Abstract
Bacterial infection is a major cause of acute exacerbations of chronic obstructive pulmonary disease (AECOPD), which are associated with significantly increased airway and systemic inflammation. However, the relationship among bacteriology, the resolution of inflammation and clinical outcomes is largely unknown. In this study, we recruited consecutive patients hospitalized for AECOPD with purulent sputum. We measured the airway and systemic inflammation levels, the COPD assessment test (CAT) score and adverse outcomes between patients with and without potentially pathogenic microorganisms (PPM). Among sputum samples collected from the 135 episodes of AECOPD, 42 (31.1%) were PPM-positive at admission. Compared with those in the PPM-negative group, more patients in the PPM-positive group had ≥2 exacerbations in previous year and Anthonisen type I at admission and higher drop in sputum neutrophil, serum hs-CRP and CAT value from exacerbation to the subsequent baseline. No significant differences in the adverse outcomes between the two groups were observed. Among the 38 PPM-positive patients who survived and were discharged from hospital, 19 remained PPM-positive (bacterial persistence group) and 19 PPM-negative (bacterial clearance group). Both inflammation indices and CAT score decreased compared to admission in the two groups, regardless of the bacteriology at discharge. Our data suggest uncultivated bacteria and/or virus might also play important roles in causing inflammation and AECOPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality throughout the world (Citation1, 2). In China, the overall prevalence for individuals with COPD greater than or equal to 40 years of age was 8.2% (Citation3). By 2012, COPD is predicted to rank third worldwide in terms of mortality (Citation2). Patients with COPD are susceptible to frequent and recurrent acute exacerbations of COPD (AECOPD), which are characterized by sustained increase in respiratory symptoms such as dyspnea, sputum volume and purulence. An AECOPD is defined as “an event in the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough, and/or sputum and beyond normal day-to-day variations, that is acute in onset and may warrant a change in regular medication in a patient with underlying COPD” (Citation4). AECOPD are associated with faster decline in lung function, poorer quality of life, increased cost of treatment and mortality (Citation5–8).
The onset of AECOPD generally reflects a flare-up of extensive inflammatory processes. Extensive studies have demonstrated increased levels of airway inflammations at AECOPD (Citation9, 10). Systemic inflammation has also been observed during AECOPD and has been correlated with airway inflammation and bacterial infection (Citation11, 12). Understanding the nature and course of systemic and airway inflammation in COPD is critical for the optimization of anti-inflammatory therapy.
The etiology of AECOPD is heterogeneous. Many factors may trigger the inflammatory process during AECOPD, but the majority of AECOPD are associated with respiratory tract infection (Citation13). While around 50% AECOPD were associated with bacteria (Citation14–16), the precise role of bacterial infection in AECOPD has been controversial (Citation17). Earlier studies showed the presence of similar bacteria in both stable and exacerbation states, complicating the distinction between bacterial colonization and infection (Citation18–20). In addition, most of these studies are cross-sectional, and the direction of the correlation is unknown.
Although the heightened inflammation at exacerbation in the bacteria-positive group might be due to bacterial infection, it is also possible that this group always has higher levels of inflammation even at stable states and has a higher chance of bacterial infection, which would rule out the involvement of bacterial infection in inflammation. On the contrary, longitudinal studies, by comparing temporal changes in inflammation between baseline and exacerbation in the same cohort of patients with and without bacteria, have greater ability to show the bacterial causalities of exacerbations.
Recently, Papi et al. investigated the roles of bacterial infection in AECOPD by comparing lung function and inflammation at exacerbation and stable convalescence. They found that patients with infectious exacerbations had longer hospitalizations and greater impairment of lung function. Although sputum neutrophils were significantly increased in all exacerbations, no significant differences in the change in neutrophils from stable convalescence to exacerbation were observed between patients with and without bacterial infection (Citation11).
