLD) by multiple logistic regression analysis. Odds ratios (OR) with 95% confidence intervals (CI).
When the analyses were performed separately for men and women, the results among men showed a similar pattern with age and smoking to being dominating risk factors. Among women, only increasing age increased the risk of having COPD (data not shown), no significant associations were found regarding exposures including passive smoking and using open fires for cooking.
Discussion
There are only a few studies based on general population samples in which the prevalence of COPD has been studied in the south-eastern part of Asia. When the study started there were no relevant data at all about the prevalence of COPD in Vietnam and the whole former Indochina. In our study, the overall prevalence of COPD was 7.1% among adults aged 23–72 years. COPD was considerably more common among men, the prevalence increased substantially by age, and this was true particularly among smokers. We found the prevalence of COPD to be high particularly taking into account the relatively young age of the population of Vietnam. Among male smokers >60 years, 48% fulfilled the GOLD criterion of COPD.
The prevalence of COPD in our study was twice as high compared to a study in Korea, 3.7%, similar to a study in China, 8.2%, but lower than found in studies in the Philippines, 20.8%, and Japan, 10.9% (Citation5,6,Citation21,22). These differences may reflect not only real differences, but may at least in part be due to differences in methods and diagnostic tools, further, to differences in smoking patterns, environmental and other exposures and to differences in the genetic background. In the age >50 years, the prevalence of COPD in our study was 14.5% (men 23.5%; women 6.8%), which is similar to estimates of prevalence in Europe, for instance 14.3% in the age >45 years in northern Sweden (Citation23) and 17.4% in Copenhagen in the age >35 years (Citation7).
As in several other studies, roughly about a half of the subjects with COPD had a mild disease (Citation4,Citation24). The prevalence of COPD in GOLD stage II-IV was 3.7% in our study, while in ages >50 years, the prevalence of COPD in GOLD stage II-IV was 7.2% (men 9.8%; women 5.1%). The results are similar, or even somewhat higher compared with the Latin-American PLATINO Study with a corresponding prevalence of 5.7% in Caracas, Venezuela, and Sao Paolo, Brazil, and 2.6% in Mexico in ages >40 years (Citation25), and similar with 6.1% in northern Sweden in ages >45 years. The prevalence of severe or very severe COPD in ages >50 years was 2.3% in our study versus 0.8% in ages >45 years in the referred Swedish study (Citation4).
The risk factor pattern among men corresponds quite well to results from Europe and northern America, where increasing age and smoking are the dominating risk factors. The fact that about 50% of elderly smokers who continue to smoke have developed COPD is in line with studies for instance in Sweden, Finland, Spain and the USA (Citation23,24,Citation26,27). As found by others, also the severity of COPD was strongly associated with the cumulated tobacco consumption (Citation4). As about a half of the subjects with COPD in our study still were smokers demonstrates a need of effective programs against smoking and for smoking cessation in Vietnam.
First, to prevent children and teenagers from taking up smoking, and quitting of smoking among those who smoke are by far the most effective, and also cost-effective, ways to reduce the risk of developing COPD, and to stop the disease progression among those who have developed COPD. Brief treatment of tobacco dependency should be offered for smokers at every visit to health care providers. More effective laws for banning of smoking in public areas in order to diminish exposure from second hand smoke, and organizing anti-smoking campaigns at schools and universities are other important measures. Reduction of the total personal exposure to air pollution from traffic jams and biomass smoke, and reduction of exposures from occupational dusts, gases and fumes are also important for primary and secondary prevention of COPD.
Only 1% of women were smokers or ex-smokers, and all women fulfilling the GOLD criteria of COPD had never been smokers. We could not find any other significant risk factor for COPD among women than increasing age. Although questions about several possible risk factors for COPD were included in the questionnaire, such as passive smoking and use of biomass fuel (Citation28,29) for cooking, none of them was found to be associated with COPD in our study. One reason is the low number of women with COPD. Another reason may be that the exposure to environmental tobacco smoke exists all over the society in Vietnam. Further, the use of biomass for cooking is common all over Vietnam, particularly in rural areas, and this was the case in 84% of the participants in the rural area.
The proportion of the subjects with doctor-diagnosed COPD varied from 26% in mild to 44% in moderate COPD. These results are similar to studies in Europe and the USA (Citation4,Citation27), but higher than in Japan where only 9% had been diagnosed before the survey (Citation22). As found by others, respiratory symptoms were more common in moderate and severe COPD than in mild COPD (Citation4,Citation24), a result that parallels to use of medicines for respiratory disorders. Only seven out of the 40 subjects fulfilling the spirometric criteria of COPD reported they previously had been diagnosed as having asthma. That supports our results and contradicts that our results to a large degree could have been biased by asthma. Further, asthma and COPD can coexist (Citation30). Only two of the 40 with COPD had previously had tuberculosis despite the fact that tuberculosis is common in Vietnam.
A weakness in our study is the low participation rate resulting in limitations for calculating risk factors and somewhat wide confidence intervals. Further, bias might have created as the actual participants may not fully represent the target population, despite the results of a good representativeness of study sample (Citation13). The strength is that the sample was randomly selected and the study was performed at the same time in the two study areas around the year thus diminishing bias caused by season. Furthermore, the examinations were performed by a limited number of trained physicians and nurses and they were consecutively followed by repeated quality controls.