Despite the advantages of longitudinal study, very few such studies have been conducted (Citation10, 11). Furthermore, these studies have been performed between exacerbation and stable convalescence (usually 8 weeks apart), finer scales analysis of the relationship between bacterial clearance, airway and systemic inflammation and clinical outcomes is still lacking. In addition, most microbiology studies have been reported in the western countries, very limited of such reports were from Asia (Citation21, 22). Here, we performed a prospective, longitudinal study, including 135 Chinese patients hospitalized for AECOPD. We examined relationships among bacterial etiology, airway and systemic inflammation and clinical outcomes between and within patients with and without bacterial infection.
Methods
Patient enrollment
Between April 1, 2009 and September 30, 2011, a prospective study was conducted including consecutive patients presenting AECOPD admitted to a tertiary hospital center in Beijing, China. For patients admitted more than once during the study period, only the first admissions were included in the analysis. The patient inclusion criteria were: COPD diagnosis and presence of exacerbation according to the Global Initiative of Chronic Obstructive Lung Disease definition (GOLD) (Citation4); and the self-reported observation of purulence in the sputum.
Exclusion criteria were: asthma, bronchiectasis, pneumonia, cancer, sleep apnea syndrome, or other forms of active lung disease; hospitalization for reasons other than COPD exacerbation including acute coronary syndrome, congestive heart failure; need for intubation or admission to ICU; length of stay (LOS) shorter than 1 day or longer than 30 day; long-term oral corticosteroids (CS) therapy (more than three months treatment with 7.5 mg per day on prednisone or equivalent); and systemic CS therapy for exacerbation for more than 48 h before presentation.
Written informed consent was obtained from all patients, and the Ethics Committee of the hospital approved the study protocol (IRB00001052-07095).
Treatment of AECOPD
According to the GOLD guidelines, patients were treated with nebulized salbutamol, ipratropium bromide and budesonide, intravenous prednisolone in a dosage of 30 to 40 mg daily. Duration of intravenous therapy was 4 days. The duration of systemic corticosteroid treatment was 10–14 days. On day 4 patients were switched to an oral tapering schedule of prednisolone. Antibiotics were administered if bacterial infection is suspected (patient-reported sputum purulence) and adjusted according to antimicrobial susceptibilities.
Mechanical ventilation was instituted by the treating physician, for indications such as respiratory arrest, deterioration in level of consciousness, and increasing partial pressure of arterial carbon dioxide despite maximal pharmacological treatment. Non-invasive ventilation was used initially whenever possible and indicated. Decisions regarding admission or transfer to ICU were made by the treating unit.
Clinical variables
Variables including demographic data, spirometric values on stable condition, co-morbid conditions, continuing management, smoking habits, exacerbation frequency in the previous year and respiratory symptoms were collected on the day of admission. Anthonisen type of AECOPD was determined according to the symptoms before treatment (Citation23). Exacerbation frequency in the previous year was based on the number of exacerbations the patient recalled for the year before recruitment. According to Hurst et al., frequent and non-frequent exacerbators were defined as exacerbation frequency ≥2 and <2 in the previous year, respectively (Citation24). The COPD assessment test (CAT) score was collected on the day of admission before treatment, on the day of discharge, and in 8 weeks after discharge (stable status).
The adverse clinical outcomes (death from any cause in hospital or within 8 weeks of discharge, need for mechanical ventilation after the second hospital day, recurrent exacerbations within 8 weeks of discharge) of the patients were collected during follow up. A recurrent exacerbation was defined in the present study as a second exacerbation, fulfilling the present criteria, occurring within 8 weeks of the discharge. Symptoms from the first exacerbation must have recovered to pre-exacerbation levels.
Sample collection and measurements
Sputum and serum samples were collected on the day of admission (before treatment), the day of planned discharge (days 10–14), and 8 weeks after discharge. Spontaneous (131/135, 97.0%) and induced (4/135, 3.0%) sputa were collected, processed within 2 h of collection and divided into two aliquots. One sputum aliquot was processed with phosphate buffered saline (Citation25, 26). Cytospins were prepared and cell-free supernatant was collected and stored in aliquots at −80°C pending analyses of soluble mediators. Differential cells were counted on May Grünwald Giemsa stained cytospins in a blinded fashion. Quantitative sputum cultures were performed using the second aliquot as described previously (Citation10). Bacterial agents were classified as potentially pathogenic microorganisms (PPMs) or non-PPMs according to Cabello et al. (Citation20). Peripheral venous blood (7 mL) was collected into a Vacutainer tube (BD Diagnostics, NJ, USA) and centrifuged at 6716 G for 10 min at 4°C. Serum was then separated and stored at −80°C till further analysis.
Sputum and serum interleukin (IL)-6 and sputum IL-8 and myeloperoxidase (MPO) were quantified using commercial sandwich ELISA kits (R&D Systems, Abingdon, UK). Serum C-reactive protein (hs-CRP) was measured by a latex agglutination test, using an Olympus AU5400 automatic biochemical analyzer. All the samples from each patient were measured in the same assay to reduce inter-assay variability. The limit of detection was: 0.7 pg/mL for serum and sputum IL-6, 7.5 pg/mL for sputum IL-8, 0.062 ng/ml for sputum MPO, and 0.1 mg/L for serum hs-CRP, respectively.
Statistical analysis
Statistical analysis was performed using SPSS version 11.5.0. Categorical and numerical variables were presented as n (%) and median (interquartile ranges), respectively. Wilcoxon's signed rank test and Mann–Whitney U-test were used to evaluate differences between 2 groups of paired and unpaired data, respectively. Categorical outcome variables were analyzed using the chi-square tests. Results were considered statistically significant at the 5% level.
Results
Patient characteristics
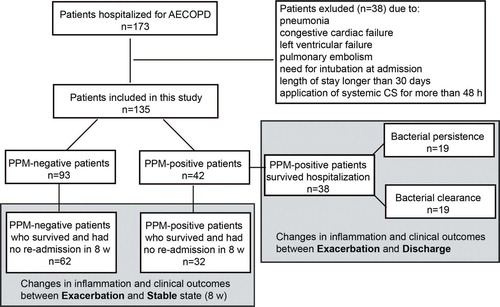
A total of 173 eligible patients were included in the present study. However, 38 patients were withdrawn from the study due to: pneumonia (n = 6); congestive cardiac failure or left ventricular failure at admission (n = 5); pulmonary embolism (n = 3); need for intubation at admission (n = 14); length of stay longer than 30 days (n = 5); and application of systemic CS for more than 48 h (n = 5) before presentation. As a result, a total of 135 patients were included in the analysis. Among sputum samples from the 135 episodes of AECOPD hospital admissions, 42 (31.1%) had positive growth of PPM (Figure ).
The patients’ characteristics of PPM-positive group and PPM-negative group were shown in Table . Compared to the PPM-negative group, most of the characteristics in the PPM-positive group were similar except the significantly higher frequency of exacerbations in the previous year and the Anthonisen type I (Table ).
Table 1. Characteristics of PPM-positive and PPM-negative group
Bacteriology
The routine sputum culture results are shown in Table . Despite the self-reported purulence, the frequency of bacterial infection at AECOPD is low. Of the 135 sputum analyzed, 47 bacterial species were detected from 42 samples (31.1%), including 13 Haemophilus influenzae (9.6%), 12 Pseudomonas aeruginosa (8.9%), 7 Streptococcus pneumonia (5.2%) and some other species (Table ). Consistent with other reports, Haemophilus influenzae was the most popular bacteria in this study, however, unlike those studies, Pseudomonas aeruginosa ranked the second. Five of the 12 Pseudomonas aeruginosa were co-infected with other pathogens such as MRSA, Acinetobacter baumanii, Escherichia coli, and Candida albicans (Table ).
Table 2. Routine sputum culture results in patients admitted with AECOPD
Comparison of the PPM-positive and PPM-negative cohorts: correlations among bacterial infection, adverse outcomes, airway and systemic inflammation and CAT score
For comparison of the adverse outcomes, all the 135 subjects were included (Figure , Table ). Among the 42 PPM-positive patients, a total of 11 (26.2%) patients had one or more adverse outcomes. Similarly, 29 out of 93 (31.2%) PPM-negative patients had at least one adverse outcome. No significant difference in adverse outcomes was observed between the PPM-positive and PPM-negative groups (chi-square test, p = 0.556).