As subjects older than 72 years of age were not included in the study, our results may be an underestimation of the prevalence of COPD, however, the participation rate was lower among the younger. Thus we believe our estimation of the overall prevalence is close to a true prevalence of COPD among adults in Northern Vietnam.
In conclusion, the prevalence of COPD among adults in northern Vietnam was 7% and it was higher among men. The prevalence increased with age and was 24% among men and 7% in women aged >50 years. Smoking and increasing age were the most important determinants of COPD, and about 50% of smoking men ≥60 years fulfilled the GOLD criterion of COPD.
Acknowledgments
We thank the field laboratory of Bavi, Fila Bavi, and the staffs of the health care centres of Bavi and Hoankiem, Hanoi, for affording localities and help with invitations of the study subjects. Further, we thank all physicians and research nurses in our work team for contributing with data collection. The authors thank Professor Anders Oden, Chalmers University of Technology Gothenburg, Sweden for statistical advice.
The Swedish government SIDA's secretariat for Research Cooperation for the bilateral cooperation between Vietnam and Sweden is greatly acknowledged for financial support. Additional funding was provided by the Swedish Heart-Lung Foundation and the Swedish Asthma-Allergy Foundation. We acknowledge the support of the European Respiratory Society, Fellowship STRTF 2013-2840.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Global Initiative for chronic obstructive lung disease. Available from http://wwwgoldcopdorg. 2010.
- Vandivier RW, Voelkel NF. The challenges of chronic obstructive pulmonary diseases (COPD)-a perspective. COPD. 2005; 2(1):177–184.
- Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD 2010; 7(5):375–382.
- Lindberg A, Bjerg A, Ronmark E, Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2006; 100: 264–272.
- Kim SJ, Suk MH, Choi HM, The local prevalence of COPD by post-bronchodilator GOLD criteria in Korea. Int J Tuberc Lung Dis 2006; 10:1393–1398.
- Idolor LF, De Guia TS, Francisco NA, Burden of obstructive lung disease in a rural setting in the Philippines. Respirology 2011.
- Fabricius P, Lokke A, Marott JL, Prevalence of COPD in Copenhagen. Respir Med 2011; 105:410–417.
- COPD prevalence in 12 Asia-Pacific countries and regions: projections based on the COPD prevalence estimation model. Respirology 2003; 8:192–198.
- Jenkins CN, Dai PX, Ngoc DH, Tobacco use in Vietnam. Prevalence, predictors, and the role of the transnational tobacco corporations. JAMA 1997; 277:1726–1731.
- Hoa NB, Tiemersma EW, Sy DN, Household expenditure and tuberculosis prevalence in VietNam: prediction by a set of household indicators. Int J Tuberc Lung Dis 2011; 15:32–37.
- Lam HT, Ronmark E, Tu'o'ng NV, Increase in asthma and a high prevalence of bronchitis: results from a population study among adults in urban and rural Vietnam. Respir Med 2011; 105:177–185.
- Lam HT, Tuong NV, Ekerljung L, Allergic rhinitis in northern Vietnam: increased risk of urban living according to a large population survey. Clinical and Translational Allergy 2011; 1: 7.
- Lam HT, Tuong NV, Lundback B, Storage mites are the main sensitizers among adults in northern Vietnam: Results from a population survey. Allergy 2011; 66(12):1620–1621.
- Chuc NT, Diwan V. FilaBavi, a demographic surveillance site, an epidemiological field laboratory in Vietnam. Scand J Public Health Suppl. 2003; 62:3–7.
- Bousquet J, Burney PG, Zuberbier T, GA2LEN (Global Allergy and Asthma European Network) addresses the allergy and asthma ‘epidemic’. Allergy. 2009; 64:969–977.
- Biino G, Rezzani C, Grassi M, ECRHS screening questionnaire scoring: a methodological suggestion for asthma assessment. European Community Health Survey. J Outcome Meas 2000; 4:740–62.
- Cruz AA, Popov T, Pawankar R, Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA(2)LEN. Allergy 2007; 62 Suppl 84:1–41.
- Lundback B, Nystrom L, Rosenhall L, Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal survey. Eur Respir J 1991; 4:257–266.
- Wanger J, Clausen JL, Coates A, Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26:511–522.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23: 932-46.
- Zhong N, Wang C, Yao W, Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007; 176:753–760.
- Fukuchi Y, Nishimura M, Ichinose M, COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004; 9:458–465.
- Lundback B, Lindberg A, Lindstrom M, Not 15 but 50% of smokers develop COPD?–Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003; 97:115–122.
- Kotaniemi JT, Sovijarvi A, Lundback B. Chronic obstructive pulmonary disease in Finland: prevalence and risk factors. COPD 2005; 2:331–339.
- Menezes AM, Perez-Padilla R, Jardim JR, Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005; 366:1875–1881.
- Sobradillo V, Miravitlles M, Jimenez CA, (Epidemiological study of chronic obstructive pulmonary disease in Spain (IBERPOC): prevalence of chronic respiratory symptoms and airflow limitation). Arch Bronconeumol 1999; 35:159–66.
- Mannino DM, Gagnon RC, Petty TL, Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2000; 160:1683–1689.
- Orozco-Levi M, Garcia-Aymerich J, Villar J, Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J 2006; 27:542–546.
- Regalado J, Perez-Padilla R, Sansores R, The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med 2006; 174:901–905.
- Weatherall M, Travers J, Shirtcliffe PM, Distinct clinical phenotypes of airway diseases defined by cluster analysis. Eur Respir J 2009; 34:812–818.