Table 3. Relationship between bacterial infection and adverse events
To compare differences in the airway and systemic inflammation and CAT scores between exacerbation and the subsequent stable states, we included 32 PPM-positive and 62 PPM-negative patients who survived, returned to baseline (pre-exacerbation) state and were not re-hospitalized in 8 weeks (Figure and Table ). At exacerbation, only the airway neutrophils in the PPM-positive group were significantly higher than those in the PPM-negative group (p = 0.028). The airway MPO and systemic hs-CRP in the PPM-positive group were also higher than those in the PPM-negative group, but did not reach statistical significance (Table ).
Table 4. Airway infection and decrease in airway and systemic inflammation from exacerbation to subsequent baseline
All the airway and systemic inflammation indices decreased significantly from exacerbation to the subsequent baseline (p < 0.5). Interestingly, the decrease in neutrophils and hs-CRP level in the PPM-positive group was significantly higher than that in the PPM-negative group. None of the baseline stage inflammation indices was significantly different between the two groups except hs-CRP, which is lower in the PPM-negative group (Table ).
We used the CAT score as another clinical outcome to assess the patient's health status and exacerbation severity (Citation27, 28). No difference in CAT score was detected between PPM-positive and PPM-negative groups either at exacerbation or the subsequent stable baseline. However, the CAT score decreased greater in the PPM-positive group than in the PPM-negative group from exacerbation to baseline (p = 0.042, Table ).
Comparison of the bacterial clearance and persistence group within the PPM-positive cohort: correlation among bacterial clearance, resolution of airway and systemic inflammation and clinical outcomes at discharge
After comparing changes in inflammation and clinical outcomes from exacerbation to the subsequent baseline between PPM-positive and PPM-negative groups, we next examined the changes in microbiology, inflammation and clinical outcomes within the PPM-positive group in a shorter period of time, from admission (day 0) to discharge (days 10–14). For this analysis, we included 38 patients who survived after hospitalization. On the day of planned discharge, 19 of the 38 patients remained PPM-positive (bacterial persistence group), whereas the other 19 became PPM-negative (bacterial clearance group).
The 19 patients in the bacterial persistence group had a higher bacterial load than the 19 patients in the bacterial clearance group at admission (p = 0.019, Figure ). The bacterial load in the bacterial clearance group dropped below the detection level at discharge. No significant change in bacterial load in the bacterial persistence group was observed (p = 0.120, Figure ), although the bacterial species changed in 7 of the 19 patients at discharge (Table ).
Figure 2. Bacterial load at exacerbation and discharge in the bacterial persistence group (A) and bacterial clearance group (B). The differences in the bacterial load at exacerbation between the two groups were evaluated using Mann-Whitney U-test and the changes from exacerbation to discharge within each group were examined using the Wilcoxon's signed rank test.
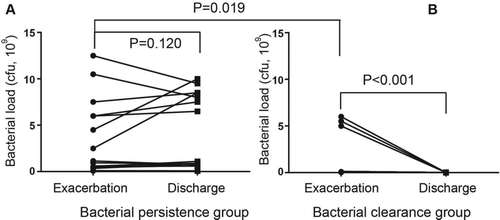
Table 5. Decreases in airway and systemic inflammatory indices and CAT score from exacerbation to the subsequent baseline
Table S1. Types of potentially pathogenic microorganisms (PPM) isolated at admission and at discharge
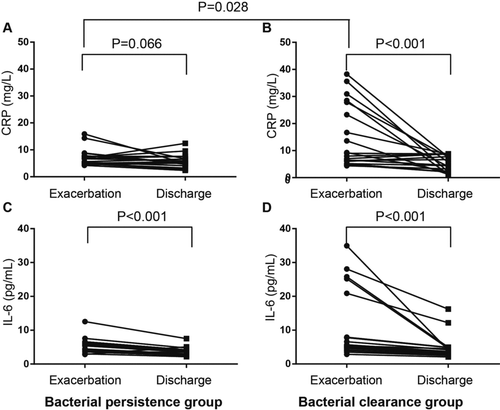
Despite the higher bacterial load in the bacterial persistence group at admission, no significant differences in the inflammatory measurements between the two groups were observed except the hs-CRP level. Surprisingly, the hs-CRP (9.0, 6.0–27.9) in the clearance group is significantly higher than that in the persistent group (6.7, 5.0–7.8, p = 0.028). Compared to admission, both airway and systemic inflammation at the day of discharge decreased significantly in the two groups (Figures and ), except hs-CRP the decrease of which did not reach statistical significance. Changes in these inflammatory indices between admission and the day of discharge in the two groups were shown in Table . Compared with bacterial clearance group, bacterial persistence group had marginally significant lower decrease in serum hs-CRP. No significant differences in inflammation were observed between the two groups at discharge.
Figure 3. Changes in airway inflammation indices including neutrophils (A and B), IL-8 (C and D) and MPO (E and F) from exacerbation to discharge in the bacterial persistence (A, C and E) and clearance (B, D and F) groups. The differences in the inflammation at exacerbation between the two groups were evaluated using Mann-Whitney U-test and the changes from exacerbation to discharge within each group were examined using the Wilcoxon's signed rank test.
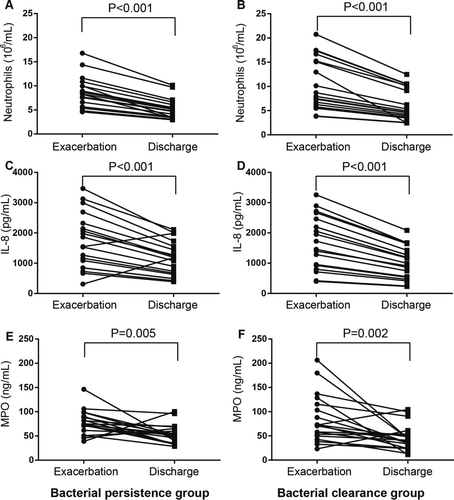
Figure 4. Changes in systemic inflammation indices including CRP (A and B) and IL-6 (C and D) from exacerbation to discharge in the bacterial persistence (A and C) and clearance (B and D) groups. The differences in the inflammation at exacerbation between the two groups were evaluated using the Mann–Whitney U-test, and the changes from exacerbation to discharge within each group were examined using Wilcoxon's signed rank test.
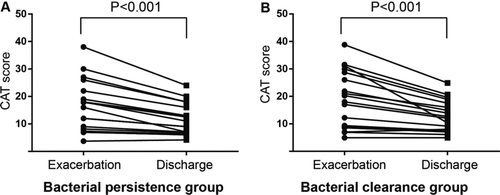
At the day of discharge, CAT value decreased significantly compared to admission (Figure ). There was no significant difference in improvement of CAT value between bacterial clearance group and bacterial persistence group (5.0, 1.5–9.8) vs 5.3, 1.3–8.0), p = 0.834) at day of discharge compared to admission. No significant differences in CAT score on the day of admission, or discharge were observed between the two groups, either.
Figure 5. Changes in CAT score from exacerbation to discharge in the bacterial persistence (A) and clearance (B) groups. The differences in the CAT score at exacerbation between the two groups were evaluated using the Mann–Whitney U-test and the changes from exacerbation to discharge within each group were examined using Wilcoxon's signed rank test.
The adverse outcomes including the rate of relapse (3/19 in bacterial clearance group vs 2/19 in bacterial persistence group, p > 0.05) or death within 8 weeks of discharge (0/19 in bacterial clearance group vs 1/19 in bacterial persistence group, p = 1.000) were comparable between bacterial clearance group with bacterial persistence group (p > 0.05).
Though we did not find significant difference in CAT scores and adverse outcomes between the bacterial persistence and clearance groups, we did observe differences in the frequency of exacerbations. Among the 38 PPM-positive patients, 21 were frequent exacerbators, while 17 were non-frequent exacerbators. The percentage of frequent exacerbators in the bacterial clearance group (36.8%, 7 out of 19) was significantly lower than that in the bacterial persistence group (73.7%, 14 out of 19) (Chi-square, p = 0.022).
Discussion
To the best of our knowledge, this is the first comprehensive characterization of bacteriology, inflammation, and clinical outcomes in both a longer (exacerbation to the subsequent baseline) and shorter (exacerbation to discharge) period of time in a Chinese population with AECOPD. Potentially pathogenic bacteria were found in 32.4% of episodes of AECOPD, based on sputum culture data. Haemophilus influenzae (9.6%) was the most popular species detected in this study, consistent with previous reports (Citation8, Citation15, Citation29, 30). However, unlike those studies, Pseudomonas aeruginosa (8.9%), rather than Streptococcus pneumonia (5.2%) or Moraxella catarrhalis (1.5%), was the second most frequent bacterium detected in this study. Interestingly, Pseudomonas aeruginosa also ranked second in two other studies focusing on Asian populations with AECOPD in Hong Kong (Citation21, 22).
Althought the differences in bacterial occurrence might be because of differences in population investigated, this discrepancy was also probably due to the differences in disease severity. Previous studies have shown that Pseudomonas aeruginosa are more commonly detected in severe exacerbations with greater lung function impairment and airflow obstruction (Citation31–33). It is worth noting that the majority of the cohort in this study has severe exacerbation, although no significant difference in FEV1% predicted was observed between Pseudomonas aeruginosa positive and Pseudomonas aeruginosa negative group (44 (34, 54) vs 52 (36, 56), p = 0.181). More studies focusing on the patients with mild exacerbation or stable state are desired to determine if the bacteriology in Chinese people is different from those in other populations.
The use of sputum purulence as an indicator of bacterial infection has been controversial. While several previous studies reported that sputum purulence was associated with a higher rate of positive cultures (Citation34, 35), others showed that the sputum purulence at exacerbation is insufficient for predicting benefit from antibiotic therapy (Citation36, 37). In our study, although we constrained our inclusion criteria to only include those with self-reported purulence in their sputum, the percentage of PPM-positive patients was still low (only 32.4%), suggesting that purulence alone is not a good predictor of infection.
A small number (4 out of 132) induced sputum specimens were included in this study. These samples were induced during the subsequent stable stages after discharge, and were equal distributed between the PPM-positive (2) and PPM-negative (2) group. Previous study has shown that airway inflammatory markers are equivalent between spontaneous and induced sputum samples (Citation38). Consistent with their data, we did not see significant differences in these markers between spontaneous and induced samples (for sputum neutrophil: 3.8 (3.2, 5.6) vs 4.4 (2.9, 6.4), p = 0.646; for sputum IL-8:1046.5 (564.6,1559.7) vs 1238.5 (732.4,1663.8), p = 0.64; for sputum MPO:31.2 (25.0, 35.2) vs 44.8(27.8, 66.0), p = 0.16).
Patel et al. found that the presence of bacterial colonization in the stable state was associated with increased exacerbation frequency (Citation39). Consistently, our data showed that the frequency of exacerbations was also positively correlated with bacterial infection at the onset of exacerbation. In the PPM-positive group, significantly more patients had ≥2 exacerbations in the previous year. In addition, the persistence of bacteria after antibiotic treatment is also positively correlated with the frequency of exacerbations. In our study, the bacterial persistence group had significantly more frequent exacerbators than the bacterial clearance group.
This is also in agreement with another study showing that frequent exacerbators have reduced responses to AECOPD therapy, which results in persistently higher systemic inflammatory markers during the recovery period (Citation40). Due to the observational nature of this study, a causality relationship could not be established. So we could not rule out the possibility that persistently higher inflammatory status in frequent exacerbators make them more susceptible to bacterial infections.
By comparing changes in inflammation levels between exacerbation and the subsequent baseline, consistent with a previous study, we found that presence of PPM in sputum was associated with a significantly larger decrease in serum hs-CRP levels and sputum neutrophil counts (Citation41), suggesting that these microorganisms were not just innocent bystanders. Because serum hs-CRP can be easily and rapidly measured in many healthcare settings our findings have important clinical implications. In addition to bacterial infections, viral infections and environmental stresses are also important triggers of AECOPD. Prescribing unnecessary antibiotics to patients with AECOPD increases the risk for bacterial resistance and other adverse effects. Our data and those from others suggest that hs-CRP level could serve as a reliable indicator of bacterial infection in patients with AECOPD and could be applied in decision-making process on whether to prescribe antibiotics for the treatment of AECOPD (Citation42, 43).
Another focus of this study was to determine if bacterial clearance is associated with the resolution of airway and systemic inflammation and improvement of clinical outcomes. Interestingly, in this study, all inflammatory index levels and CAT score at the day of discharge decreased significantly in both bacterial persistent group and bacterial clearance group, reflecting the response to antibiotic therapy. Although we did not find any difference in decrease of pulmonary inflammatory cytokines after AECOPD treatment between the two groups, we did observe marginally significantly higher decrease in hs-CRP level in the bacterial clearance group (3.1 (0.2, 20.2)) than in the bacterial persistence group (1.2 (−0.7, 2.7), Table ), suggesting that hs-CRP might be used as a marker for bacterial response to antibiotic treatment.
Because bacterial infection and changes in inflammation and clinical indices are the major focus of this study, antibiotics usage could potentially affect the bacteriology and related measures. We did not find any significant differences in the duration and types of antibiotics administered either between the PPM-positive and PPM-negative (Table ), or between the bacterial persistence and clearance groups (Table ), suggesting that the effect of antibiotics on bacteria are comparable between groups.
Table S2. Duration and types of antibiotic usage between potentially pathogenic microorganisms (PPM) positive and negative groups
Table S3. Duration and types of antibiotic usage between bacterial clearance and persistence groups
One limitation of this study is that we used culture-based approach to study the bacterial infection in AECOPD, which only capture the common pathogens. Recent studies using culture-independent pyrosequencing-based approaches revealed that the bacterial communities infecting patients with COPD are much more complex than previously appreciated (Citation44, 45). Therefore, the resolution of inflammation in the bacterial persistence group might be due to the eradication of other bacteria in the communities that were not detected by culture. Those bacteria either play direct roles in causing inflammation or indirect roles by synergistic interaction with the persistent pathogen. The eradication of those bacteria, thus, led to the decrease in inflammation levels.
Of note, despite their common presence in AECOPD and potential to confound the result, viral (co)infections were not investigated in this study due to the relatively small sample size. The small sample size might also lead to the lack of sufficient power in cross-sectional comparisons of the inflammatory indices between groups, although significant changes in these measures were observed longitudinally. Another limitation of this study is that the majority of the subjects in this study have severe disease. Some of the findings in this study might not be generalizable to the mild and moderate patients.
Conclusions
In conclusion, bacterial infections are positively associated with exacerbation frequencies and decrease in neutrophils and hs-CRP levels from exacerbation to the subsequent baseline. Within the PPM-positive group, decreases in both airway and systemic inflammation were observed, regardless of the status of bacterial clearance, suggesting the existence of virus and/or uncultivated bacteria and their potential roles in causing inflammation and exacerbations.
Funding
This study was supported by a Special Fund of the Chinese Medical Association for the scientific research of chronic respiratory tract diseases (07010440052).
Declaration of Interest Statement
The authors declared that they have no conflict of interests. The authors are responsible for the writing and the content of this paper.
References
- Bozinovski S, Hutchinson A, Thompson M, MacGregor L, Black J, Giannakis E, Karlsson AS, Silvestrini R, Smallwood D, Vlahos R, Irving LB, Anderson GP. Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Resp Crit Care 2008; 177(3):269–278.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Zhou Y, Liu S, Wang X, Wang D, Lu J, Zheng J, Ran P. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med [Research Support, Non-U.S. Gov't]. 2007; 176(8):753–760.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555.
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57(10):847–852.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157(5 Pt 1):1418–1422.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60(11):925–931.
- Anzueto A, Sethi S, Martinez FJ. Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007; 4(7):554–564.
- Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005; 60(4):293–300.
- Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173(1):71–78.
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006; 173(10):1114–1121.
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007; 370(9589):786–796.
- Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax 2006; 61(3):250–258.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359(22):2355–2365.
- Murphy TF. The role of bacteria in airway inflammation in exacerbations of chronic obstructive pulmonary disease. Curr Opin Infect Dis 2006; 19(3):225–230.
- Parameswaran GI, Sethi S, Murphy TF. Effects of bacterial infection on airway antimicrobial peptides and proteins in COPD. Chest 2011; 140(3):611–617.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181(2):150–157.
- Monso E, Ruiz J, Rosell A, Manterola J, Fiz J, Morera J, Ausina V. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med 1995; 152(4 Pt 1):1316–1320.
- Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, Hernandez C, Rodriguez-Roisin R. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998; 157(5 Pt 1):1498–1505.
- Cabello H, Torres A, Celis R, El-Ebiary M, Puig de la Bellacasa J, Xaubet A, Gonzalez J, Agusti C, Soler N. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J 1997; 10(5):1137–1144.
- Ko FW, Ip M, Chan PK, Fok JP, Chan MC, Ngai JC, Chan DP, Hui DS. A 1-year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest 2007; 131(1):44–52.
- Ko FW, Ng TK, Li TS, Fok JP, Chan MC, Wu AK, Hui DS. Sputum bacteriology in patients with acute exacerbations of COPD in Hong Kong. Respir Med 2005; 99(4):454–460.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106(2):196–204.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128–1138.
- Louis R, Shute J, Goldring K, Perks B, Lau LC, Radermecker M, Djukanovic R. The effect of processing on inflammatory markers in induced sputum. Eur Respir J 1999; 13(3):660–667.
- Efthimiadis A, Pizzichini MM, Pizzichini E, Dolovich J, Hargreave FE. Induced sputum cell and fluid-phase indices of inflammation: comparison of treatment with dithiothreitol vs phosphate-buffered saline. Eur Respir J 1997; 10(6):1336–1340.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34(3):648–654.
- Mackay AJ, Donaldson GC, Patel AR, Jones PW, Hurst JR, Wedzicha JA. Usefulness of the Chronic Obstructive Pulmonary Disease Assessment Test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med 2012; 185(11):1218–1224.
- Mackay AJ, Hurst JR. COPD exacerbations: causes, prevention, and treatment. Immunol Allergy Clin North Am 2013; 33(1):95–115.
- Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3(3):245–251.
- Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177(8):853–860.
- Beasley V, Joshi PV, Singanayagam A, Molyneaux PL, Johnston SL, Mallia P. Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis 2012; 7:555–569.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M, COPD SGBI. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest 1999; 116(1):40–46.
- Allegra L, Blasi F, Diano PL, Cosentini R, Tarsia P, Confalonieri M, Dimakou K, Valenti V. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Resp Med 2005; 99(6):742–747.
- Soler N, Agusti C, Angrill J, Puig De la Bellacasa J, Torres A. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease. Thorax 2007; 62(1):29–35.
- Brusse-Keizer MG, Grotenhuis AJ, Kerstjens HA, Telgen MC, van der Palen J, Hendrix MG, van der Valk PD. Relation of sputum colour to bacterial load in acute exacerbations of COPD. Respir Med 2009; 103(4):601–606.
- van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C, Hendrix R. Clinical predictors of bacterial involvement in exacerbations of chronic obstructive pulmonary disease. Clin Infect Dis 2004; 39(7):980–986.
- Bhowmik A, Seemungal TA, Sapsford RJ, Devalia JL, Wedzicha JA. Comparison of spontaneous and induced sputum for investigation of airway inflammation in chronic obstructive pulmonary disease. Thorax 1998; 53(11):953–956.
- Patel IS, Seemungal TAR, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002; 57(9):759–764.
- Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Mullerova H, Donaldson GC, Wedzicha JA. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J 2007; 29(3):527–534.
- Bathoorn E, Liesker JJ, Postma DS, Koeter GH, van der Toorn M, van der Heide S, Ross HA, van Oosterhout AJ, Kerstjens HA. Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis 2009; 4:101–109.
- Stockley RA, O'Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 2000; 117(6):1638–1645.
- Daniels JM, Schoorl M, Snijders D, Knol DL, Lutter R, Jansen HM, Boersma WG. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest 2010; 138(5):1108–1115.
- Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One 2012; 7(10):e47305.
- Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, Martinez FJ, Huffnagle GB. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 2011; 6(2):e16384.